Autonomic functions and gastric motility in children with functional abdominal pain disorders
2019-01-16AmaranathKarunanayakeShamanRajindrajithHitanaduraAsitadeSilvaSampathGunawardenaNirangaManjuriDevanarayana
Amaranath Karunanayake, Shaman Rajindrajith, Hitanadura Asita de Silva, Sampath Gunawardena,Niranga Manjuri Devanarayana
Abstract BACKGROUND Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs)are the most common cause of recurrent abdominal pain in children. Despite its high prevalence, the underlying pathophysiology of this condition is poorly understood.AIM To assess the role of gastric dysmotility and autonomic nervous system dysfunction in the pathophysiology of AP-FGIDs.METHODS One hundred children, fulfilling Rome III criteria for AP-FGIDs, and 50 healthy controls, aged 5 to 12 years, were recruited after obtaining parental consent. All patients were investigated for underlying organic disorders. Gastric motility and cardiovascular autonomic functions were assessed using validated non-invasive techniques.RESULTS The main gastric motility parameters assessed (gastric emptying rate [45.7 vs 59.6 in controls], amplitude [48.7 vs 58.2], frequency of antral contractions [8.3 vs 9.4],and antral motility index [4.1 vs 6.4]) were significantly lower in children with AP-FGIDs (P < 0.05). The post-prandial antral dilatation at 1 min after the test meal significantly correlated with the severity of abdominal pain (P < 0.05).Assessment of autonomic functions in AP-FGID patients showed neither a significant difference compared to the control group, nor a correlation with gastric motility abnormalities (P > 0.05). The duration of pain episodes negatively correlated with the parasympathetic tone (maladaptive parasympathetic tone) (P< 0.05).CONCLUSION Children with AP-FGIDs have abnormal gastric motility but normal cardiovascular autonomic functions. There is no relationship between abnormal gastric motility and autonomic functions. The pathogenesis of AP-FGIDs is not related to cardiovascular autonomic dysfunction.
Key words: Abdominal pain; Functional gastrointestinal disorders; Autonomic function;Gastric motility
INTRODUCTION
Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs) are one of the most recognized groups of gastrointestinal disorders in children across the world. It has an estimated global prevalence of 13.5% in community- and schoolbased surveys[1]. This group of disorders consists of irritable bowel syndrome (IBS),functional abdominal pain (FAP), functional dyspepsia (FD) and abdominal migraine[2]. Although not directly related to mortality, AP-FGIDs have a considerable effect on health-related quality of life and healthcare expenditure[3,4]. Even with the highly advanced modern technologies, pathophysiological mechanisms of FGIDs are not yet clearly understood. The recognized pathophysiological mechanisms include visceral hypersensitivity, altered gastrointestinal motility, immunological dysfunction, altered gastrointestinal microbiota, altered intestinal permeability,genetic factors and psychosocial disturbances[5].
Abnormalities in gastrointestinal motor function have been suggested as a potential pathophysiological mechanism in AP-FGIDs. They include, dilated gastric antrum at fasting period[6,7], delayed gastric emptying[6,8-11], impaired initial distribution of a meal[12], impaired gastric accommodation to a meal[13]and antral hypomotility[8-11,14].
The autonomic nervous system (ANS) is an integral part of the brain-gut axis that is involved in regulating gastrointestinal motility. Some studies have demonstrated dysfunctions in both sympathetic and parasympathetic divisions of the ANS in children and adults with functional gastrointestinal disorders (FGIDs)[15-17]. Elsenbruch and Orr have noted a significant correlation between vagal response and postprandial abdominal symptoms in patients with diarrhoea-predominant IBS[18].Abnormalities of gastric motility and underlying vagal defects have been demonstrated in adult patients with IBS[15,18]. In addition, the ANS is thought to play an important role in modulating visceral sensitivity in FGIDs[19]. However, the relationship between autonomic function and gastric motility has not been studied in affected children.
The main objective of this study was to assess ANS functions in paediatric patients with AP-FGIDs and its relationship with gastric motor functions.
MATERIALS AND METHODS
Study design
This is a comparative, cross-sectional study to assess cardiovascular autonomic functions and gastric motility in children with AP-FGIDs.
Recruitment of the patients
All consecutive patients aged 5-12 years who were eligible according to the inclusion criteria were recruited from the paediatric out-patient clinics of North Colombo Teaching Hospital, Ragama, Sri Lanka and investigated in the Gastroenterology Research Laboratory, Faculty of Medicine, University of Kelaniya, Sri Lanka. A detailed history was taken from each subject and his or her parents after obtaining written informed consent. Details regarding pain characteristics and autonomic symptoms were obtained using an interviewer-administered pre-tested questionnaire.AP-FGIDS were diagnosed using Rome III criteria[2].
Inclusion criteria
(1) Fulfilment of Rome III criteria for at least one AP-FGID AND;
(2) Abdominal pain at least once per week for at least 2 mo prior to diagnosis AND;
(3) Pain severity more than 25 mm on a 100-mm visual analogue scale and severe enough to interrupt the activities of the child (e.g., sleep, play, schooling, etc).
All patients were screened for organic diseases using a detailed history, and complete physical examination, including growth parameters, stool microscopy, urine microscopy and culture, full blood count, C-reactive protein, liver function tests, renal function tests and ultrasound scan abdomen. Special investigations performed, based on clinical judgment of the consultant paediatrician who assessed the patients,included upper and lower gastrointestinal endoscopy, serum amylase and X-ray kidney-ureter-bladder. Patients were not screened for coeliac disease since it is extremely rare in Sri Lanka[20].
Exclusion criteria
(1) Clinical or laboratory evidence suggestive of an organic pathology.
(2) Chronic medical or surgical diseases other than AP-FGIDs.
(3) Long-term medication for any illness other than AP-FGIDs.
(4) Previous abdominal surgery.
(5) Subjects who had received prokinetic drugs or any other drugs that can alter gastrointestinal motility during the 30 d prior to the diagnosis being made.
Recruitment of controls
An age- and sex-compatible group of children were recruited from the community of the same geographical area as controls after obtaining written parental consent. None of the controls had acute or chronic disease or symptoms related to the gastrointestinal tract.
Subject preparation for testing
Autonomic functions and gastric motility were assessed on the same day (gastric motility from 8:30 am to 9:30 am and autonomic function test from 9:30 am to 10:30 am) under thermo-neutral conditions (26°C). All girls who had attained menarche underwent laboratory investigations during the proliferative phase of their menstrual cycles. All medications with adrenergic and cholinergic properties were discontinued for a period of at least five times the half-life of the specific medication. All subjects were advised to refrain from ingesting beverages containing caffeine, nicotine or alcohol for at least 8 h prior to testing. They were in a fasting state for at least six hours prior to the study. A standard breakfast was given with water after completion of gastric motility assessment. The autonomic function was assessed in all subjects 30 minutes after completion of breakfast.
Assessment of gastric motility
Gastric motility was measured in all children with AP-FGIDs and the controls by a previously reported and validated ultrasound method[21]using a high-resolution realtime scanner (Siemens ACUSON X300™) with 1.8 MHz to 6.4 MHz curve linear transducer and with facilities to record and playback. All gastric motility parameters were assessed by the same investigator (NMD) who was blind to the diagnosis and results of the autonomic function tests. The main gastric motility parameters assessed were fasting antral area, gastric emptying rate, frequency and amplitude of antral contractions and antral motility index.
Assessment of cardiovascular autonomic functions
All subjects underwent autonomic cardiovascular tests according to the test battery described by Ewing et al[22], using the standard procedures described. All autonomic functions were assessed by the same investigator (AK) who was blind to the gastric motility status. The test battery consisted of four autonomic function tests conducted in the following order, and the results were recorded in a data sheet.
(1) Blood pressure (BP) response to standing from lying down position.
(2) Heart rate response to standing from lying down position.
(3) Heart rate variation with deep breathing.
(4) Valsalva test.
Before the test, the procedures were explained and mimicked for the benefit of each subject.
After instrumentation, children were subjected to ten minutes of mandatory rest. At the end of the rest period, the electrocardiogram (ECG) recording from lead II was started along with the BP recording. Thereafter, two readings of BP and heart rate were obtained at an interval of two minutes between two consecutive recordings. The average of the two readings was recorded as resting heart rate and BP.
Test 1 - BP response to standing from lying down position: BP readings were recorded one minute after the unaided standing up, maintaining the arm cuff at the level of the heart. The one-minute systolic BP was compared with the resting systolic BP, and the postural change in systolic BP was calculated. An automated BP machine(A&D Medical®) with a paediatric cuff, which was calibrated against a standard mercury sphygmomanometer, was used. The BP response to standing is dependent upon sympathetic adrenergic function[23].
Test 2 - Heart rate response to standing from lying down position: ECG was recorded for a further 60 seconds after standing. The heart rate ratio (30:15 ratio) was calculated as the ratio between the longest R-R interval at around the 30thbeat (R-R 30)and the shortest R-R interval at or around the 15thbeat after standing (R-R 15). The 30:15 ratio was calculated as R-R 30/R-R 15. An increment in the 30:15 ratio was considered an increased parasympathetic response[24].
Test 3 - Heart rate response to deep breathing: Subjects were instructed to sit quietly and to breathe deeply at six breaths per minute (five seconds in and five seconds out).The investigator guided them through the manoeuvre by counting. Continuous ECG recording (Lead II) was completed for three consecutive artefact-free cycles of deep inspiration and expiration. The difference between maximum and minimum heart rates during each cycle was calculated, and the mean difference of the three cycles was obtained. Impairment in heart rate variability is a sign of parasympathetic dysfunction[23]. Increased parasympathetic response is indicated by widening of the difference[25].
Test 4 - Valsalva test: Subjects were asked to exhale into a mouthpiece connected to a mercury manometer and to maintain the expiratory pressure at 20 mmHg for 15 s in the sitting position. ECG was recorded during this manoeuvre and for 45 seconds afterwards. Pre-testing showed that most of the children were not able to achieve 40 mmHg expiratory pressure proposed by Ewing. Therefore, we set the value at 20 mmHg, which was achieved by the children. The child was allowed to rest for one minute before repeating the Valsalva manoeuvre. The Valsalva ratio was calculated by dividing the maximum R-R interval following Valsalva manoeuvre with the minimum R-R interval during the Valsalva procedure. The mean ratio of the two attempts was calculated. A reduced ratio indicates parasympathetic dysfunction[23].
Tools used to assess symptoms
Autonomic symptoms were assessed by a modified composite autonomic symptom scale (commonly known as COMPASS) which was translated and validated for the local language[26].
Gastrointestinal symptoms were assessed using a translated and validated Rome III questionnaire[27,28]. The severity of abdominal symptoms was recorded on a 100-mm visual analogue scale.
Statistical methods
The sample size was calculated using the 30:15 ratio taken from a previous study done on obese children aged 5-10 years in India[29]. The similarity with the race and age group was considered for selecting values from the Indian study. At a power of 90%and significance level of 95%, the minimum sample required was 26 per group.
All statistical analyses were completed using PSPP version 0.8.3-g5f9212 statistics software (Free Software Foundation, Inc. http://fsf.org/). Means and standard deviations were calculated for continuous variables, and frequencies and percentages were taken for categorical variables. For continuous data, nonparametric, a Mann Witney U test was used. For dichotomous data, a chi-square test was used to assess differences between the two groups. A two-tailed level of significance of 0.05 was used for the analysis.
Ethical approval
This study protocol was approved by the Ethics Review Committee, Faculty of Medicine, University of Kelaniya, Sri Lanka.
RESULTS
Sample characteristics
A total of 100 patients with AP-FGIDs [39 (39%) boys, mean age 7.9 years (SD 2.1 years)] and 50 healthy controls [20 (40%) boys, mean age 8.6 years (SD 1.9 years)] were recruited for this study. The AP-FGID group consisted of 54 (54%) children with FAP,33 (33%) with IBS, and 13 (13%) with FD.
Autonomic symptoms in study subjects
The autonomic symptoms related to the gastrointestinal tract were significantly higher among the AP-FGID group (Table 1). The extra-intestinal symptoms, with the exception of the presence of cold feet, were higher among the AP-FGID group, though the difference was not statistically significant.
Autonomic parameters in study subjects
The autonomic parameters were not significantly different between AP-FGIDs and control groups (Table 2). All parasympathetic parameters were lower in the AP-FGIDs group, but these were not statistically significant. Resting heart rate, which is under parasympathetic inhibition, was higher among the AP-FGIDs group but with no statistical significance.
When pain characters of children with AP-FGIDs were correlated with autonomic parameters, the duration of pain episode negatively correlated with the 30:15 ratio (r =-0.21, P = 0.024, Pearson correlation coefficient). Other characteristics had no such correlation.
Gastric motility in study subjects
Gastric emptying rate, frequency of antral contractions, the amplitude of antral contractions and antral motility index were significantly lower in AP-FGIDs group(Table 3). Pain severity positively correlated with the antral area at 1 min (r = 0.2, P =0.02).
Correlation between autonomic functions and gastric motility
In healthy controls, the gastric emptying rate and the frequency of antral contractions positively correlated with the 30:15 ratio. Furthermore, the Valsalva ratio positively correlated with the frequency of antral contractions in healthy controls (Table 4).There was no such correlation between autonomic functions and gastric motility in patients with AP-FGIDs.
DISCUSSION
The current study assessed the cardiovascular autonomic functions and gastric motility in children with AP-FGIDs. The assessment of autonomic functions in APFGID patients showed no significant difference when compared with the control group. The gastric motility parameters were significantly impaired in children with AP-FGIDs. None of the autonomic function tests showed significant correlation with any of the gastric motility parameters in the AP-FGIDs group.
The lack of differences in the autonomic parameters in the two groups indicates the possibility of normal autonomic function in children with AP-FGIDs. Since there are no tests that can directly assess the autonomic function of the gastrointestinal system and its interactions with the brain, the cardiovascular autonomic functions are used asa proxy to assess autonomic function of the gastrointestinal system[30]. Chelimsky et al[16]noted orthostatic intolerance in six out of eight patients (reflected by excessive increase in heart rate or reduction in BP) and low Valsalva ratio in two patients. Heart rate response to deep breathing, which exclusively assesses parasympathetic function,was within the normal limits in all eight patients[16]. However, this was an observational study with no controls. In addition, the authors did not classify recurrent abdominal pain to definitive functional gastrointestinal disorders using the standard Rome criteria. Several studies have assessed heart rate variability (HRV) in adult patients with IBS using different methods[31]. However, no significant difference in vagal activity and sympatho-vagal balance between children with FAP/IBS and healthy controls have been shown in HRV assessments[32]. Furthermore, meta-analysis of studies assessing HRV found that there could be a significantly lower vagal influence in IBS patients compared to controls[33]. The studies included in these metaanalyses used a different method to assess autonomic function, and therefore, we cannot directly compare our findings with the meta-analyses. Similar to our findings,some other studies have failed to demonstrate a significant association between autonomic functions and functional gastrointestinal disorders[34,35].

Table 1 Autonomic symptoms among children with abdominal pain-predominant functional gastrointestinal disorders and controls, n (%)
In addition, the lack of significant difference of extra-intestinal autonomic symptoms between children with AP-FGIDs compared to controls potentially indicates that children with AP-FGIDs do not have generalized autonomic dysfunction. Similarly, Chelimsky et al[16]did not find extra-intestinal autonomic symptoms in children with AP-FGIDs.
Current autonomic tests are only a proxy measure of gastrointestinal autonomic function. Apart from that, contribution of the autonomic input of the local neuronal network within the stomach has not been taken into account during the testing. These factors may at least partially contributed to the lack of differences in autonomic function in children with AP-FGIDs. However, it is essential to understand that with the current knowledge and available tests, this is the closest that we could come to making a reasonable assessment of autonomic functions.
Gastric motility abnormalities have been reported in children and adults with FGIDs[6,8-11,14,36-38]. In the current study, we also noted a similar pattern of abnormalities in children with AP-FGIDs. In addition, we found a positive correlation between abdominal pain and abnormalities in gastric motility, similar to previous studies[6,10,11,39-41]. These findings suggest a potential pathophysiological relationship between gastric motility abnormalities and AP-FGIDs.
When we correlated gastric motility with autonomic parameters, we did not find a clear correlation between them in children with AP-FGIDs. However, the finding of a significant correlation in controls indicates parasympathetic control of gastrointestinal motor function. None of the other studies in children have assessed the association between autonomic function and gastric motility, and therefore, we could not make a clear comparison.
The ANS is a physiological stress system. It is involved in adapting to various stimuli. Available literature has shown that autonomic activity may present as being normal[38], hypo-functioning[42]or hyper-active[43]in functional abdominal pain.Dysfunction of the ANS can cause significant gastrointestinal problems[44]. At the central level, there is a strong connection between autonomic activation and nociception, which is supported by the anatomical and functional overlap of pain processing structures and autonomic regulating structures[45]. The interaction between pain and autonomic response becomes maladaptive in chronic pain[46]. In some chronic pain states, sympathetic hyperactivity contributes to increased sensitivity topain[47]. In contrast, the pain can result in reduced parasympathetic activity[48].Association of pain and low parasympathetic flow has been reported in women with IBS[49].
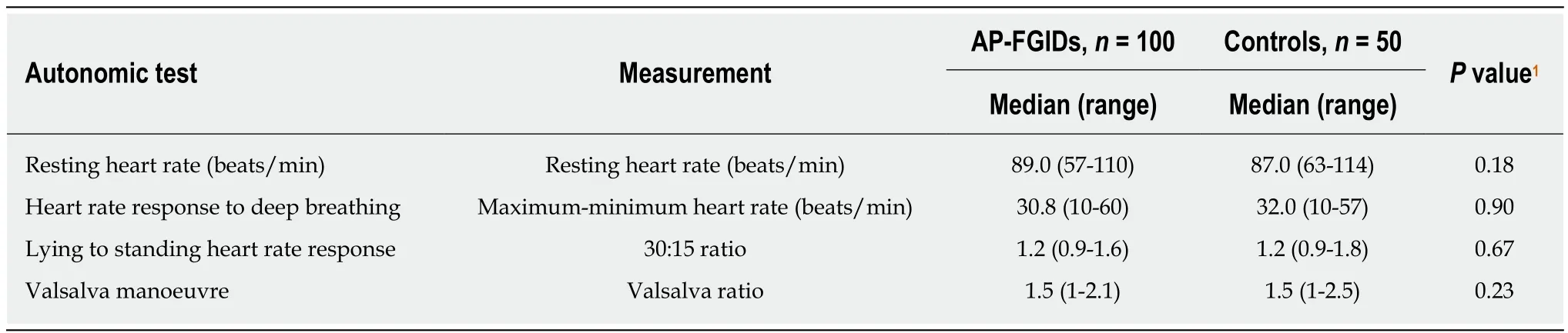
Table 2 Comparison of autonomic parameters between children with abdominal pain-predominant functional gastrointestinal disorders and controls
In the current study, pain duration negatively correlated with the 30:15 ratio(parasympathetic), which can be interpreted as increased pain duration when parasympathetic activity is reduced. The 30:15 ratio is a sensitive index to detect autonomic abnormalities in children[50]. Therefore, we suggest that parasympathetic division may adapt to the initial phase of the disease, as shown in Figure 1. However,a decrease in the parameters after 12 mo of disease may be a feature of mal-adapting autonomic flow. Furthermore, progressive autonomic dysfunction over time has been demonstrated in adults with IBS[51]. Therefore, the degree of parasympathetic functional impairment may present as a spectrum extending from normal to severe impairment. In this context, we may not see a similar response in every FGID patient.
The three cardinal findings in this study, such as lack of difference in extraintestinal autonomic symptoms between AP-FGIDs and controls, lack of differences in autonomic functions between the two groups and lack of correlation between gastric motility and autonomic parameters in those with FGIDs, suggest that the ANS does not play a major role in the pathogenesis of AP-FGIDs in children. In this context,abnormalities in parasympathetic flow are unlikely to be the primary cause for impaired motility in AP-FGIDs. Therefore, the stomach’s unresponsiveness to extrinsic autonomic signals (functional extrinsic denervation) would be a possible underlying primary pathophysiological mechanism for gastric motility abnormalities seen in AP-FGIDs.
Based on these observations, we have developed a hypothetical model to explain the possible mechanism of pathogenesis of AP-FGIDs, which we term ‘’automatic stomach in AP-FGIDs’’. According to the proposed model, functional extrinsic denervation is able to impair motility by three mechanisms (Figure 1). Both impaired motility and maladapted parasympathetic flow have an impact on pain. Possible functional extrinsic denervation demonstrated in the current study would affect gastric motility by increasing dopaminergic inhibition on the stomach, possibly via DAR2 receptors, leading to an “automated stomach” that does not directly respond to the outflow of the autonomic nervous system (Figure 1). Additionally, we have incorporated the modulatory effects of peripheral dopamine receptors on the central dopaminergic system as another possible effect of functional extrinsic denervation[52].
There are several strengths of this study. We have employed well established, non -invasive techniques to assess cardiovascular autonomic functions and gastric motility.The two investigators who assessed gastric motility and autonomic functions were blinded to the diagnosis of the study subjects. In addition, the impact of diurnal variation on motility was minimized by conducting the study from 8:30 am to 10:30 am. The large sample size (100 AP-FGIDs and 50 controls) and detailed evaluation of patients (using history, examination and investigations) to exclude possible underlying organic disorders were the other strengths of the study. Therefore, we believe that our results can be applied to the whole population of children with APFGIDs. However, we did not separate children with AP-FGIDs into specific disease entities such as IBS, FD and FAP. In addition, we used cardiovascular autonomic functions to assess the autonomic functions of the gastrointestinal tract, which is only a proxy measure at best.
In conclusion, children with AP-FGIDs showed abnormal gastric motility parameters, while their cardiovascular autonomic functions were normal. There was no correlation between gastric motility parameters and autonomic functions,indicating that abnormalities in the autonomic nervous system do not play a major role in the pathogenesis of AP-FGIDs. However, we believe maladaptiveparasympathetic flow and the proposed automated stomach model can shed some light upon the pathophysiology of AP-FGIDs in children.
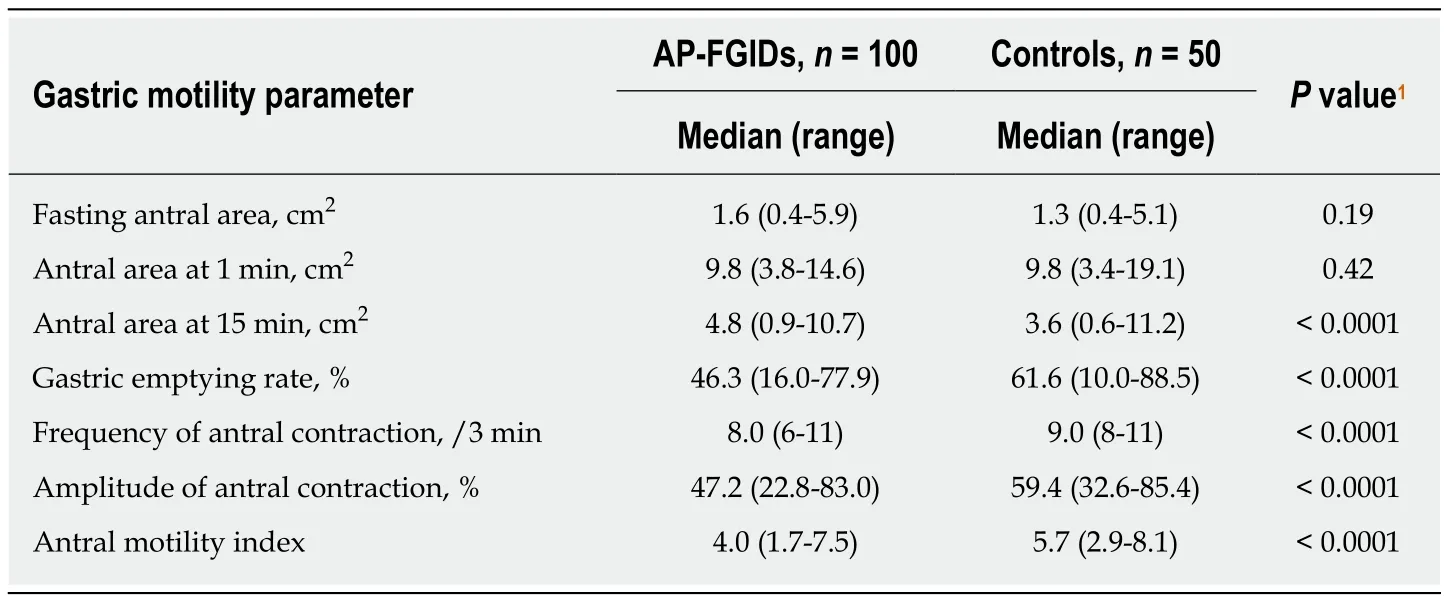
Table 3 Comparison of gastric motility parameters between children with abdominal painpredominant functional gastrointestinal disorders and controls
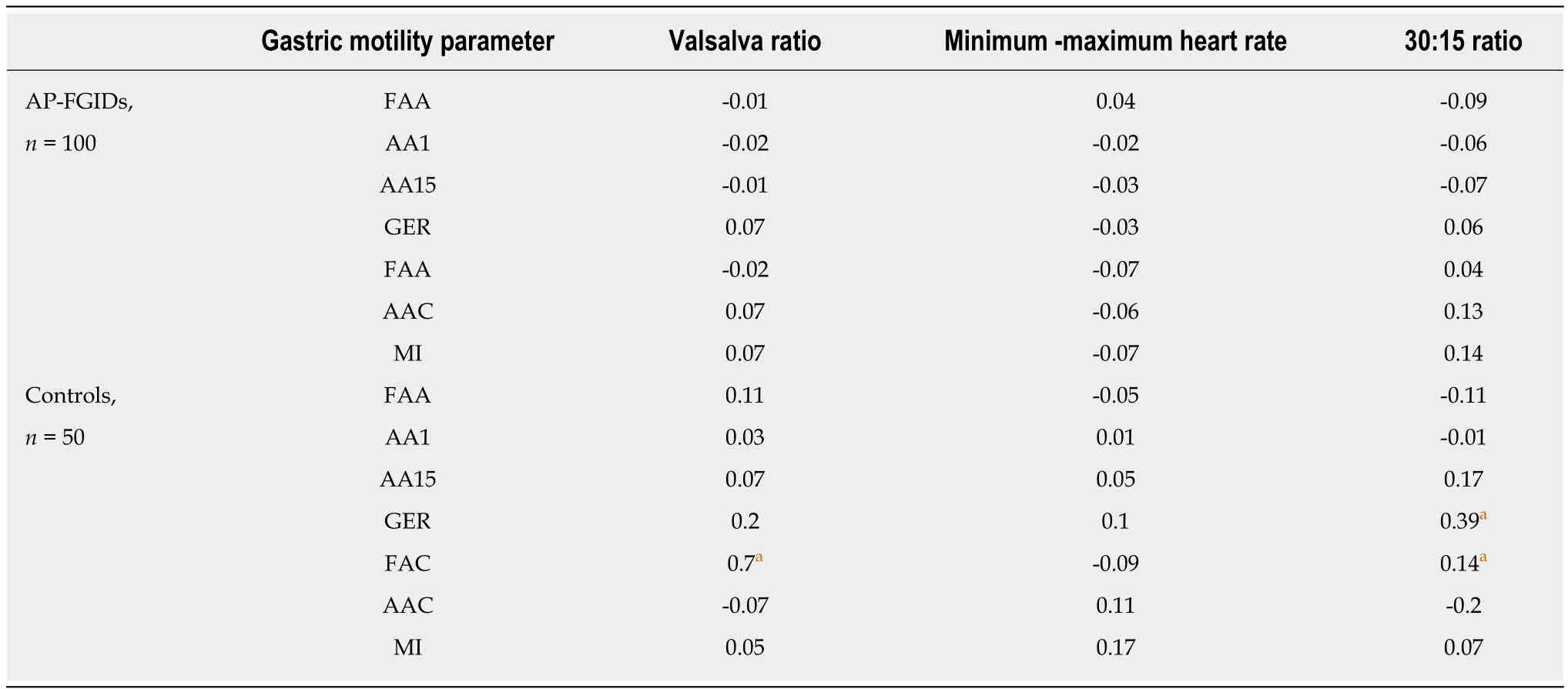
Table 4 Correlation between autonomic parameters and gastric motility parameters among children with abdominal pain-predominant functional gastrointestinal disorders and controls
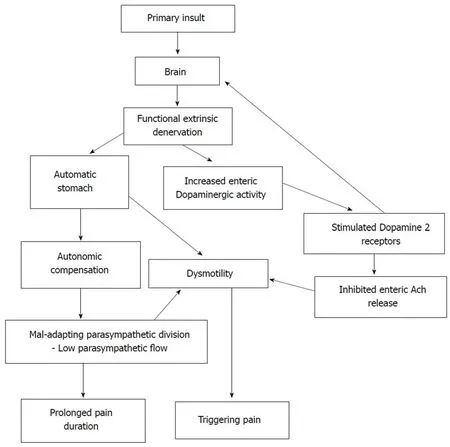
Figure 1 Development and consequences of automatic stomach in abdominal pain-predominant functional gastrointestinal disorders according to the proposed model. Ach: Acetylcholine.
ARTICLE HIGHLIGHTS
Research background
Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs) are a common clinical problem in paediatric practice across the globe, with an estimated prevalence of 13.5%.Although thought to be benign in nature, as a group they are known to associate with poor health-related quality of life and high healthcare burden.
Research motivation
The pathophysiology of AP-FGIDs is not clearly understood. Previous studies have shown abnormalities in gastroduodenal motility, such as delayed gastric emptying, impaired antral motility, and impaired gastric accommodation as potential pathophysiological mechanisms in children. Studies among adults have found autonomic dysfunction in patients with IBS.However, the association between autonomic dysfunction and gastric motility in children with AP-FGIDs had not been previously evaluated.
Research objectives
The main objective of our study was to assess the autonomic functions in children with APFGIDs and their relationship to gastric motor functions.
Research methods
One hundred children fulfilling Rome III criteria for AP-FGIDs and 50 healthy controls aged 5 to 12 years were recruited for the study. All patients were thoroughly investigated to rule out underlying organic disorders. Gastric motility and cardiovascular autonomic functions were assessed using validated, non-invasive techniques.
Research results
Gastric emptying rate, amplitude of antral contractions, and antral motility index were significantly lower in children with AP-FGIDs. Autonomic functions, including blood pressure and heart rate responses to standing from lying down position, heart rate response to deep breathing, and Valsalva test, showed no difference between children with AP-FGIDs and controls. These parameters did not show any correlation with gastric motor functions. However,the duration of pain episodes negatively correlated with the parasympathetic tone.
Research conclusions
Although children with AP-FGIDs have abnormal gastric motility parameters, their cardiovascular autonomic functions are normal. In addition, there is no correlation between autonomic functions and gastric motility. Our findings indicate that the autonomic nervous system is not chronically abnormal in patients with AP-FGIDs. Based on currently available evidence, we propose maladaptive parasympathetic flow and an automated stomach model as a potential pathophysiological mechanism for AP-FGIDs.
ACKNOWLEDGMENTS
We would like to acknowledge Mrs. Liyanayage JCD and Mrs. Ariyawansa J,Technical Officers, Department of Physiology, Faculty of Medicine, University of Kelaniya, Sri Lanka, for their assistance in gastric motility and autonomic function tests. We also acknowledge Dr. Perera BJC, Joint Editor of Sri Lanka Journal of Child Health for editing the manuscript.
杂志排行
World Journal of Gastroenterology的其它文章
- Endoscopic foregut surgery and interventions: The future is now.The state-of-the-art and my personal journey
- Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma
- Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity?
- Initial management for acute lower gastrointestinal bleeding
- Endoscopic trans-esophageal submucosal tunneling surgery: A new therapeutic approach for diseases located around the aorta ventralis
- Usefulness of urinary trypsinogen-2 and trypsinogen activation peptide in acute pancreatitis: A multicenter study in Japan
