A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors
2019-02-15YuChenLianchaoLiuDiminWuYuPengHeAngLi
Yu Chen,Lianchao Liu,Dimin Wu,Yu-Peng He*,Ang Li,*
a College of Chemistry,Chemical Engineering and Environmental Engineering,Liaoning Shihua University,Fushun 113001,China
b State Key Laboratory of Bioorganic and Natural Products Chemistry,Center for Excellence in Molecular Synthesis,Shanghai Institute of Organic Chemistry,Chinese Academy of Sciences,Shanghai 200032,China
Key words:Click chemistry Azide–alkyne cycloaddition Alkenyl tri fl ates Lithium chloride
ABSTRACT On the basis of the mild transformation of alkenyl tri fl ates into alkynes promoted by LiCl,a one-pot protocol using alkenyl tri fl ate precursors was developed for copper-mediated azide–alkyne cycloaddition.This protocol may provide an opportunity of sequentially click reactions for the construction of bifunctional probes in chemical biology studies.
The emergence and evolution of click chemistry have greatly facilitated the development of the fields of chemical biology and material science[1].Thecopper catalyzed azide–alkynecycloaddition(Cu AAC)pioneered by Sharplessand co-workers[2]remainsoneof the most powerfultoolsofclick chemistry,despitenumerousendeavorsto develop various click reactions[1c,d,g].CuAACprovidesan expedient and modular approach to prepare small molecule probesof biological interest;biotin,photoactivatable groups,and fluorophores can be readily installed onto the small molecule core at a late stage [23_TD DIFF1b].In recent years,sequential click reactions have received increasing attention[3].The programmed reaction sequence allows rapid construction of multifunctional small molecule probesand polymers.For instance,an af fi nity-based bifunctional probe for cellular target identi fi cation usually contain a photoactivatable group(such as a diazirine)for crosslinking with the target protein and a biotin tag for pull-dow n[4],and it is more practical to introduce the photoactivatable group by aclick reaction at a late stage rather than to carry on the fairly labile functionality through ade novo synthesis.In this case,sequential click reactionsare highly desired.Although one can envision a variety of combinations of click reactions,the most attractive and straightforw ard strategy of using double Cu AAC reactions to install different functionalities is hampered by the narrow w indow for differentiation of the substrate reactivity.Recently,we reported the LiCl mediated elimination of alkenyl tri fl ates for the preparation of alkynes under mild conditions[5],which permits a strategy of masking terminal alkynes as alkenyl tri fl ates in a click process.Thus,we envision a programmed sequence of tw o copper mediated cycloaddition reactions starting from asubstrate containing a terminal alkyne and an alkenyl tri fl ate which can beeasily converted into an terminal alkyne.The fi rst click event should occur under standard Cu AAC conditions,while the second step requiresan efficient one-pot protocolon thebasisof our previous discovery[5].Herein,we describe such a protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors.
We investigated the conditions for the one-pot elimination/azide formation/cycloaddition sequence,using readily available alkenyl tri fl ate 1[5]as a precursor.The resultswere summarized in Table 1.In the presence of LiCl,elimination of 1 proceeded in DMF at ambient temperature to give the desired terminal alkyne[5].Benzyl azidewasthen generated in situ from NaN3and Bn Br through an SN2 reaction;DMFalso served as a good solvent for the substitution.Thus,weneeded a suitable copper salt or complex asa promoter for the cycloaddition,which had to be compatible to the reaction system.The commonly used Cu I[6]proved to be ineffective under these conditions(entry 1).Interestingly,copper(I)thiophene-2-carboxylate(CuTC)[5,7]led to formation of asmall amount of 1,2,3-triazole2(entry 2).Increasing theequivalentsof Cu TCimproved the yield of 2 to 30%,which was still unsatisfactory from a synthetic perspective.To our delight,Et3N was found to be a crucial additive for this one-pot process,and 2 was obtained in 78%isolated yieldfrom 1.Et3N presumably buffered the acid generated through the elimination processand might promotetheformation of thecopper acetylide intermediate as well.Of note,the amine can be added before the SN2 reaction;this order change had no bearing on the overall ef fi ciency.
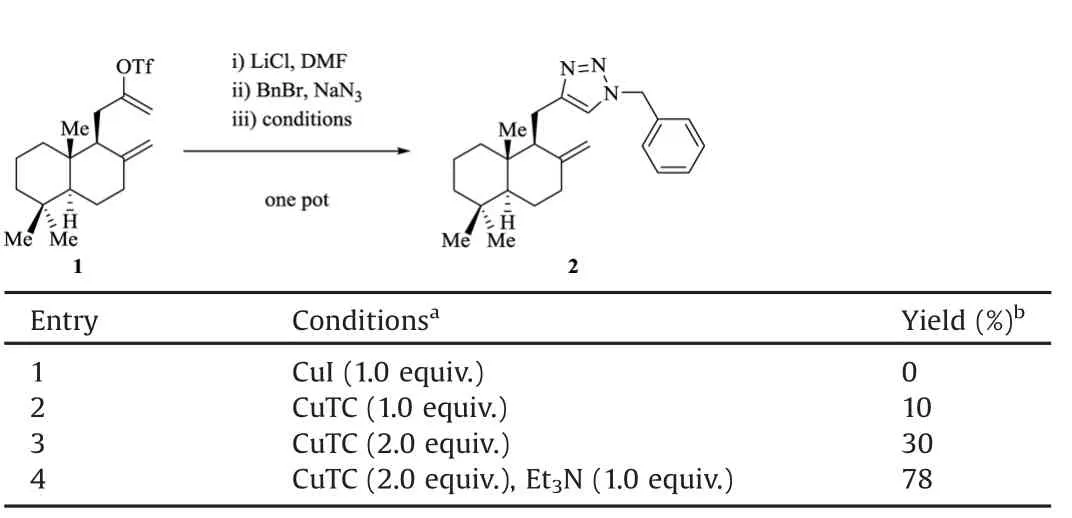
Table 1 Studies of the conditions for the one-pot tri fl ate elimination/azide formation/cycloaddition sequence.
Having established the optimal conditions,we examined a series of azides(generated in situ from NaN3and corresponding alkyl bromides)for the one-pot process,as show n in Table 2.1,2,3-Triazoles 3–11 were obtained in good to excellent yields(entries1–9).Substituted benzyl azides with electron-rich(entry 1)or electron-de fi cient(entries 2 and 3)aromatic rings all performed well under the standard conditions.α-Azido carbonyl compounds(entries 4–7)were suitable substrates as well,which w ould allow facile incorporation of a photoactivatable group or a biotin tag.To our delight,the reactions of allyl azides(entries 8 and 9)proceededwith good ef fi ciency,which may offer an alternative type of linkers of improved stability compared with esters and amides.
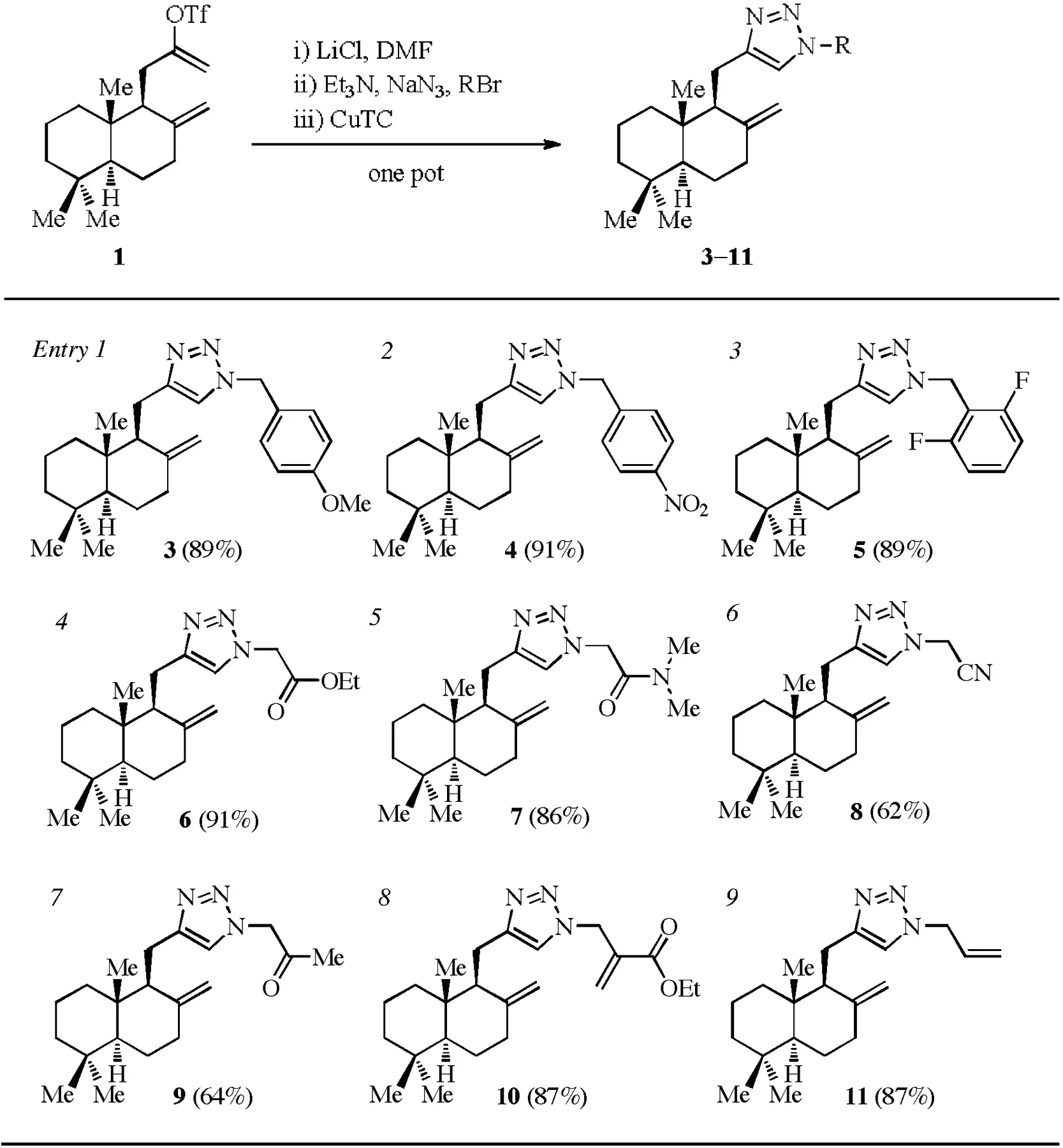
Table 2 Scope of the azides(generated in situ)suitable for the one-pot sequence.
A general procedure for the one-pot sequence is described below.To a stirred solution of alkenyl tri fl ate 1 (34.2 mg,0.090 mmol)in DMF(300 m L)was added anhydrous LiCl(15.3mg,0.361 mmol)at 22?C.The mixture was allowed to stir at that temperature for 1.5h before Et3N(9.1 mg,12.5 m L,0.090 mmol),NaN3(8.8 mg,0.135 mmol),and the bromide(0.135 mmol)were sequentially added.The resultant mixture was stirred at 22[13_TD DIFF]?Cfor 2.5 h,and Cu TC(34.3 mg,0.180 mmol)wasadded.The mixture was allowed to stir at that temperature for 2h before it was quenched with saturated aq.NaHCO3(2m L)and extracted with EtOAc(5?2 m L).The combined organic phases were washed with brine(5m L),dried over anhydrous Na2SO4,fi ltered,and concentrated under vacuum.The residue was subjected to preparative thin layer chromatography for puri fi cation using EtOAc/petroleum ether(1:3)as eluent to give the 1,2,3-triazole.Characterization of compounds 2–11 is included in the Supporting information.
In summary,we have developed an efficient protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors.Three sequential reactions,namely the LiCl-promoted elimination for alkyne formation,the nucleophilic substitution for azide formation,and the cycloaddition for the triazole formation,proceeded smoothly in one pot.This protocol may fi nd further use in the construction of bifunctional small molecule probesfor target identi fi cation,imaging,and other chemical biology studies.
Acknow ledgm ents
We thank Xiaowen Yang and Dr.Wenhao Zhang for helpful discussions.Financial support was provided by the National Natural Science Foundation of China(Nos.21525209,21621002,21772225,and 21761142003),the Chinese Academy of Sciences(Strategic Priority Research Program(No.XDB20000000)and Key Research Program of Frontier Sciences(No.QYZDB-SSW-SLH040)),Shanghai Science and Technology Commission(Nos.15JC1400400 and 17XD1404600),the National Program for Support of Top-Notch Young Professionals of China,and the K.C.Wong Education Foundation.
Appendix A.Supplem entary data
Supplementary material related to thisarticle can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2018.06.001.
杂志排行
Chinese Chemical Letters的其它文章
- Information for authors
- One-pot synthesis of tetrahydroindoles via a copper catalyzed N-alkynation/[4+2]cycloaddition cascade
- Gram-scale preparation of dialkylideneacetones through Ca(OH)2-catalyzed Claisen-Schmidt condensation in dilute aqueous EtOH
- Tetra-phthalimide end-fused bi fl uorenylidene:Synthesis and characterization
- Water bridges are essential to neonicotinoids:Insights from synthesis,bioassay and molecular modelling studies
- Novel dual inhibitors against FP-2 and PfDHFRas potential antimalarial agents:Design,synthesis and biological evaluation
