One-pot synthesis of tetrahydroindoles via a copper catalyzed N-alkynation/[4+2]cycloaddition cascade
2019-02-15ChunJiangPiaoPiaoYuQingZhangHuaDongXuMeiHuaShen
Chun Jiang,Piao-Piao Yu,Qing Zhang,Hua-Dong Xu*,Mei-Hua Shen*
School of Pharmaceutical Engineering&Life Science,Changzhou University,Changzhou 213164,China
Keywords:Tetrahydroindole N-Alkynation Intramolecular[4+2]cycloaddition Copper catalysis Ynamide Relay reaction
ABSTRACT A Cu SO4 catalyzed ynamide formation/[4+2]cycloaddition relay reaction was achieved in one-pot.Various 4,7-cis-dihydroindolines were produced in good to high yields.The one-pot operation in conjunction with the cheap and benign copper catalysis renders this reaction highly sustainable.
N-Heterocycles are ubiquitous structures that found w idely in nature products and manmade compounds with diverse functionalities[1].One effectual strategy to construct these scaffolds is cyclization reactions between tw o reacting partners tethered through a nitrogen atom[2].Particularly,if a cycloaddition between the tw o reacting partners takes place,then a bicyclic system w ill be formed simultaneously[3].Our group have used this strategy to achieve several elegant synthesis of valuable nitrogen containing bi,tri and polycyclic skeletons[4].Intramolecular[4+2]reaction,as a powerful tool for the building of 6-membered rings,has been successfully employed for this purpose[5].Here,we w ould like to report a copper catalyzed N-alkynation/[4+2]cascade that can conveniently give rise to 4,7-dihydroindolidines[6]in a one-pot operation.
In line with our efforts in devising methodologies for N-heterocycle synthesis,we became interested in intramolecular[4+2]reaction of dienes with ynamides,a class of alkynes with unique electronic properties that distinguish them from others[7].Extensive literature searching has resulted in only few reports on this type of reaction[8].In 2003,Witulski and co-workers described an intramolecular[4+2]cycloaddition of ynamideswith pendent dienes producing 4,7-dihydroindoline.In their report,[RhCl(PPh3)3]/AgSbF6catalyst combination was identi fied as the best catalytic conditions,and only limited number of substrates were revealed[9].We felt there should be much room to improve for this important reaction and were encouraged to carry out optimization of this transformation.
Initially,dienyl sulfonamide 1a was submitted to react with alkyne bromide 2a in conditions similar to that reported for ynamide synthesis by Hsung’s group[10],namely,a mixture of 1a(1 equiv.),2a(1.3 equiv.),Cu SO4?5H2O(10 mol%),1,10-phenanthroline(20 mol%)and K2CO3(2 equiv.)in toluene was heated in 60?C for 10 h under nitrogen atmosphere.To our delight,dihydroindoline 3aa(50%yield)was isolated and identi fied,accompanied with 35%1a recovered(Table 1,entry 1).Evidently,3aa was a product of an intramolecular[4+2]cycloaddition of the in situ generated ynamide with the tethered diene.
When the reaction temperature was raised to 80?C,the starting material was fully consumed in 10 h,to give a clean reaction mixture from which 85%3aa was collected(entry 2).Cs2CO3and K3PO4were also viable bases for this reaction,while inferior yields were obtained(entries 3–4).The reaction took place smoothly in DCM,DCEor THFas well but with 10%–20%drop in yields(entries 5–7).The current ynamide formation/[4+2]cyclization cascade feathered tw o merits that w orth to note:1)The employment of cheap and nontoxic copper salt as catalyst renders this reaction extremely economically practicable and environmentally sustainable;2)The one-pot operation improves the synthetic ef fi ciency significantly.
To explore the scope of substrates,more dienyl sulfonamides 1 were made and subjected to react with phenyl alkynylbromide 2aunder the optimized conditions.The outcomes were summarized in Table 2.Phenyldienyl substrates 1b-1f with substituents of divergent electronic properties on the aromatic ring all underwent the one-pot cascade successfully to give corresponding 4,7-dihydroindolidines 3ba-3fa in good to high yields.1 g with a liable furan group also tolerated the conditions to couple with 2a followed by cycloaddition smoothly giving 3ga in 62%yield.Pleasingly,tosylamides with aliphatic dienes(1h-1i)showed comparable reactivity and underwent the cascade to afford 3 ha and 3ia in good yields.
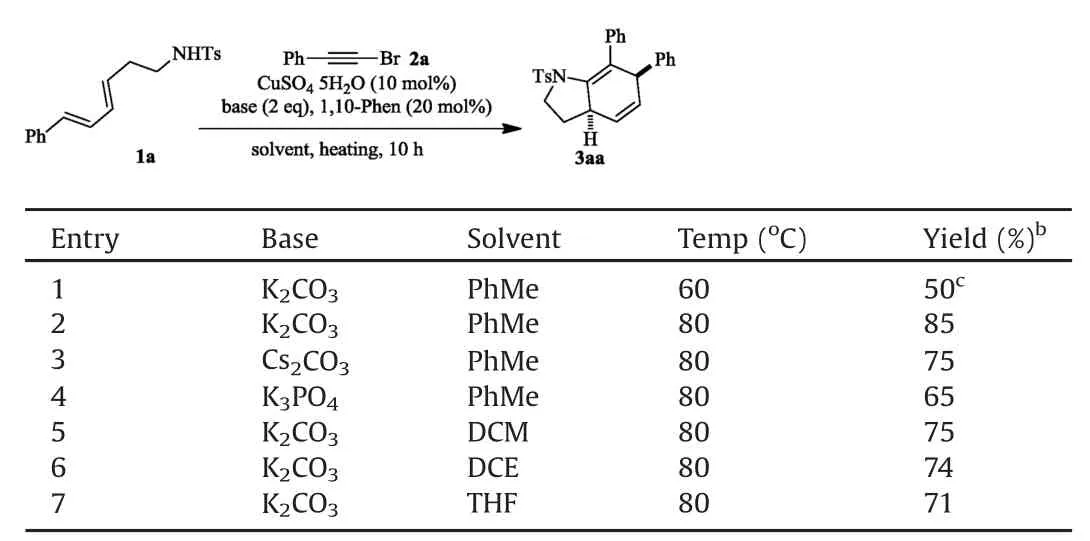
Table 1 Conditions optimization for N-alkynation/[4+2]cycloaddition cascade.a
Then the scope of alkynyl bromides wasinvestigated using 1a or 1e as reacting partner(Table 3).Substitution on the phenyl ring of the alkynyl bromide has negligible impact on the cascade reaction as comparable outcomes were observed for 2b-2f.Furan and thiophene substituted alkynyl bromides 2g-2 h also underwent this one-pot reaction with 1a successfully,albeit corresponding products 3ag and 3 ah were formed in lower yields.The reactions of aliphatic alkynyl bromides 2i-2j,with vulnerableα-methylene group,also took place nicely to give related 3ai and 3aj in good yields.To our delight,bromopropionate 2k,with different electronic property w hen compared with its aryl and alkyl congeners,was also viable for the one-pot cascade reaction to give rise to 3ak in 72%yield.The relative stereochemistry of 3ed was established unequivocally to be 4,7-cis by X-Ray analysis(Fig.1)and that of the others was assigned by analogy.
In order to extend this reaction to hexahydroquinoline synthesis,dienyl sulfonamide 1 j was made and submitted to react with alkynyl bromide 2a under the standard conditions.Surprisingly,no desired[4+2]cycloaddition product 3ja was detected,even after the temperature was raised to 130?C.Instead,N-alkynation product 4ja was isolated in 74%yield(Scheme 1,top).In order to classify if tetrahydroindoles 3 were formed via the intermediacy of the corresponding NH alkynation products of 1ak,we have made to collect small amount of 4aa(17%)from the reaction of 1a with 2a by decreasing the reaction temperature to 45?Cand shortening the reaction time to 5 h(Scheme 1,bottom).These evidencesindicate that dienyne 4 is the real substrate for the[4+2]reaction.
The products with versatile alkene groups can serve as intermediates for further transformations.For example,OsO4catalyzed dihydroxylation of 3aa afforded 4 in 80% yield.Treatment of 3aa with DQQ delivered dihydroindole 5 in 72%yield(Scheme 2).The experiment details,characteristics of compounds,copies of NMR spectra can be found in Supporting information.
In summary,a rapid entry to tetrahydroindoles has been realized in a one-pot procedure.The copper catalyzed N-alkynation is followed by an intramolecular Diels-Alder cycloaddition of the nascent ynamide with the linked diene.Aryl and alkyl alkynyl bromides and dienes all are feasible for this reaction.
Acknow ledgm ents
We thank the National Natural Science Foundation of China(No.21672027),QingLan Project of Jiangsu Province(2016)and Six-Talent-Peaks Program of Jiangsu(2016)for fi nancial support.
Appendix A.Supplem entary data
Supplementary materialrelated to thisarticlecan befound,in the online version,at doi:https://doi.org/10.1016/j.cclet.2018.05.040.

Table 2 The scope of dienyl sulfonamide.a
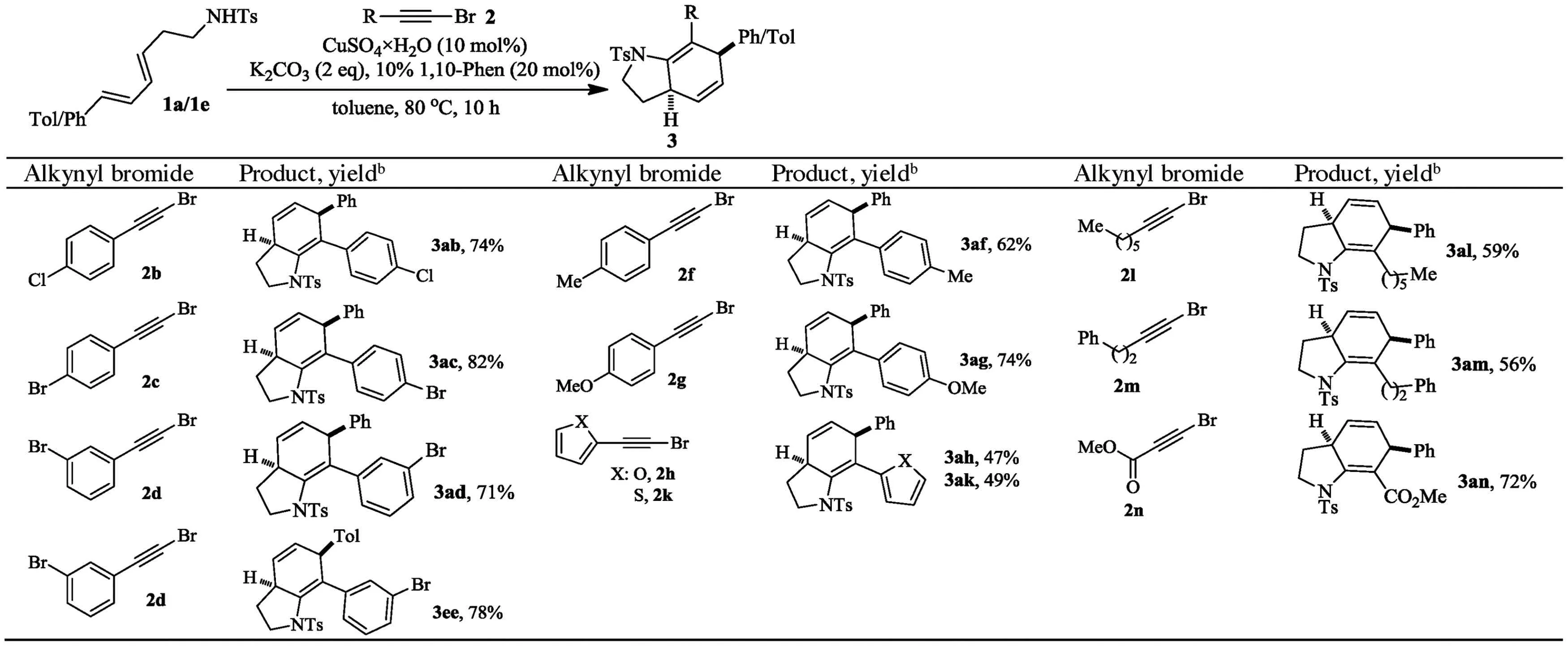
Table 3 The scope of alkynyl bromidea.
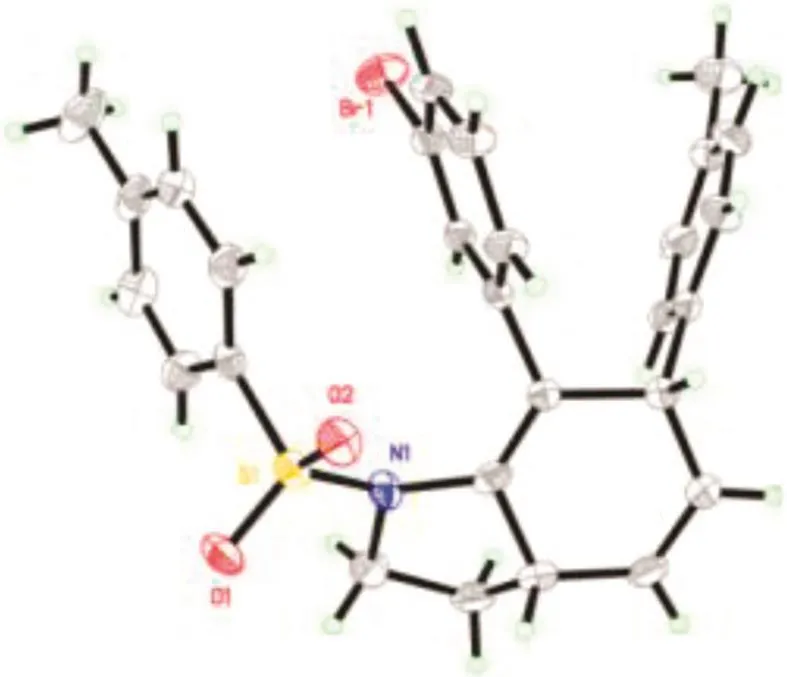
Fig.1.X-ray crystal structure for 3ed.
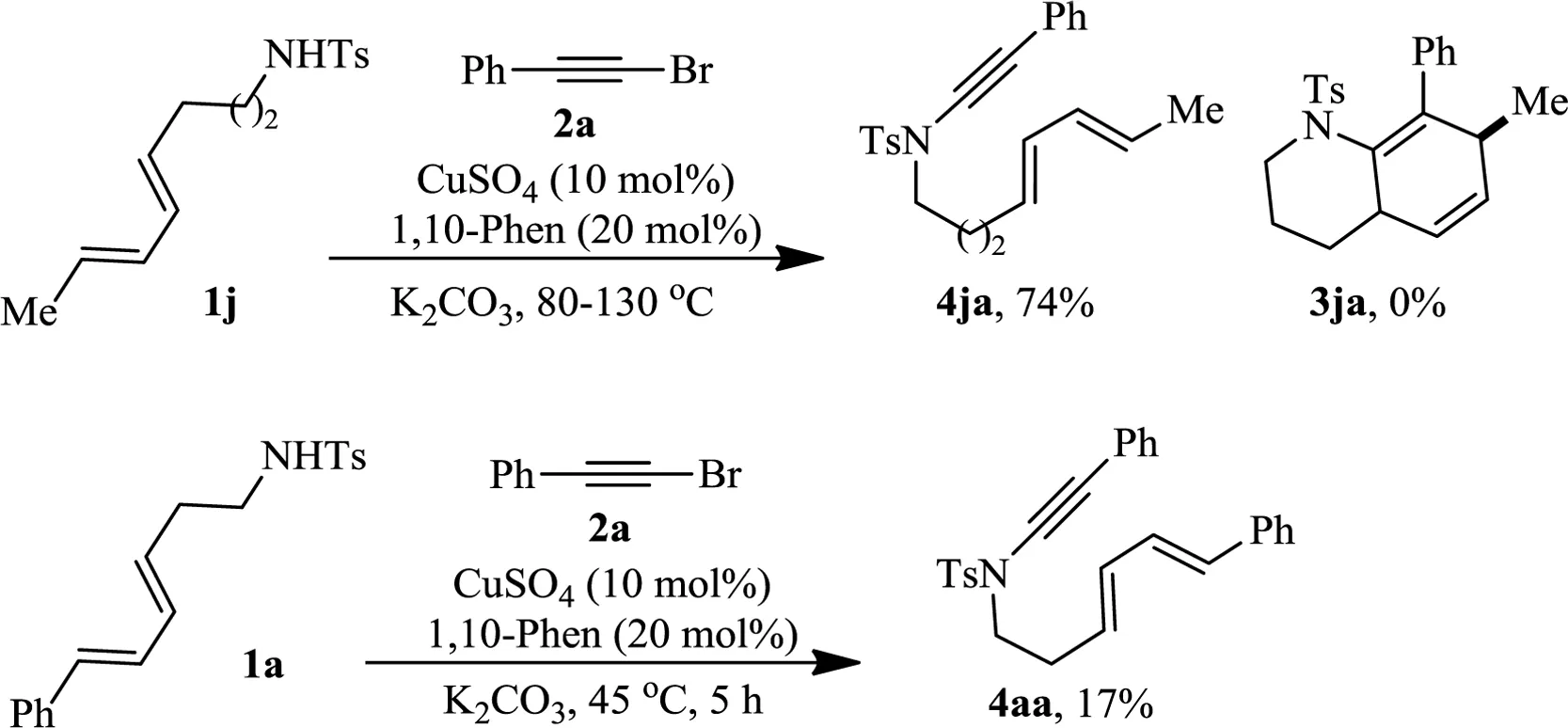
Schem e 1.Preparation of intermediates 4ja and 4aa.
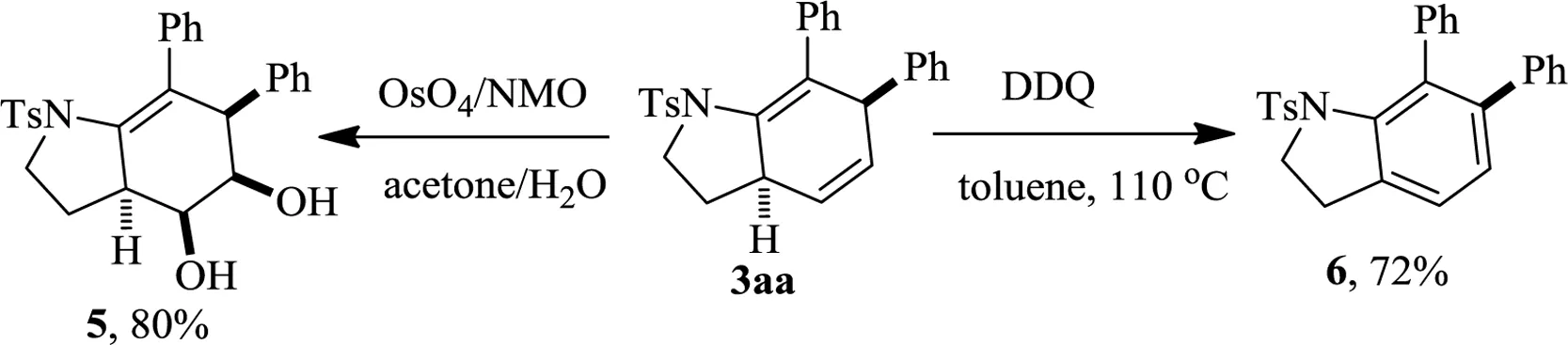
Schem e 2.Dihydroxylation and oxidation of 3aa.
杂志排行
Chinese Chemical Letters的其它文章
- Information for authors
- A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors
- Gram-scale preparation of dialkylideneacetones through Ca(OH)2-catalyzed Claisen-Schmidt condensation in dilute aqueous EtOH
- Tetra-phthalimide end-fused bi fl uorenylidene:Synthesis and characterization
- Water bridges are essential to neonicotinoids:Insights from synthesis,bioassay and molecular modelling studies
- Novel dual inhibitors against FP-2 and PfDHFRas potential antimalarial agents:Design,synthesis and biological evaluation
