Gram-scale preparation of dialkylideneacetones through Ca(OH)2-catalyzed Claisen-Schmidt condensation in dilute aqueous EtOH
2019-02-15HaoZhangMengtingHanChenggenYangLeiYuQingXu
Hao Zhang,Mengting Han,Chenggen Yang,Lei Yu,*,Qing Xu,*
a Guangling College,Yangzhou University,Yangzhou 225002,China
b School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,China
Key words:Claisen-Schmidt condensation Acetone Aldehyde a,b-Unsaturated ketone Dialkylideneacetone
ABSTRACT A synthetic method of dialkylideneacetones has been developed.Compared with known protocols,the method employed catalytic Ca(OH)2 as the cheap,mild base catalyst and dilute aqueous EtOH(20%,v/v)as the green and safe solvent.The procedure was easily operated:In most cases,the product could be isolated by a simple fi ltration,and puri fied by washing with w ater.This paper provided experimental details of the reactions,which could be applied in gram-scale synthesis and should be a very reliable and practical protocol to prepare these useful compounds in laboratory and at the industrial level.
Dialkylideneacetones serve as the key precursors to construct/synthesize many useful organic skeletons or natural products,such as pyrimidines,2,7-disubstituted tropones,cystodytins,etc.[1–3].They are also w idely employed as bioactive components in antiangiogenic reagent,quinine reductase inducer and arginine methyltransferase inhibitor in biochemistry studies[4–7],as agrochemical,pharmaceutical and perfume intermediates and ligands in fine chemical industry[8–11],and as liquid crystalline polymer units in materials science [12]. Like simple a,b-unsaturated ketones,dialkylideneacetones could be prepared through the Claisen-Schmidt reactions of acetone with aldehydes for the high atom utilization ef fi ciency form the industrial view point[13,14].However,the present methods were catalyzed/promoted by strong acids or bases[15–19],Lew is acid catalysts such as Cu(OTf)2[20],FeCl3[21],TiCl3(OTf)[22],Sm I3[23],Yb(OTf)3[24],etc.,noble metal catalysts such as Ru Cl3[25],TMSCl/Pd-C[26],or supported catalysts/dehydrants such as KFAl2O3[27],P2O5/SiO2[28],etc.,which were corrosive,expensive,or required tedious fabrication methods or high catalyst loadings.Moreover,although there are many reports on symmetrically substituted dialkylideneacetones,method for the synthesis of dialkylideneacetones with different alkylidenes has not been well documented yet.
Our group aims to develop the green synthetic methods with industrial application potential[29–34].Recently,it was found that Ca(OH)2,the cheap,mild and common but rarely used inorganic base,could serve as the efficient catalyst in dialkylideneacetone synthesis.The method required very low catalyst loading(10 mol%)and could use dilute alcohol as the green solvent(20%aqueous solution,v/v).It could be expanded to at least 100 mmol scales to produce the related dialkylideneacetonesin gram scale.Herein,we w ish to report the details of the method.
The Ca(OH)2-catalyzed reaction of(E)-4-phenylbut-3-en-2-one(1a)with benzaldehyde(2a)were initially tested.After heating 1 mmol of 1a with equivalent 2a in 1 m Lof EtOH in the presence of 0.2 mmol of Ca(OH)2at 60?Cfor 48 h,the desired product 3a could be isolated in 47%yield by preparative thin layer chromatography(Scheme 1).In the process,partial of the Ca(OH)2wasinsoluble and precipitated at the bottom of the reaction tube.Therefore,w ater was then introduced into the solvent to enhance the Ca(OH)2solubility.The product yield was enhanced with elevated H2O content and 20%aqueous EtOH(volume concentration)was found to be the most preferable solvent,affording 3a in 80%yield(Fig.1).No reaction occurred in the absence of EtOH,although Ca(OH)2dissolved completely in the case.This was probably due to the low solubility of the organic reactants in w ater,as after the addition of 2 mol%of sodium dodecyl sulfate or benzyldodecyldimethylammonium bromide as surfactant,the same reactions in w ater could afford the desired product 3a in 87%and 39%yields,respectively.Thus,EtOH,as the w ater-soluble organic solvent,might facilitate the contact of the organic reactants with Ca(OH)2to promote the reaction,and it was better than the sodium dodecyl sulfate surfactant for the solid waste-free process.
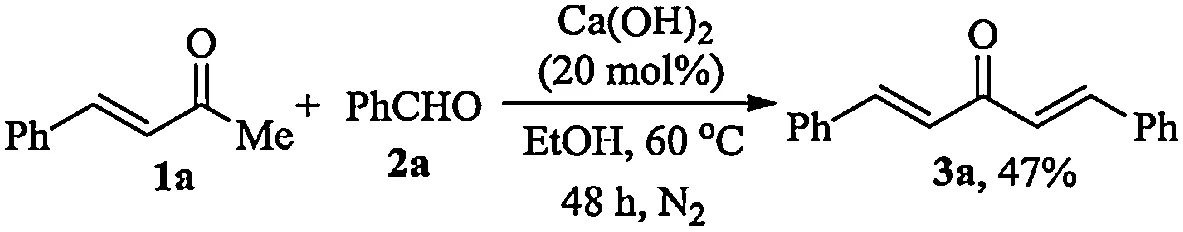
Schem e 1.Synthesis of dialkylideneacetones catalyzed by Ca(OH)2.
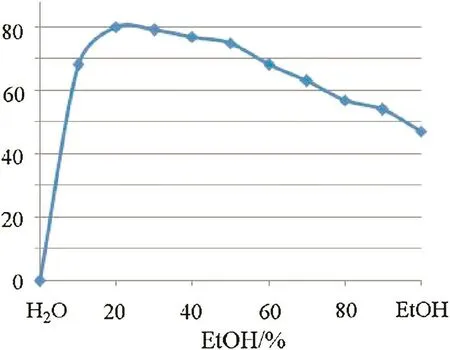
Fig.1.Experimental resultsof the reactionsperformed in aqueous EtOH at different volume concentrations.
Additional parallel experiments were conducted to optimize the reaction conditions.Elevated Ca(OH)2amount could hardly improve the reaction,while the excess catalyst precipitated due to itslow solubility(Table 1,entry 1).Considering the higher turnover number and the acceptable product yield,Ca(OH)2/1a=10%was then screened out to be the better condition(Table 1,entries3 vs.1,2,4).The reaction was retarded at reduced temperature(Table 1,entry 5),and afforded 3a in the highest yield(83%)at 80?C(Table 1,entries 7 vs.6,8).
Under the optimized conditions,the reaction of 1a with benzaldehyde(2a)was magni fied into 10 mmol scale and the desired product 3a could be obtained in 81%yield through column chromatography isolation(Table 2,entry 1).Further magni fied reaction at 100 mmol was conducted and in the process,the product 3a precipitated and could be isolated by a simple fi ltration(Fig.2a).After washing with water to remove the Ca(OH)2catalyst and drying under infrared lamp irradiation,pure 3a was obtained as a yellow solid in 80%yield(Fig.2b and Table 2,entry 2).4-Methylbenzaldehyde(2b),the electron-enriched aldehyde,could also react with 1a to produce the related dialkylideneacetone 3b in good yield at 100 mmol scale(Table 2,entry 3),but for 4-methoxybenzaldehyde(2c),since a series of unidenti fied byproduct was generated,no precipitation was observed in the100 mmol-scale reaction and the product was isolated in 51%yield by column chromatography in a 10 mmol-scale reaction(Table 2,entry 4).Notably,electron-de fi cient aldehydes,such as 2d–e,were preferable for the reaction,affording the desired products in excellent to almost quantitative yields(Table 2,entries 5 and 6).The reactions with heterocycle-substituted aldehydes 2f–g also led to excellent product yields(Table 2,entries 7 and 8).Aliphatic and alkenyl aldehydes 2h–i were also applicable to produce 3h–i inIFF]32%–55%yields(Table 2,entries 9 and 10).Because Ca(OH)2catalyst was used in very low loading(10 mol%)and might be deactivated by the carboxylic acid impurities,the aldehydes should be puri fied by distillation or recrystallization before using.
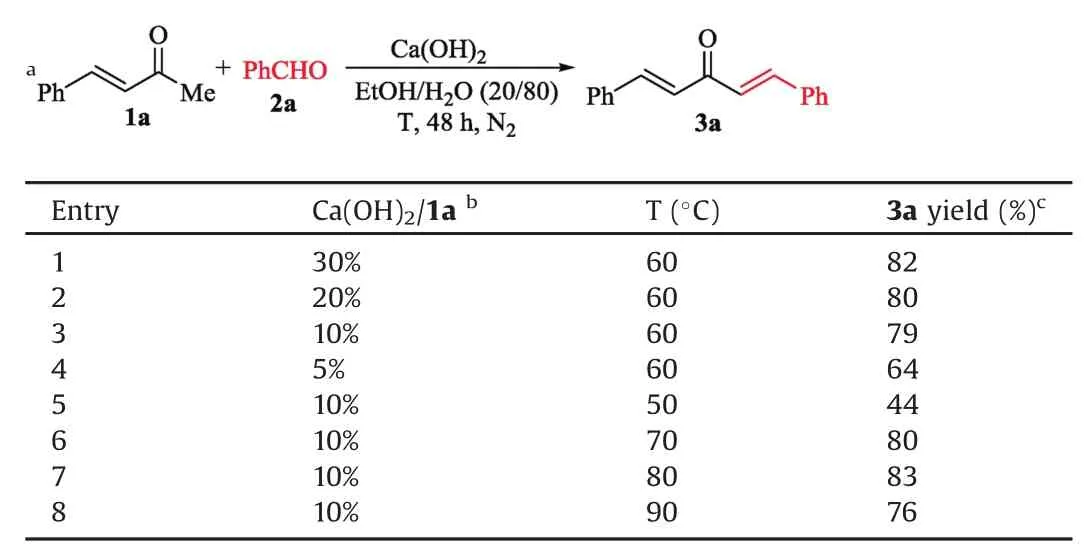
Table 1Condition optimizations for the reaction of(E)-4-phenylbut-3-en-2-one(1a)with benzaldehyde(2a).
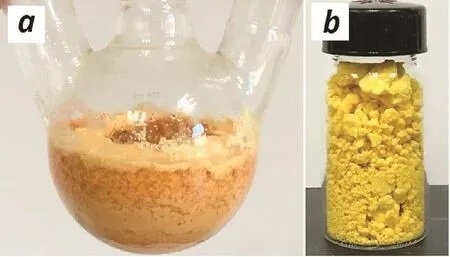
Fig.2.Photographs of the reaction fl ask after the reaction(a)and the puri fied product 3a(b).
As(E)-4-phenylbut-3-en-2-one(1a)was synthesized through the Ca(OH)2-catalyzed condensation of benzaldehyde(2a)with acetone,we then tried to combined the tw o reaction steps in one pot to prepare dialkylideneacetone 3a.In the process,2a fi rst reacted with acetone to produce 1a.The excess acetone and solvent were then removed by distillation and another solution of benzaldehyde 2a in fresh EtOH/H2O was added.After heating at 80?Cfor 48 h,the product 3a precipitated and could be isolated by fi ltration and puri fied by washing with w ater(Table 3,entry 1).Other dialkylideneacetones 3b–i were smoothly prepared in similar way(Table 3,entries 2–9).
Moreover,the order of introducing functional groups was reversible.As show n in Table 4,substituted aldehydes 2 fi rst reacted with acetone to generate the intermediate 1 in situ,which could then react with another benzaldehyde(2a)to produce the same products3 in moderate to excellent yields(Table 4,entries1–8).Notably,the yields of 3b–c could be enhanced by using this protocol(Table 4,entries 1 and 2 vs.Table 3,entries 2 and 3).
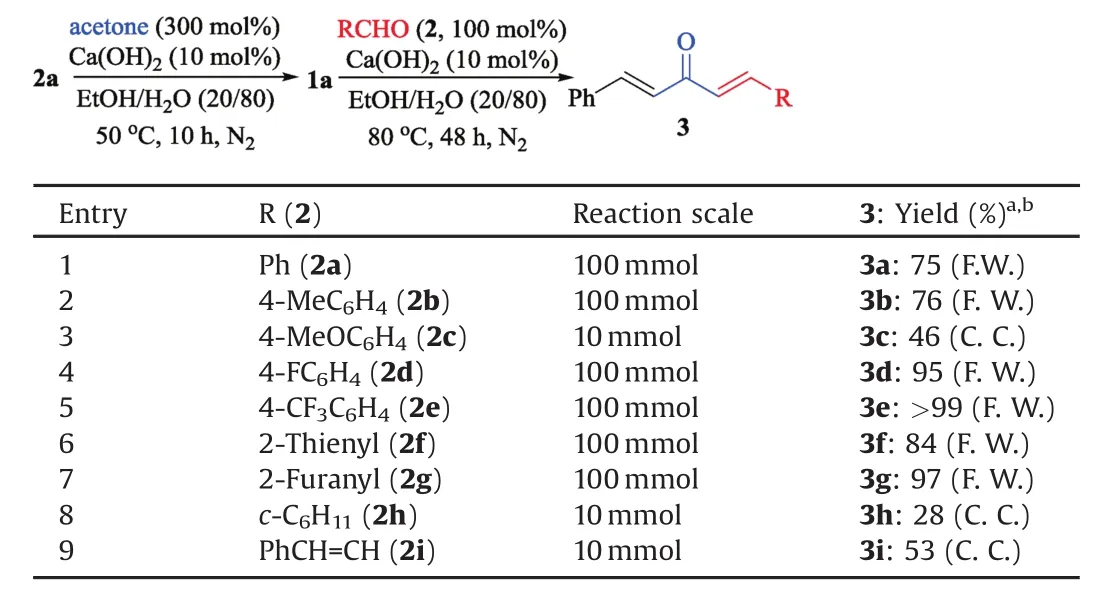
Table 3 One-pot synthesis of dialkylideneacetone 3 from 2a.
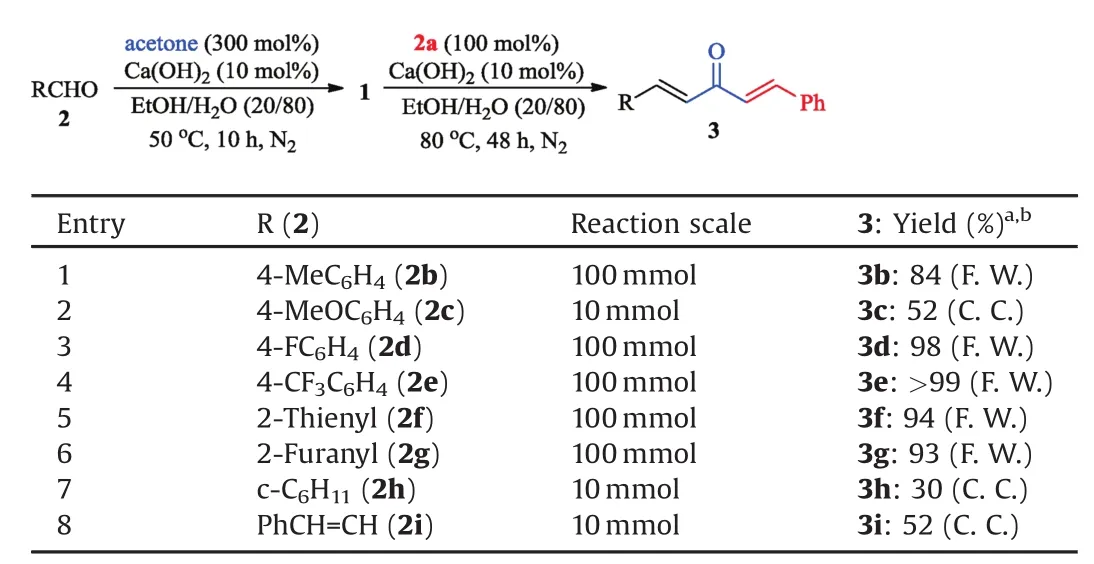
Table 4One-pot synthesis of dialkylideneacetone 3 from substituted aldehydes 2.
In conclusion,we developed a practical method for the synthesis of dialkylideneacetones through the Ca(OH)2-catalyzed Claisen-Schmidt condensations in dilute ethanol.The cheap,lowloading and mild catalyst,green solvent and the easily-operated procedures are the advantages of the protocol over many know n methods.The reaction wasscalable to at least 100 mmol to produce the desired dialkylideneacetones in grams,show ing very good practicability for both laboratory synthesisas well as the industrial level production.
Acknow ledgm ents
We thank the National Natural Science Foundation of China(Nos.21202141,21672163),Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD),Top-notch Academic Programs Project of Jiangsu Higher Education Institutions(TAPP),the high level talent support project of Yangzhou University(top-notch talent,L.Yu),Yangzhou Natural Science Foundation(No.YZ2014040)and the Natural Science Foundation of Guangling College(No.ZKZD17005)for fi nancial support.
杂志排行
Chinese Chemical Letters的其它文章
- Information for authors
- A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors
- One-pot synthesis of tetrahydroindoles via a copper catalyzed N-alkynation/[4+2]cycloaddition cascade
- Tetra-phthalimide end-fused bi fl uorenylidene:Synthesis and characterization
- Water bridges are essential to neonicotinoids:Insights from synthesis,bioassay and molecular modelling studies
- Novel dual inhibitors against FP-2 and PfDHFRas potential antimalarial agents:Design,synthesis and biological evaluation
