Tetra-phthalimide end-fused bi fl uorenylidene:Synthesis and characterization
2019-02-15DebinXiaFangyuanZhangZhigangLiuLinghaoMengRuiqingFanYulinYang
Debin Xia*,Fangyuan Zhang,Zhigang Liu,Linghao Meng,Ruiqing Fan*,Yulin Yang*
MIIT Key Laboratory of Critical Materials Technology for Newenergy Conversion and Storage,School of Chemistry and Chemical Engineering,Harbin Institute of Technology,Harbin150001,China
Key words:Bi fl uorenylidene Polycycles Electron acceptor Tw isting Three dimensional
ABSTRACT A novel electron acceptor,namely,tetra-phthalimide end-fused bi fl uorenylidene(3D-imide)was designed and synthesized.In comparison to its framework analogue 12,12'-bidibenzo[b,h]fl uorenylidene(BF-3),the electron withdraw ing capability and optical absorption were strengthened and enlarged,respectively.Moreover,photoluminescence quenching experiments were carried out,which con fi rmed 3D-imide could be a good candidate for nonfullene organic photovoltaics.Finally,we gained more insight into its electronic properties through density functional theory calculation.
In recent years,bi fl uoreylidene and its derivatives have attracted great attention,since their unique conformational behavior[1,2]and they are promising as new generation of electron acceptors in organic electronics[3–6].Now adays,the structures of related molecules are limited,which can mainly be divided into three types,namely,BF-1[7–13],BF-2[3,14–15]and BF-3[3,14,16](Fig.1).As for compounds BF-1 with functional groups in different positions,they present various conformations and physicochem ical properties.For instance,the dihedral angle between tw o fl uorene p-planes is range from 31?to 52?[12].For compounds BF-2 and BF-3[3],due to the effect of the enhanced p-conjugation,their absorption onsets of UV–vis spectra can be shifted to long w avelengths of 560 nm and 603 nm,respectively,which is bene fi cial to harvest more sunlight.Even though BF-2 based organic photovoltaics devices realized a power conversion ef fi ciency(PCE)of 1.7%[3],its narrow light absorption range and high lowest unoccupied molecular orbital(LUMO)level strongly restrict further device ef fi ciency improvement.
To overcome the aforementioned issues and further enrich the bi fl uoreylidene structure library,the tetra-phthalimide endcapped bi fl uorenylidene(3D-imide)is designed based on the follow ing considerations:(1)The light absorption onset can redshift since effective p-conjugation is elongated;(2)The electron-withdraw ing dicarboximides units are introduced to decrease the LUMO energy level;(3)Alkyl chains can be easily attached at the nitrogen atoms of the end-capped dicarboximides to ensure the good solubility in common solvents.Herein,we report the synthesis,physical and electrochemical properties of a novel 3D acceptor.In the end,density functional theory(DFT)calculation method is employed,w hose results are well in agree with the experimental discoveries.
The target compound 3D-imide could be synthesized via tw o routesasillustrated in Scheme 1.2,2',3,3',6,6',7,7'-Octamethyl-9,9'-bi fl uorenylidene(2)was synthesized in 32%yield through Lawesson’s reagent-assisted reductive coupling with 2,3,6,7-tetramethyl-9H-fl uoren-9-one(1)as starting material.Thereafter,radical bromination was done with bromine in CCl4upon irradiation with a 250 W halogen lamp[17],which afforded 2,2',3,3',6,6',7,7'-octakis(dibromomethyl)-9,9'-bi fl uorenylidene(3)in 75%yield.Precursor 3 wasdirectly used in the next step without further puri fi cation.Finally,the target compound 3D-imide was obtained as purple solid in 50%yield via a Diels-Alder cycloaddition.It is noted that using our reported precursor 4[17]as starting material can also yield 3D-imide.Its chemical structure was unambiguously con fi rmed by high-resolution mass spectrometry(HRMS).Fig.2 displays a single peak at m/z 1704.1512 Da,which is consistent with its expected molecular mass of 1704.1529 Da.Also,the isotopic distribution observed perfectly matches with the simulated pattern.A well-resolved1H NMRspectrum of 3D-imide could be recorded in CDCl3at room temperature.As show n in1H NMRspectrum(see the Supporting information),we can assign the protons near nitrogen atoms to the chemical shift of 3.6 ppm and the four single peaks(8.0–9.5 ppm)are assigned to the varying environmental aromatic protons of 3D-imide,w hose FT-IR spectrum was also recorded(Fig.S1 in Supporting information).3D-imide presents very good solubility in common organic solvents and excellent stability in the solid state,facilitating all further characterizations.Note that a resolved13CNMRspectrum could not be obtained,even though we enhanced the concentration of the CDCl3solution and extended the accumulation time of NMR experiments,which is similar to the reported results[18].
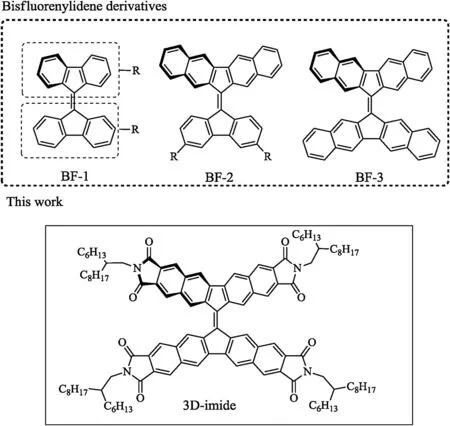
Fig.1.Chemical structuresof bis fl uorenylidene derivatives(Rrepresents functional groups)and 3D-imide.
The solution(chloroform)and thin-fi lm UV–vis absorption spectra of 3D-imide are show n in Fig.3.The fi rst band peaking at 326 nm is corresponding to the p–p*transitions of the diphthalimide fused fl uorene,while the band in the long-w ave length region is ascribed to the p–p*transition of the tetraphthalimide end-capped bi fl uorenylidene conjugated structure.No obvious spectral shift is observed from solution to thin fi lm,indicating that there is no appreciable intermolecular aggregation,which might be caused by the 3D geometry.Notably,the absorption edge of 3D-imide is 655 nm,which is redshifted with respect to that of compound BF-3,due to the extension of the effective conjugation length by the incorporation of the imide moieties[3].The optical gap of 3D-imide is determined to be 1.90 eV based on the equation Eopt=1240/lonset.
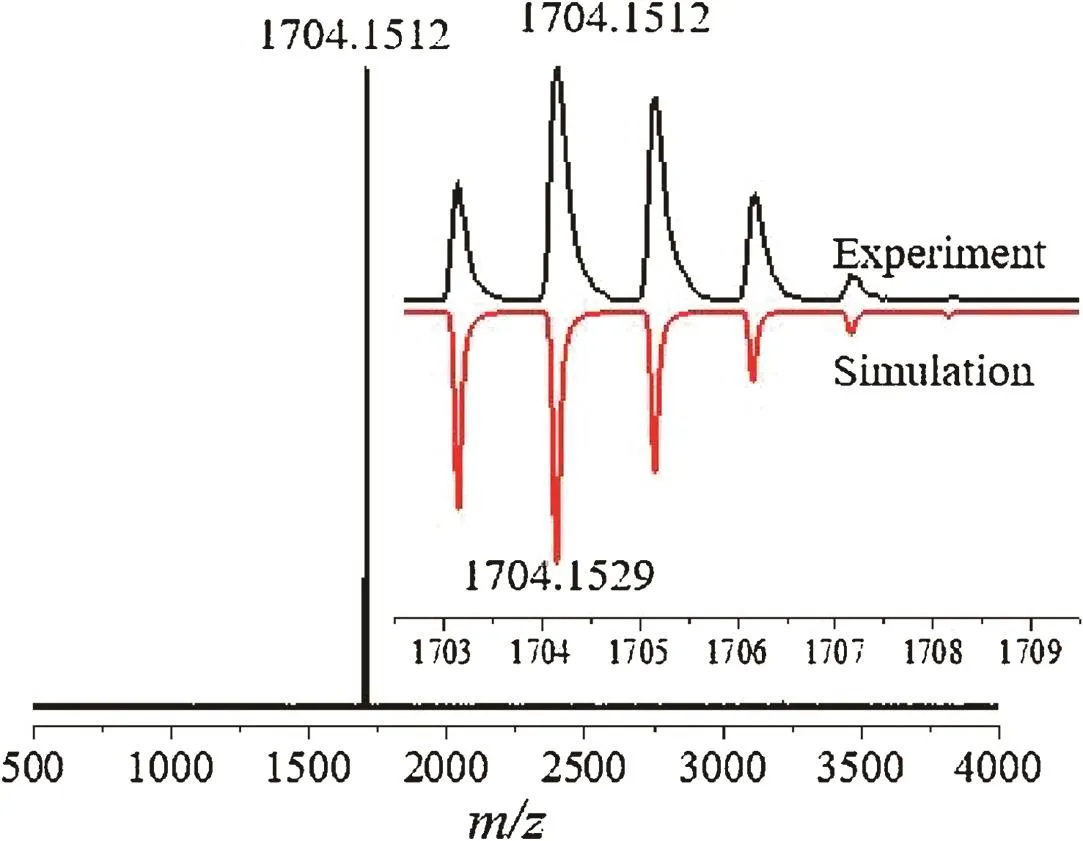
Fig.2.MALDI-TOF mass spectrum of 3D-imide.Inset:the corresponding experimental and simulated isotopic distributions.
To investigate the photoinduced charge transfer and charge separation between poly(3-hexylthiophene)(P3HT)and acceptor 3D-imide,the photoluminescence(PL)quenching experiments were carried out in the solution state.Fig.4 displays the PLspectra of P3HT solution and the mixtures of P3HT/3D-imide solutions.With the increasing concentration of 3D-imide,the PLintensity of the P3HT is decreasing correspondingly.Notably,we observe that the intensity of 581 nm emission peak for P3HTdecreases faster in comparison with its shoulder peak,which is different to the reported results[8].With the 1?10?4mol/L 3D-imide addition,the 581 nm emission can be almost completely quenched.Therefore,based on the pioneering works[15,19–20],we can conclude that 3D-imide represents a promising novel electron acceptor for organic electronics.
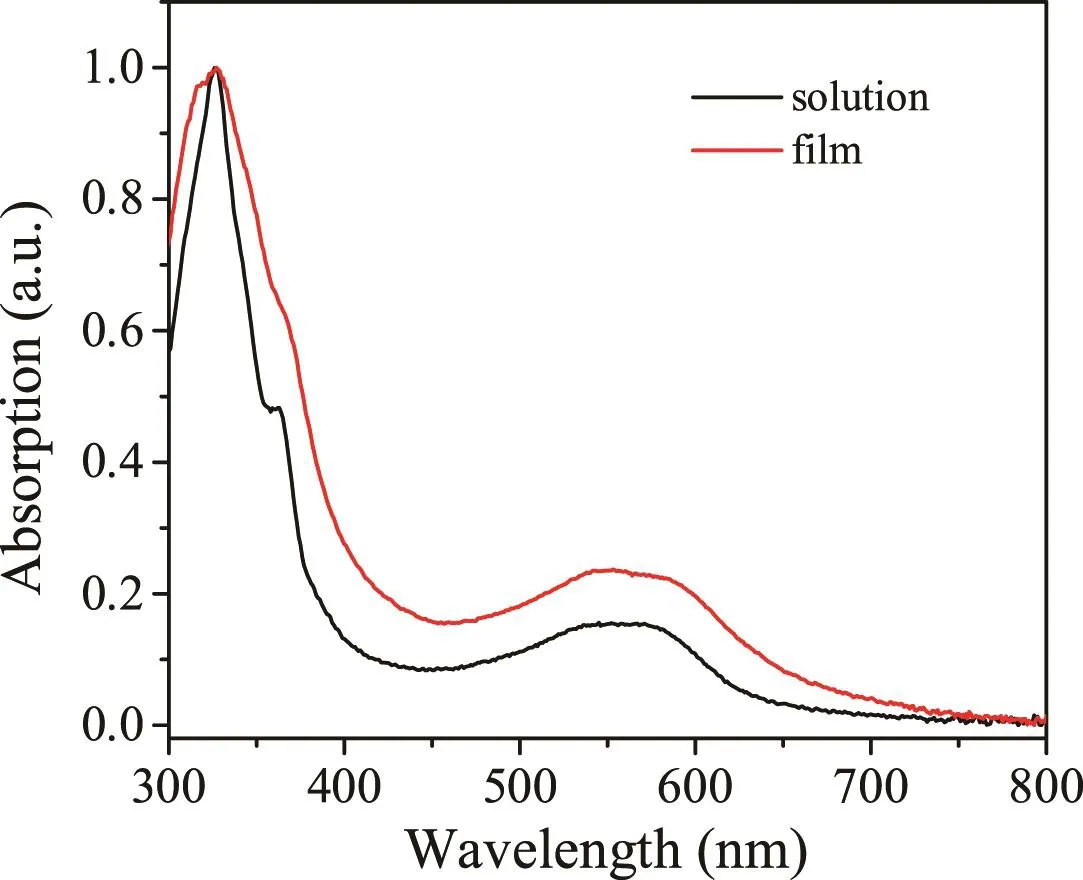
Fig.3.UV–vis absorption spectra of 3D-imide in chloroform and in thin fi lm.
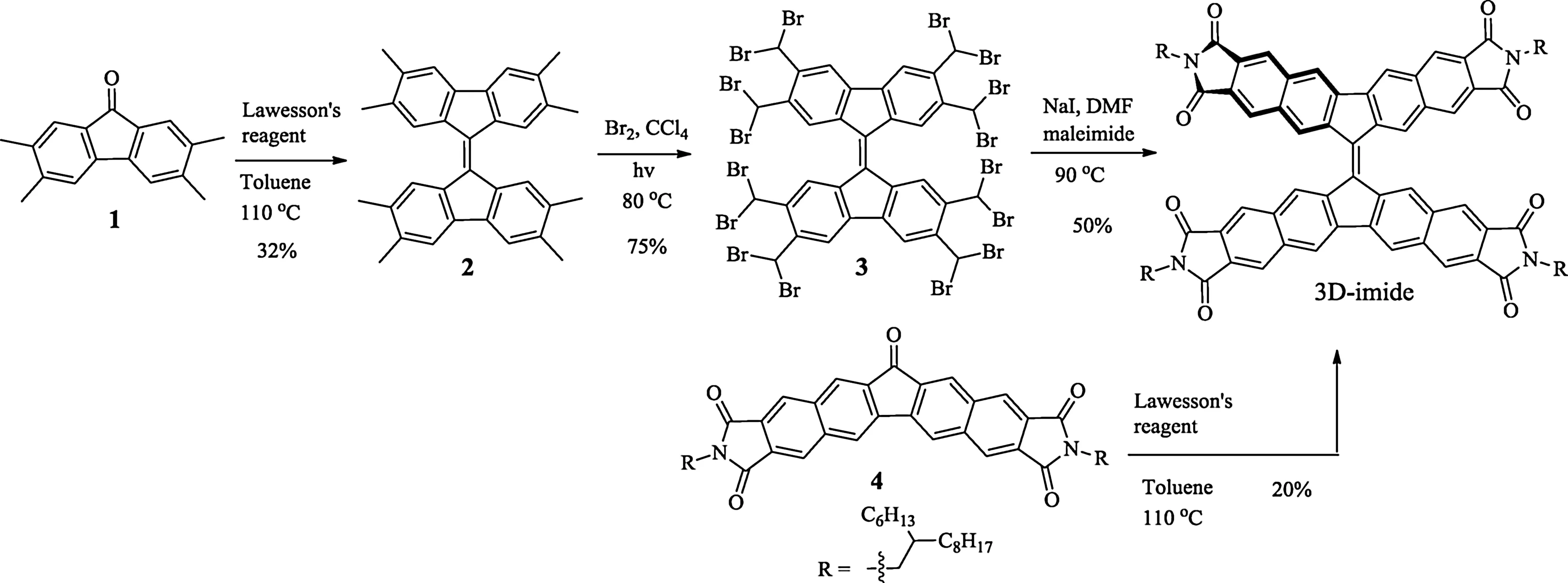
Schem e 1.Synthetic route to 3D-imide.
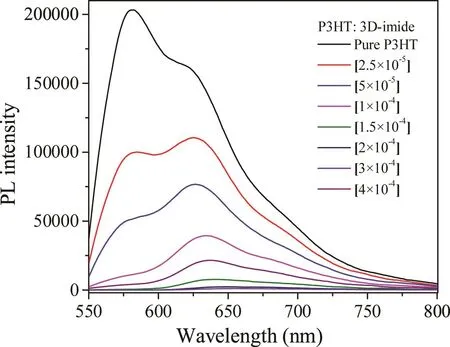
Fig.4.PL quenching spectra of P3HT:3D-imide in CHCl3 with increasing concentration of 3D-imide(l Ex=540 nm).
To investigate the electrochemical properties of 3D-imide,cyclic voltammetry was employed.As show n in Fig.5,tw o reversible reduction waves are recorded,which are assigned to the formation of the corresponding radical anion and di-ion[19].By measuring the difference between the onset of reduction and the half-w ave potential of the ferrocene standard,lowest unoccupied molecular orbital(LUMO)is calculated to be?3.76 eV,according to the equation:LUMO=?[Ered/onset?E(Fc+/Fc)+4.8]eV.This low LUMO level is comparable with that of conventional electron acceptors,such as dicyanovinylene substituted tetraindenospirofl uorene[21],perylene bis(dicarboximide)analogues[22]and B!N bridged aromatic units[23–25].In comparison with the LUMO energy level(?3.35 eV)of BF-3,such deep LUMO of 3D-imide can be attributed to the strong electron-withdraw ing character of end-capped imide units.The HOMO energy of 3D-imide is?5.66 eV,calculated from its optical gap according to the equation HOMO=LUMO?Eg.
To gain more insights into the electronic propertiesof 3D-imide compared with its carbon analogue BF-3,density functional theory(DFT)calculations were performed on the basis of B3LYP/6-311G(d,p).The optimized geometries of 3D-imide model and BF-3 reveal similar dihedral angles(?35?)between their planar backbones.Also,as show n in Fig.6,the introduction of imide moieties presents almost no influence on the electron delocalization over the w hole conjugated systems.Moreover,the frontier energy levels of 3D-imide are deeper than those of BF-3,which is well in agree with the experimental results.This can further emphasize the function of the imide units.According to timedependent DFT(TD-DFT)calculations,the lowest-energy absorption of 3D-imide and BF-3,can be assigned to the HOMO!LUMO transitions,which is corresponding to the absorption bands at?602 nm and?577 nm,respectively.
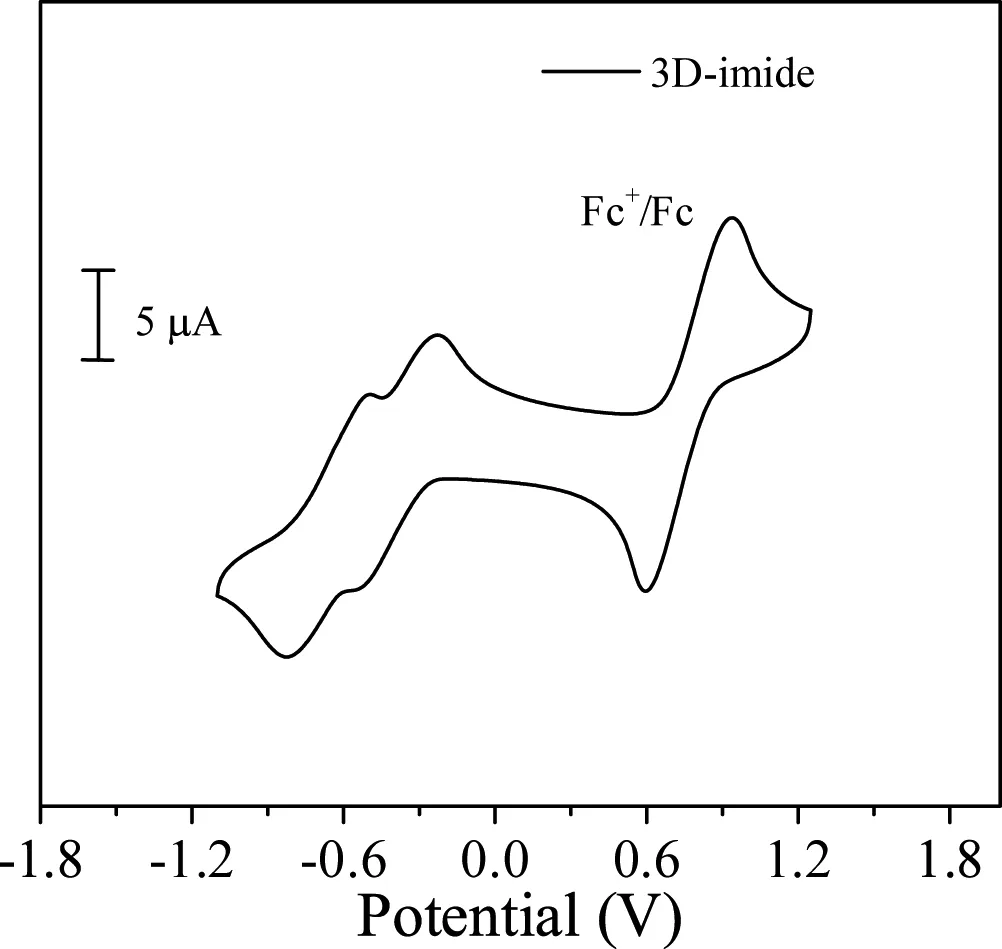
Fig.5.Cyclic voltammetric pro fi le of 3D-imide in dichloromethane with 0.1 mol/L Bu4NPF6 as supporting electrolyte.
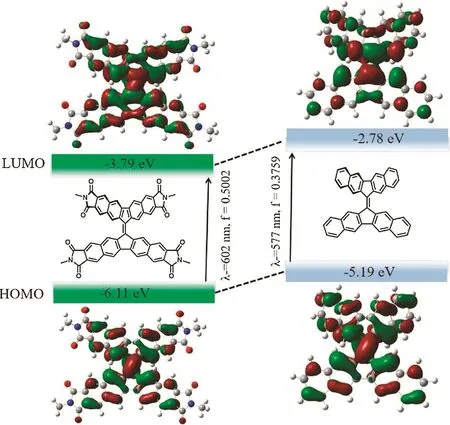
Fig.6.The HOMO–LUMO transition of 3D-imide(left)and BF-3(right)using TDDFT at the B3LYP6-311G(d,p)level.
In conclusion,we have fi rst time introduced the imide groups into the bi fl uorenylidene system.The yielding 3D-imide presents stronger electron accepting capability,with a LUMO energy as lowas?3.76 eV.P3HT emission quenching experiments con fi rm its potential as acceptor in the fi eld of photovoltaics.We expect that 3D-imide can be w idely used in electronic device.
Acknow ledgm ent
This work is supported by National Natural Science Foundation of China(No.51603055)
Appendix A.Supplem entary data
Supplementary data associated with thisarticle can be found,in the online version,at https://doi.org/10.1016/j.cclet.2018.01.033.
杂志排行
Chinese Chemical Letters的其它文章
- Information for authors
- A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors
- One-pot synthesis of tetrahydroindoles via a copper catalyzed N-alkynation/[4+2]cycloaddition cascade
- Gram-scale preparation of dialkylideneacetones through Ca(OH)2-catalyzed Claisen-Schmidt condensation in dilute aqueous EtOH
- Water bridges are essential to neonicotinoids:Insights from synthesis,bioassay and molecular modelling studies
- Novel dual inhibitors against FP-2 and PfDHFRas potential antimalarial agents:Design,synthesis and biological evaluation
