Contralateral pneumothorax in the subacute phase after pacemaker implantation: lead retention and follow-up
2019-01-16XiaoDongCHENXuXUJiaWenJIHaiLeiLIUJianPingSHEN
Xiao-Dong CHEN, Xu XU, Jia-Wen JI, Hai-Lei LIU , Jian-Ping SHEN,
1Department of Cardiology, Affiliated Hospital of Intergrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine,Nanjing, China
2Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing, China
3Department of Radiology, Affiliated Hospital of Intergrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine,Nanjing, China
4Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Keywords: Atrial lead perforation; Contralateral pneumothorax; Pacemaker implantation
An 89-year-old woman [body mass index (BMI) = 20.27 kg/m2)] with hypertension underwent implantation of a dual-chamber pacemaker because of dizziness and syncope due to sick sinus syndrome. Pacemaker implantation via the left subclavian approach was performed on June 3, 2017. A right ventricular active lead (TendrilTM STS 2088TC, St Jude Medical, Penang, Malaysia) and an atrial active lead(OptisenseTM 1999, St Jude Medical, Penang, Malaysia)were placed in the right ventricular apex and anterior wall of the right atrium, respectively. Atrial lead parameters were normal (Table 1). The ventricular pacing threshold was 0.6 V at 0.5 ms, and the pacing impedance was 640 Ω. Sensed R wave was 17.2-18.4 mV. A postoperative chest radiograph showed normal findings with appropriate lead position (Figure 1A).
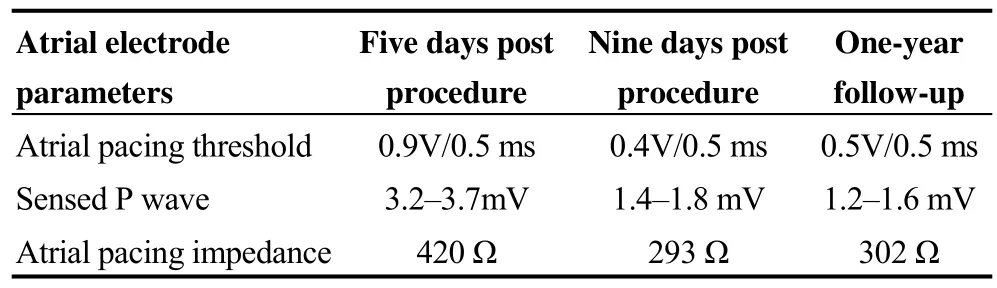
Table 1. Atrial electrode parameters in Case 1.

Figure 1. Images of chest X-ray and CT scan post pacemaker implantation during follow-up in case 1. (A): Five days after procedure with proper location of atrial and ventricular leads on X-ray; (B & C): extrusion of atrial lead helix through the right atrium (B, red arrow)caused pneumothorax and pneumopericaridum (C, red arrows) on CT scan nine days post procedure; and (D & E): no pneumothorax, pneu mopericaridum and pleural effusion still existed on CT scan at one-year follow-up.
However, the patient was referred for assessment of nonspecific pain in the upper abdomen and mild dyspnea nine days after the operation. Auscultation revealed diminished respiration with dry and moist rales over the right chest, and a small amount of right-sided pneumothorax without obvious displacement of the electrodes was found on chest computed tomography (CT). Pneumopericardium,right pleural effusion, and extrusion of the screw-in atrial lead to the left lung, yet without gross displacement, were clearly observed on the chest CT scan (Figures 1B and 1C).Pacemaker interrogation results were as follows: slight changes in atrial lead parameters, a lower sensed P wave and decreased pacing impedance. Ventricular lead parameters remained stable. The patient’s symptoms relieved immediately after thoracic drainage. As the interrogation parameters were stable and chest radiography revealed resolution of pneumothorax in the following three days, the chest tube was removed subsequently and the atrial lead was not repositioned. During a 1-year follow-up period, the pacemaker parameters remained stable, and no residual pneumothorax, pneumopericardium, and pericardial effusion were found on CT scans (Figures 1D and 1E).
In another case, a 93-year-old woman (BMI = 18.31 kg/m2) with hypertension underwent implantation of a dual-chamber permanent pacemaker for symptomatic sick sinus syndrome on June 26, 2017. Both atrial lead (Vitatron model, Crystalline Actfix ICQ09B-52B; Medtronic, Puerto Rico, USA) and ventricular lead (Vitatron model, Crystalline Actfix ICQ09B-58B, Medtronic) were inserted via the left subclavian vein and actively fixed in the right atrial appendage and right ventricular septum, respectively. Atrial pacing parameters were normal (Table 2). The ventricular pacing threshold was 0.6 V at 0.5 ms, with a pacing impedance of 640 Ω. Sensed R wave was 16.8-18.4 mV. Postoperative chest radiography showed normal findings with an appropriate lead position (Figure 2A).
Seven days after the procedure, the patient experienced pain over the right chest and shortness of breath. Diminished respiration and moist rales were auscultated over the right chest. Chest radiography revealed moderate right-sided pneumothorax without obvious electrodes displacement.Chest CT was performed and revealed extrusion of the atrial lead into the left lung via the left atrial appendage (Figures 2B and 2C). Then, a chest tube was introduced, and the patient’s symptoms relieved promptly. As pneumothorax resolved and the atrial lead was stable with acceptable parameters, the chest tube was removed, and the atrial lead was retained. At the one-year follow-up, no discomfort wasreported. No residual pneumothorax and pericardial effusion were found on CT scanning (Figures 2D and 2E).

Table 2. Atrial electrode parameters in Case 2.

Figure 2. Images of X-ray and CT scan post pacemaker implantation during follow-up in case 2. (A): Five days after procedure with proper location of atrial and ventricular leads on X-ray; (B & C): extrusion of atrial lead helix through the right atrium caused pneumothorax(B, red arrow) and right pleural effusion (C, red arrow) on CT scan seven days post procedure; and (D & E): no pneumothorax and pleural effusion still existed on CT scan at one-year follow-up.
Contralateral pneumothorax is a rare complication during pacemaker implantation,[1-6]and it mostly occurs in the acute phase (< 24 h) after pacemaker implantation because of active fixation leads perforating the lung via the right atrial wall. Subacute contralateral pneumothorax associated with atrial lead perforation is easily overlooked because of the phase during which it occurs,[1,5]and most patients undergo atrial lead repositioning.[7,8]We report two unusual cases of contralateral pneumothorax in elderly female patients with a low BMI in the subacute phase after pacemaker implantation, despite which the atrial leads were retained in situ.
The risk factors for atrial perforation are as follows: old age (> 80 years), female sex, low BMI, an atrial lead positioned on the right free wall, corticosteroid therapy, lead repositioning, and so on.[1,2,5,9-11]Both our patients were elderly women with a low BMI who had atrial leads positioned in the right appendage or anterior wall of the right atrium. However, the reason for subacute contralateral pneumothorax despite stable atrial lead performance remains unknown. We speculated that because of the low profile of recent leads, the tip of the helix was micro-displaced, and perforated the pleura via the atrial wall, yet it plugged the small defect caused by perforation, avoiding pericardial effusion. Furthermore, cardiomyocyte contraction and fibrosis over the lead might play an important role in this phenomenon, preventing recurrence of pneumothorax after the subacute phase. However, in the early phase,the lead tip was not covered by fiber, and its repeated contact with the lung during inspiration gradually resulted in pneumothorax in the subacute phase. However, the electrode remained in contact with atrial myocardium, resulting in stable and normal lead parameters.
Although no serious conditions were caused in our cases,we should try our best to avoid such complications as no long-term follow-up results have been reported and we surgical operations in such elderly patients might be risky.Firstly, thorough evaluation of the risk factors listed above is critical to guide pacemaker implantation. Secondly, the atrial septum should be preferred in high-risk patients. Alternatively, excessive pressure on the lead should be avoided when screwing in the atrial lead. During follow-up,chest radiography, echocardiography, pacemaker interrogation, and even chest CT should be performed in patients with right chest or upper abdomen pain and dyspnea. Thoracic drainage could help relieve symptoms if a large amount pneumothorax is confirmed. However, the atrial lead should be retained in the case of stable and acceptable atrial lead parameters. If the atrial lead parameters are unstable, the atrial lead should be repositioned through surgery.
A micro-displaced atrial lead after pacemaker implantation could result in contralateral pneumothorax in the subacute phase, even without pericardial effusion or sudden changes in the pacemaker parameters. This condition can easily be identified by CT, and no progressive treatment is necessary besides chest intubation and close follow-up.
Acknowledgment
The authors declare that there are no conflicts of interest.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Applicability of the PRECISE-DAPT score in elderly patients with myocardial infarction
- Heart failure mortality compared between elderly and non-elderly Thai patients
- Value of cystatin C in predicting atrial fibrillation recurrence after radiofrequency catheter ablation
- Perspective of delay in door-to-balloon time among Asian population
- Characterization of coronary atherosclerotic plaques in a homozygous familial hypercholesterolemia visualized by optical coherence tomography
- Hypertension, abnormal blood pressure circadian pattern, and frailty:data from the literature
