Gastric electrical stimulation: An emerging therapy for children with intractable gastroparesis
2019-02-12AniruddhSetyaPriyankaNairSamXianjunCheng
Aniruddh Setya, Priyanka Nair, Sam Xianjun Cheng
Abstract
Key words: Gastroparesis; Gastric electrical stimulation; Nausea; Vomiting; Prokinetics
INTRODUCTION
Gastroparesis (GP) is defined as a syndrome of objectively delayed gastric emptying in the absence of mechanical obstruction. Symptoms include early satiety,postprandial fullness, bloating, nausea, vomiting, and abdominal pain. Since similar symptomatology may also be seen with other etiologies, including functional dyspepsia, incidence and prevalence is varied. In adults, the age-adjusted prevalence of definite gastroparesis varies from 2.4 to 9.6 per 100000 persons for men and 9.8 per 100000 persons for women[1]. In children, the overall prevalence remains unknown,and the data available are limited.
Even though multiple conditions including diabetic, postsurgical and autoimmune causes have been associated with GP, up to 70 % of pediatric cases are idiopathic, 18% drug-induced and only 12 % are post-surgical[2]. Infections have been implicated as a cause of GP in children and can self-recover overtime sometimes as long as 24 mo[3].
In adults, it is standard practice to diagnose gastroparesis utilizing a gastric emptying study as a “gold standard” diagnostic tool but there is no consensus for a standard in pediatrics. A delayed gastric emptying is defined as when a solid meal has a retention of greater than 90% after 1 h, greater than 60% at 2 h, and greater than 10% at 4 h[4]. Although the percentage of the patient population having gastroparesis is small, management of it is challenging, particularly in pediatric patients. It often takes a significant amount of time and effort of the treating physician and at times can be frustrating. Patients often require frequent hospitalizations for nutritional support and/or use of multiple medications for symptom control. According to a recent report of 97 pediatric patients followed up to 9.5 years (mean = 3.5 years), all patients had used promotility agents (100%), and most patients were on antiemetic (72%),antireflux (79%), and/or pain meds (52%). Mean hospitalization rates were 3.6 per year. Because of the persistence of symptoms, their quality of life was compromised with frequent school missing or restriction of normal activities. A restricted solid diet was required by 46% and 6% were maintained on a liquid diet only as they were unable to tolerate any kind of solid food. Tube feeding was required by 39% and 4%required parenteral nutrition. Two thirds (66%) of patients had to undergo surgical procedures, however, this latter option is generally considered too radical for a growing child[5]. A “bridging” therapy after failed medical interventions and before surgery is needed.
SUCCESS AND FAILURE OF CURRENT THERAPIES
Supportive care and pharmacological therapies for symptoms remain the mainstay treatment (See Figure 1). Although they are effective for mild and some moderately severe cases, often times they do not work for severe disease. According to the severity of symptoms, GP can be stratified/graded into three groups: mild, moderate or compensated, and severe or gastric failure[6]. Grade 1 (mild) gastroparesis is characterized by intermittent, easily controlled symptoms with the maintenance of weight and nutritional status on dietary modification. Grade 2 (compensated)gastroparesis is characterized by partially controlled symptoms on pharmacological agents and rare hospitalizations. Grade 3 (gastric failure) gastroparesis patients are neither responsive to dietary modification nor to medication, cannot maintain nutrition or hydrationviathe oral route, and require frequent emergency department or inpatient care.
Dietary modification
It includes eating small, frequent meals of low fat and low fiber diet or liquid diet alleviates the constant feeling of fullness. Diets low in Fermentable Oligosaccharides,Disaccharides, Monosaccharides, and Polyols can be effective in some people although the mechanism is not clearly understood.
Prokinetic agents
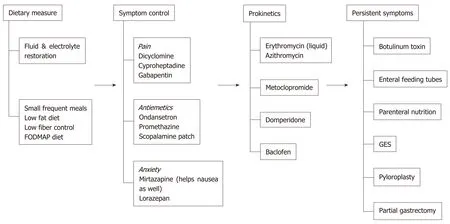
Figure 1 Management of gastroparesis. GES: Gastric electrical stimulation.
Prokinetic agents are medications that enhance gastrointestinal motility and transit of content in the gastrointestinal tract, mainly by amplifying and coordinating the gastrointestinal muscles. A recent literature review talks about the common prokinetic agents used for the management of gastroparesis in pediatrics (Table 1)[7].Metoclopramide is an antagonist of dopamine 2 (D2)-receptor and promotes gastric emptying, as well as binds to the 5-hydroxytryptamine receptor 4 to stimulate cholinergic neural pathways in the stomach. Domperidone works as a D2 receptor antagonist, which enhances antral duodenal contraction and leads to improvement in peristalsis. Through its effect on the chemoreceptor trigger zone, it also exhibits antiemetic properties. These are considered safe and effective drugs in the treatment of gastroparesis, however, there are reports about possible side-effects. Both drugs cause hyperprolactinemia and may cause galactorrhea. Metoclopramide can have extrapyramidal dyskinetic reactions and domperidone can lead to cardiac arrhythmias[8,9]. Macrolide antibiotics at reduced antimicrobial dosages, such as erythromycin oral suspension and azithromycin act as motilin agonists and have a significant prokinetic effect. Its safety and efficacy in improving feeding intolerance have been demonstrated in multiple studies with premature infants and children[10].Baclofen, an antispasmodic and muscle relaxant, is another agent that that is used in patients with gastroparesis. It probably works by having an inhibitory role on the lower esophageal sphincter relaxation through its stimulation of gammaaminobutyric acid (GABA) B receptors. It also reduces gastric emptying time[7].
Symptom treatment of nausea, vomiting, pain, and anxiety
Other than prokinetics, the symptomatic treatment of thesesymptoms remains empirical. These drugs are commonly used off -label from the indications for nonspecific nausea and vomiting, palliative care and chemotherapy-induced side effects.(1) Pain is often controlled by medications such as dicyclomine which is an anticholinergic and blocks the action of acetylcholine at parasympathetic sites in smooth muscle. Cyproheptadine is widely used in the pediatric population due to its efficacy in dyspepsia and appetite stimulation[11,12]. It is a potent antihistamine and serotonin antagonist with anticholinergic effects. Gabapentin, a GABA analog is reserved for patients with chronic abdominal pain; (2) The most commonly prescribed antiemetic drugs are phenothiazine derivatives and antihistamine agents including promethazine. There is concern about sedation and cardiac toxicity (prolongation of QT)[13]. Ondansetron, the 5-HT 3 -receptor antagonist is a reasonable second-line drug for effective nausea control[14], even though it has not been found to be superior to metoclopramide and promethazine in reducing nausea. Transdermal scopolamine is effective for nausea associated with motion sickness and is often used for nausea and vomiting of gastroparesis, although there is no peer-reviewed published report to support this practice; and (3) Symptoms of anxiety may be addressed by the 5-HT 2 receptor antagonist, mirtazapine. It is an atypical antidepressant that works as antiemetic and an appetite stimulant which has been reported efficacious in a singlereport in gastroparesis[15]. But, like most of the gastroparesis medications, it also prolongs QT[16]. Lorazepam, a benzodiazepine has been used for controlling nausea based on anecdotal chemotherapy studies[17]
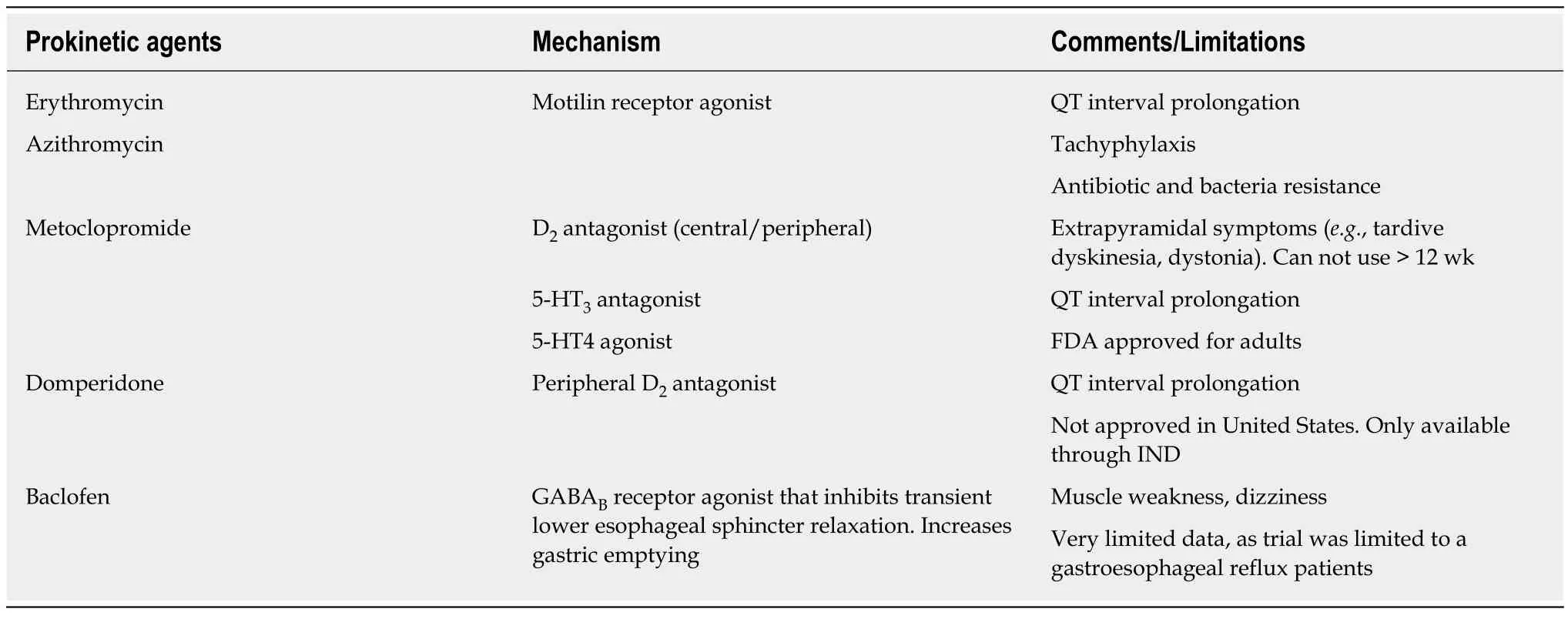
Table 1 Synopsis of commonly used prokinetic agents for gastroparesis[7]
Alternative therapies for refractory gastroparesis
In addition to the aforementioned therapies, there are a few other therapies in use(Table 2).
Endoscopic pyloric botulinum injection
Botulinum toxin A is a purified neurotoxin that inhibits the release of acetylcholine into the neuromuscular synaptic cleft, which causes a localized reduction in muscle contractility[18]. Pediatric data on its use in gastroparesis is limited, however, a retrospective study looked at the endoscopic, submucosal injection of the toxin into the pylorus to reduce the pylorospasm[19]. Two double-blind, placebo-controlled studies, showed some improvement in gastric emptying with endoscopic pyloric botulinum injection, but no improvement in symptoms compared with the placebo[20,21].
Enteral feeding
Children with severe, complicated gastroparesis who are unresponsive to conservative intervention and have had a weight loss of more than 10% due to refractory symptoms of gastroparesis can be offered enteral feeding devices. These are reversible and often temporary. There should be a trial of nasoenteric postpyloric feedings prior to jejunostomy feeding tube placement. In 2 large pediatric series,surgical placement of either a gastrostomy or jejunostomy tube was required to aid with management in a small percentage (4%, 19/469) children[2,3]. A large study in adults with gastroparesis reported improvement following jejunostomy tube placement with almost 81% patients reporting improvement in overall health status,56% reporting improved nutrition, 52% with fewer hospitalizations and 39%reporting improved nausea and vomiting[22].
Parenteral nutrition
Intravenous nutrition is rarely required when hydration and nutritional state cannot be maintained. Enteral feeding should always be preferred over parenteral nutrition for a wide range of practical reasons, such as costs, excessive healthcare utilization and the potential for complications. Parenteral nutrition is also a less “natural” way of delivering nutrition.
Surgical options
Pyloroplasty & gastrectomy have also been used in the management of gastroparesis.These gastric emptying procedures have been attempted but with limited success in children[23]. Moreover, these approaches are permanent and involve the risk of surgical complications, which are not always amenable to the parent or the child.Novel therapeutic approaches, particularly less invasive and reversible “bridging”therapies, are needed after failed medical interventions before surgery.
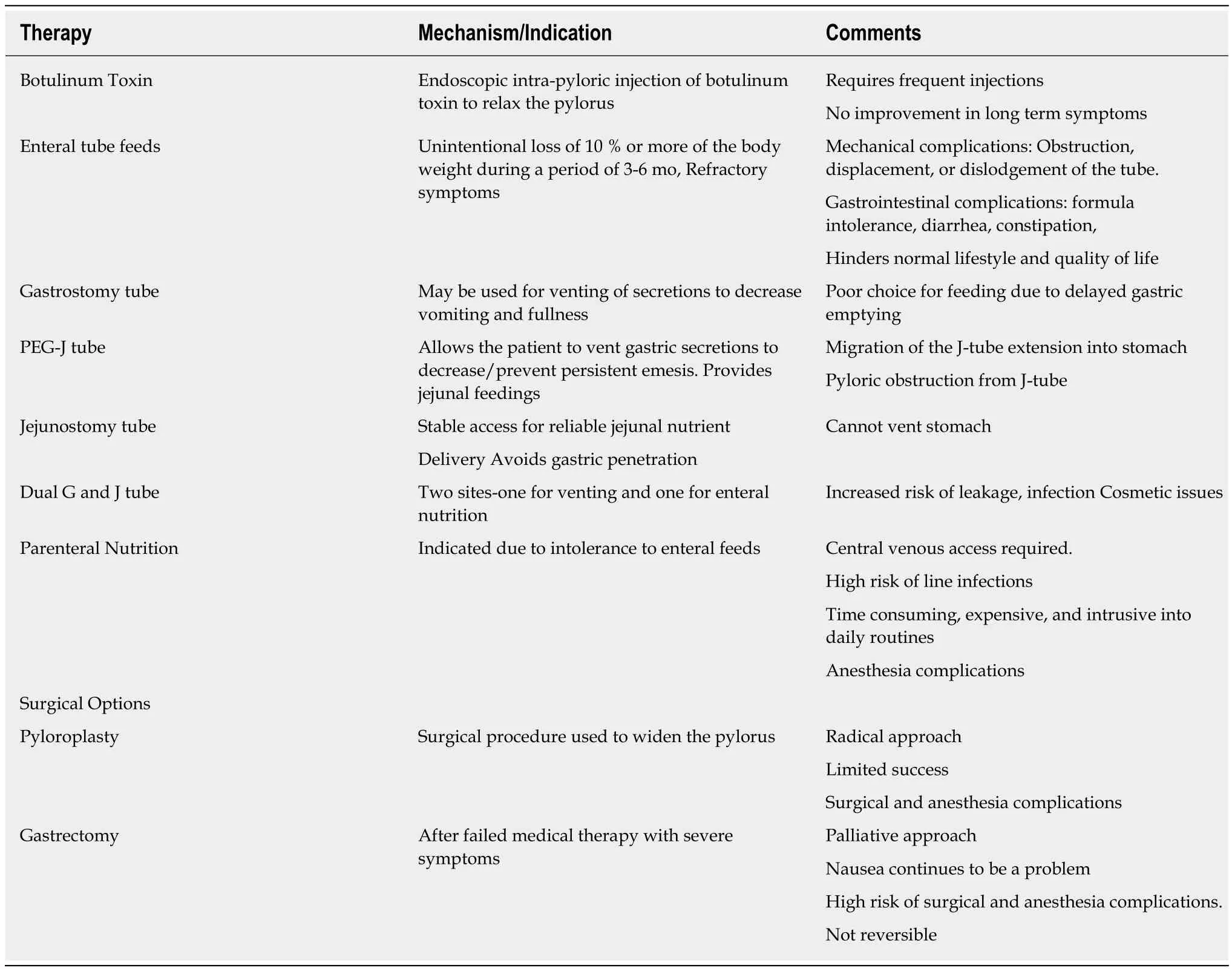
Table 2 Alternative therapies for refractory gastroparesis[2,3,19,21,22]
Gastric electrical stimulation is an emerging “bridging” therapy for pediatric gastroparesis
In 2000, the United States Food and Drug Administration (FDA) approved the use of Enterra (Medtronic Inc., Minneapolis, MN) gastric electrical stimulator (GES) under the “humanitarian device exemption” for the treatment of diabetic and idiopathic gastroparesis[24]. Since then, many prospective cohort studies and randomized controlled crossover studies have been published for adult patients[25]. These studies show that GES is effective and safe and can improve the severity of symptoms in adults. However, in pediatrics, we have very limited data with only six published studies so far (Table 3). The largest study has been done in 97 pediatric patients over a period of 10 years. GES was found to be safe and effective in pediatric gastroparesis with continued symptomatic improvement at 1 year and beyond[5].
Understanding of the gastric electrical physiology may lead to the development of novel therapies for treating gastroparesis
Normal gastrointestinal motor function is a complex series of events requiring coordination of the sympathetic and parasympathetic nervous systems, neurons and pacemaker cells [called interstitial cells of Cajal (ICCs)] within the stomach and the gut. ICCs are the “pacemaker cells” for the smooth muscle apparatus of the gastrointestinal tract. The frequency and direction of the phasic motor activity are regulated by the gastric slow wave, a rhythmic electrical oscillation, which is generated by the ICC located in the upper part of the fundus along the greater curvature. Slow waves are generated in the “pacemaker area” (like the SA node in the heart) and migrate distally to the pylorus at the rate of 3 cycles per minute (cpm) or approximately every 20 seconds. Abnormalities of this process can lead to impairments in gastric emptying. Gastroparesis occurs when ICCs are lost, their activity reduced or conduction blocked. The exact cause of gastroparesis is unknown,but the stomach is not paralyzed.
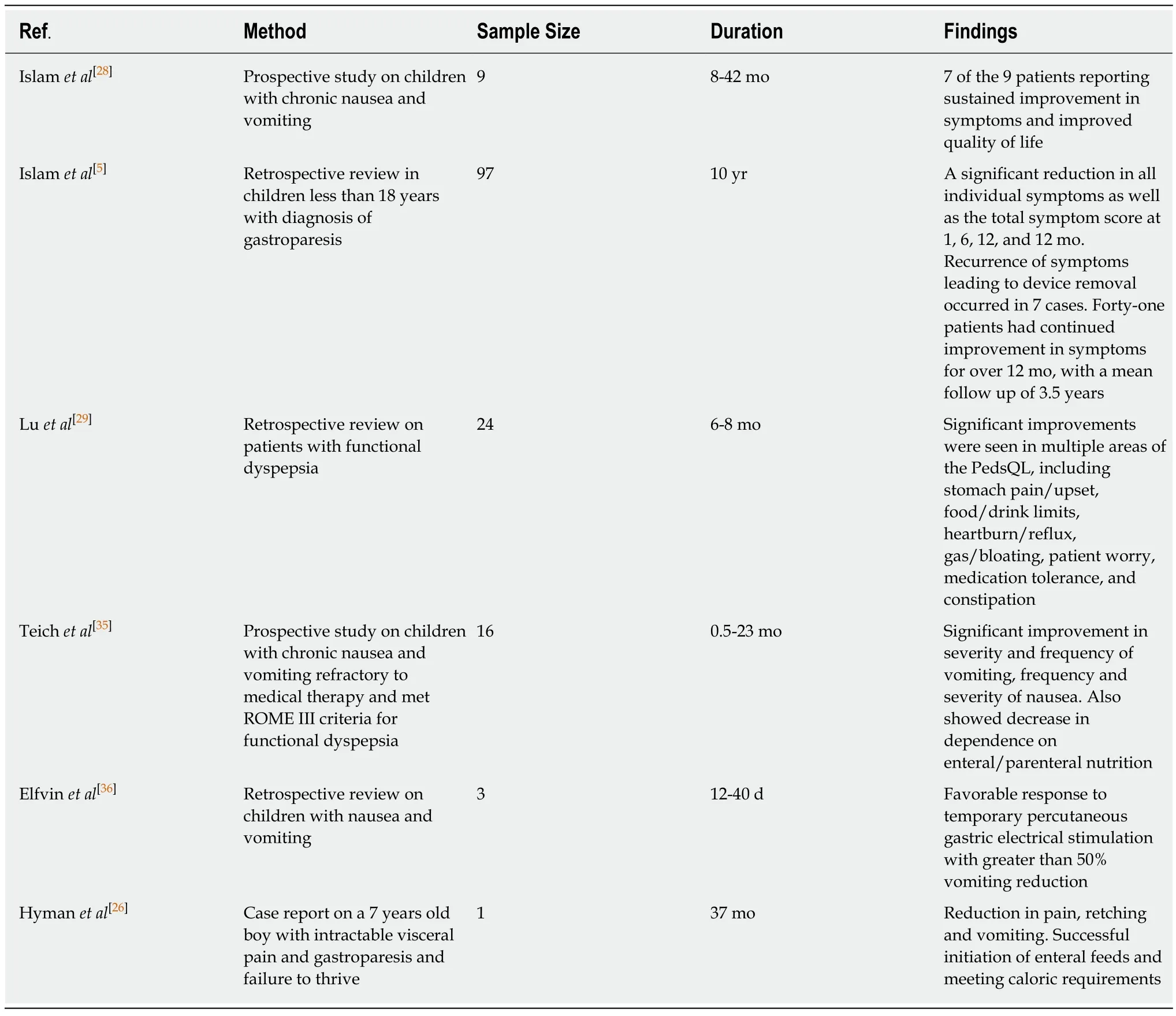
Table 3 Comparison of pediatric studies on gastric electrical stimulation
HOW DOES GES WORK?
GES, although its mechanism is not completely understood, is thought to ameliorate symptoms of nausea and vomiting by improving gastric accommodation. This is done via stimulation of the enteric nervous system in addition to central effects mediated through the vagus nerve. Like in the heart, an external pacemaker, a medical device that generates electrical impulses delivered by electrodes to stimulate and restore the function of the stomach, can be used. Currently, there are two approaches to stimulate the function of the stomach: (1) Gastric pacemaker, which delivers low-frequency high energy electrical stimulus timed to synchronize gastric rhyme (e.g., 3 cycles/min in the gastric antrum and 12 cycles/min in the duodenum) and stimulate muscle movement. This is an ideal method yet is in a developing stage, as there is no implantable power source at present; and (2) GES in which the current uses low energy and high frequency. It does not alter motility patterns[26]. This is the method in current use.
Procedure
Similar to a cardiac pacemaker, the gastric electrical stimulator is an implantable device. It contains an electronic circuit and a battery and is implanted subcutaneously in the abdominal wall. The electrical pulses delivered by the stimulator are provided by the electronic circuit, and the battery provides the energy needed for 5 to 10 years of operation. Both battery and electronic circuits are encapsulated in a titanium housing, referred to as the pulse generator unit[26]. The procedure has been detailed in previous studies[27,28]. In a nutshell, placement of temporary GES (tGES) electrodes is carried out under direct visualization using endoscopy. One wire lead is secured into the stomach mucosal wall. This wire exits from one nostril and is connected to the pulse generator which is secured on the outside of the body. A minimum of 3-days with the tGES is required to determine if the individual patient will benefit from a permanent GES. In the permanent GES, a tiny pulse generator and two-wire leads with small electrode ends are surgically implanted in the stomach. Typical initial GES settings are started at 5-7 volts, 14 Hz frequency, 1 second “on” and 4 seconds “off”,and pulse width 330 microseconds with impedance in the range of 400-800 Ω.
GES THERAPY HAS ADVANTAGES FOR CHILDREN WITH GASTROPARESIS
It provides a “bridge” treatment option before the radical life-altering surgical options. Unlike other therapies, GES neither needs medications nor gastrectomy;rather, it treats through the use of microelectrodes to deliver high frequency low energy electric stimulation to the pacemaker area of the stomach. Based on the 6 published studies summarized in Table 3, GES is feasible, tolerable and safe in children. Like in adult patients, GES improves symptoms in children, improves nutrition, and enhances the quality of life; it also helps wean off medications and eliminate many needs for hospitalization.
GES helps improve gastrointestinal symptoms in children with gastroparesis
(1) It reduces overall symptoms in as many as 69% of children, male or female, age 2-18 years with chronic gastroparesis. The most dramatic improvements were emesis score (baselinevsGES 1 movsGES 12 mo = 2.8vs0.4vs0.3) and bloating score (2.2vs0.4vs0.9), followed by pain (3.6vs0.9vs1.6), nausea (3.8vs1.1vs1.6), and satiety (2.6vs0.9vs1.2)[5]; (2) There is a substantial drop in medication use after GES therapy.Most notably in the use of antiemetics (68%vs44%), and prokinetics (41%vs2%).However, there is neither a difference in the use of pain medications (opioids, nonopioids, and neuropathic medications) nor in antireflux medications[5]. Another pediatric study showed a decrease in the use of medications after GES[29]; (3) Up to 85% of patients report a significant quality of life improvement[5]; (4) There is a threefold reduction in tube feed requirements (46% to 13 %) and a two-fold reduction in the need for parenteral nutrition (25% to 13 %) with the application of GES[30]. Thus,the use of GES helps transition to normal oral feed; (5) There is a reported reduction in the total number of hospitalizations (5% to 2.9%) for a year for all the associated comorbidities and complications arising from other modalities of treatment[5]. This, in turn, means reduced costs for both patient and the healthcare network; and (6) Islamet al[5]also showed the nutritional effects of GES are related primarily to decrease GI symptoms. These were accompanied by increased weight and body mass index, an improvement in pancreatic function, increased albumin, and a decreased need for enteral tubes or parenteral nutrition. As a result, only 15% (vs46% before GES)patients required using a restricted solid diet and no patient (vs6% before GES)needed to maintain on a liquid diet.
GES is feasible and safe in children with gastroparesis and causes few complications
Feasibility and tolerability: GES has been placed in patients with a mean duration of symptoms of 3.5 years but has been put as early as one month as well. It is found to be most successful in female adolescents but has also been used in children as young as 2 years of age[5]. At least 4 institutions in the world have reported using this modality to treat over 100 pediatric patients with gastroparesis (Table 3) of varying etiology. Both temporary and permanent GES have been used. The former is placed for only a few days, whereas the latter is for months and years. The longest duration of GES placed is over a 9-year period and was performed by Islamet al[5]from the University of Florida. During this period, GES remained to function properly without events except for battery replacement. Thus, GES is feasible and well-tolerable in children.
Safety and complications: GES is also safe in children and causes few complications.It has been found to be relatively safe in both short term and long term use in the pediatric population with a complication rate of 16% as reported by Islamet al[5]and 20% as reported by Luet al[29]with abdominal pain being the commonest complication. Most of these complications were easily fixed as they are relatively benign such as battery replacement, replacement of electrodes but about 8% had to have the stimulator explanted and 2% developed an infection. This data seems to reflect better tolerability than adults. Long term tolerability has been reported as long as 3.5 years in children, although the effectiveness seems to plateau and/or decrease after 12 months[5]. The maximum effectiveness is apparent with a decrease in symptoms with the temporary stimulator and at the 1-mo mark and even though it tends to wear off as time progresses, the cumulative symptom score is better when compared to baseline.
Some unanswered questions
What if GES is removed after symptom im provement? Few data are available and more studies are needed to address this question. However, in their study, Islamet al[5]observed two patients whose symptoms remained in remission for 2-3 years following GES removal although disease relapsed eventually in both cases.
Who responds to GES? Can responders be predictable? Future studies are needed.Based on current data, it appears that nausea/vomiting-dominant gastroparesis responded better than pain-dominant gastroparesis. Those with narcotic-dependence or with a history of psychosis or eating disorders tended to respond poorer than those without. Female adolescents seem to have responded the best.[25]
Do we really understand the mechanism behind it? The mechanism behind the improvement seen with GES therapy remains poorly understood. Systematic reviews reveal that gastric electrical stimulation has not been shown to affect gastric emptying in a consistent manner. It does not appear to entrain gastric muscle, either. The observations that GES works better for symptoms of nausea and vomiting and not in pain predominant constellation of symptoms and that it does not accelerate gastric emptying time even after symptomatic improvement[31]seem to suggest that GES may improve symptoms centrally through altering central nervous system control of nausea and vomiting via the gut-brain axis. Indeed, human studies before and during gastric stimulation have shown increases in EGG amplitude, vagal activity, and positron emission tomography-imaged activity in the thalamic and caudate nuclei of the brain during chronic GES, yet the demonstration of effect centrally does not imply causation, because the alterations in propagation velocity with GES may reflect enteric and/or autonomic changes induced by electrical stimulation[32]. Alternatively, GES may improve symptoms by increasing gastric accommodation, as revealed by Xing JH,et al[33]. Finally, a cross-over study done on patients with gastroparesis placed with GES showed that the symptoms did not significantly improve in blinded ON vs. Off(Phase I) but did in open-label (Phase II), suggesting that there may also be a component of a placebo effect[34]. Large randomized controlled trials are needed to verify this.
TAKE HOME MESSAGE
GES can be a suitable option for children and adolescents with medically refractory gastroparesis, even though currently GES is used as an “off label” indication, it can lead to long-term significant improvements in all symptoms that translates to a sustained decreased medication usage, the number of hospitalizations, healthcare costs and improved quality of life.
The system is not risk-free; however, it provides a better alternative to permanent enteral feeding tubes, central lines for parenteral nutrition and overuse of medications. This may be a more cost-effective solution in the long run for patients with medically refractory gastroparesis as it decreases then need for medications,hospitalizations, specialty formulas, etc. Gastric Electrical Stimulation helps the patient return to a more “normal” daily routine and even though some studies consider it to have a placebo effect, it is worthwhile in children and adolescents who are otherwise a very vulnerable group.
CONCLUSION
We think if GES is approached in a systematic manner, some of the potential complications can be averted in children who then can be provided with a more longterm symptom-free solution. Although gastric pacing may be the answer to such a complex disease syndrome, it is currently in the stage of infancy in terms of development for practical use. GES provides a bridge therapy option for medically refractory gastroparesis and a pediatric gastroenterologist should be aware of this new relatively unexplored modality of treatment. Finally, GES is a reversible procedure and carries no mortality risk.
杂志排行
World Journal of Gastroenterology的其它文章
- Comprehensive multi-omics analysis identified core molecular processes in esophageal cancer and revealed GNGT2 as a potential prognostic marker
- Diagnostic and prognostic value of lncRNA cancer susceptibility candidate 9 in hepatocellular carcinoma
- Operative complications and economic outcomes of cholecystectomy for acute cholecystitis
- Hepatitis C virus eradication with directly acting antivirals improves health-related quality of life and psychological symptoms
- Significance of postoperative follow-up of patients with metastatic colorectal cancer using circulating tumor DNA
- Pulmonary tumor thrombotic microangiopathy of hepatocellular carcinoma: A case report and review of literature
