Initial management for acute lower gastrointestinal bleeding
2019-01-16TomonoriAokiYoshihiroHirataAtsuoYamadaKazuhikoKoike
Tomonori Aoki, Yoshihiro Hirata, Atsuo Yamada, Kazuhiko Koike
Abstract Acute lower gastrointestinal bleeding (LGIB) is a common indication for hospital admission. Patients with LGIB often experience persistent or recurrent bleeding and require blood transfusions and interventions, such as colonoscopic,radiological, and surgical treatments. Appropriate decision-making is needed to initially manage acute LGIB, including emergency hospitalization, timing of colonoscopy, and medication use. In this literature review, we summarize the evidence for initial management of acute LGIB. Assessing various clinical factors,including comorbidities, medication use, presenting symptoms, vital signs, and laboratory data is useful for risk stratification of severe LGIB, and for discriminating upper gastrointestinal bleeding. Early timing of colonoscopy had the possibility of improving identification of the bleeding source, and the rate of endoscopic intervention, compared with elective colonoscopy. Contrast-enhanced computed tomography before colonoscopy may help identify stigmata of recent hemorrhage on colonoscopy, particularly in patients who can be examined immediately after the last hematochezia. How to deal with nonsteroidal antiinflammatory drugs (NSAIDs) and antithrombotic agents after hemostasis should be carefully considered because of the risk of rebleeding and thromboembolic events. In general, aspirin as primary prophylaxis for cardiovascular events and NSAIDs were suggested to be discontinued after LGIB. Managing acute LGIB based on this information would improve clinical outcomes. Further investigations are needed to distinguish patients with LGIB who require early colonoscopy and hemostatic intervention.
Key words: Lower gastrointestinal bleeding; Predictive model; Colonoscopy; Computed tomography; Medication
INTRODUCTION
Traditionally, gastrointestinal bleeding (GIB) was classified into upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). LGIB was defined as bleeding from the lesion distal to the ligament of Treitz, including the small and large bowels. In the last decade, the availability of advanced diagnostic innovations such as capsule endoscopy and balloon-assisted enteroscopy has led to better understanding of the etiological profile of small bowel bleeding. Thus, some recent reports adopted three categories of GIB: Upper-, mid-, and lower GIB[1].
Acute LGIB has increased due to aging of the population, and with the increasing use of antithrombotic agents[2-4]. Although many UGIB events can be prevented by proton pump inhibitors (PPIs) and eradicating Helicobacter pylori, there are few effective methods for preventing LGIB recurrence. The estimated hospitalization rate for LGIB is 33-87 per 100000 population[2,5,6], with mortality rates of 2.5%-3.9% during hospitalization[7-9]and rebleeding rates of 13%-19% after 1 year[10,11].
Several concerns exist when managing acute-onset hematochezia suspected as acute LGIB. First, the causes of bleeding varied from many types of colonic diseases to UGIB and small-bowel bleeding. Most cases experience spontaneous cessation with conservative therapy. In contrast, patients with vascular diseases, such as diverticular bleeding and angioectasia, often suffer from continuous and/or recurrent bleeding,requiring hemostatic intervention and blood transfusion[9,12]. A few cases die during hospitalization. Therefore, risk stratification tools for severe LGIB would be useful for deciding on emergency hospitalization or an early intervention. However, unlike UGIB, predictive clinical scores for severe acute LGIB are not well defined.
Second, colonoscopy, which is essential for diagnosis and therapy of LGIB[13],requires bowel preparation to identify the bleeding source, unlike upper endoscopy.The timing of time-consuming and laborious colonoscopy should be optimized, but the utility of early colonoscopy remains controversial.
Third, there are numerous antithrombotic agents, including dual antiplatelet therapy and direct-acting oral anticoagulants (DOACs), and the management of antithrombotic agent use requires considering the conflicting risks of ongoing/recurrent bleeding and thromboembolic events.
Thus, appropriate decision-making is needed when managing acute LGIB.Fortunately, novel findings in the acute LGIB setting have accumulated in recent years, such as predictive scores for severe bleeding, the clinical significance of contrast-enhanced computed tomography (CE-CT) before colonoscopy, the utility of early colonoscopy, and the management of DOACs.
The purpose of this literature review is to summarize findings regarding the initial management of acute LGIB, in line with the 2016 American College of Gastroenterology guideline for acute LGIB and the 2016 American Society for Gastrointestinal Endoscopy guideline for the management of antithrombotic agents on gastrointestinal endoscopy[13,14].
INITIAL ASSESSMENT
History-taking, physical examination, and laboratory testing are important at the time of presentation of patients with presumed acute LGIB. The need for intravenous fluid resuscitation and blood transfusion should be determined by evaluating hemodynamic status according to history of syncope, level of consciousness, and vital signs, including postural changes. Hematochezia with hemodynamic instability requires attention, as brisk UGIB can also result in that type of stool. A blood urea nitrogen/creatinine (BUN/Cr) ratio > 30 (likelihood ratio, 7.5) and nasogastric aspirate/lavage with blood or coffee grounds (likelihood ratio, 9.6) are the features of UGIB[15], being useful to distinguish UGIB from LGIB. In addition, in a report of patients with hematochezia, the systolic blood pressure was significantly lower in UGIB than in LGIB (mean pressure, 114 mmHg vs 133 mmHg)[16]. If the likelihood of UGIB is high based on these factors, upper endoscopy is recommended.
The presence of certain symptoms can suggest the source of LGIB[13]. Patients with colitis (ischemia, infection, or inflammatory bowel disease) often present with diarrhea and abdominal tenderness, whereas those with vascular diseases, such as diverticular bleeding, hemorrhoid, angioectasia, and rectal ulcers, usually do not present with lower gastrointestinal symptoms. Weight loss and altered bowel habits suggest malignancy.
Risk stratification
Although most patients with acute LGIB experience spontaneous hemostasis, some suffer from severe, persistent hemorrhage and rebleeding. The frequency of adverse outcomes varies by cause of LGIB. The causes of acute LGIB in the Western countries are as follows[17]: Diverticular bleeding (30%-65%), ischemic colitis (5%-20%),hemorrhoids (5%-20%), colorectal polyps/neoplasms (2%-15%), angioectasia (5%-10%), post-polypectomy bleeding (2%-7%), inflammatory bowel disease (3%-5%),infectious colitis (2%-5%), rectal ulcer (0-5%), colorectal varices (0-3%), radiation proctitis (0-2%), drug-induced colitis (0-2%), and Dieulafoy’s lesion (rare). On the other hand, in the tropical countries, colorectal polyps/neoplasms (29%-53%) and colitis (23%-38%) are the common causes, and diverticular bleeding is less common(4%-19%)[16,18]. The rate of rebleeding is 22%-38% in patients with diverticular bleeding[12]. Ischemic colitis cases have significantly lower blood transfusion requirements (4%) compared with other forms of LGIB[19].
Physicians must understand the predictive factors for severe LGIB to improve triage of appropriate patients for emergency hospitalization or early intervention.Several studies have investigated risk factors for adverse outcomes (rebleeding, severe bleeding, need for emergent hospitalization, need for intervention, adverse events, or death) in patients with acute LGIB[7,8,20-28]. These include older age, presenting symptoms (no abdominal tenderness, no diarrhea, altered mental status, or blood on rectal examination), vital signs, comorbidities, medication use [nonsteroidal antiinflammatory drugs (NSAIDs) and antithrombotic agents], and laboratory data[hemoglobin (Hb), hematocrit, albumin, BUN, Cr, and prothrombin time (PT)] (Table 1). We also previously reported a predictive model of severe LGIB (NOBLADS score),which included NSAID use, no diarrhea, no abdominal tenderness, systolic blood pressure ≤ 100 mmHg, albumin level < 3.0 g/dL non-aspirin antiplatelet drug use,Charlson comorbidity index score ≥ 2, and syncope[24]. Several predictive models have been validated in other settings (Table 2)[21,22,24,27,28]. Applying these models to manage LGIB could improve clinical outcomes and resource utilization. However, compared with established models of severe UGIB[29,30]such as the Blatchford score, predictive models of severe LGIB require further validation and improvements in accuracy.
INITIAL MANAGEMENT
Intravenous fluid resuscitation with crystalloids should be initiated, particularly in hemodynamically unstable patients[13,31]. In a review of fluid administration in bleeding patients, the best fluid resuscitation strategy was not determined on the basis of the timing, volume, and type of fluid[32]. Another review on critically ill patients indicated that colloids do not improve the mortality rate and are more expensive compared with crystalloids[33].
Although patients with LGIB often require a blood transfusion[9], transfusion strategies specific to LGIB have not been investigated. In a recent meta-analysis of five randomized controlled trials (RCTs) comparing restrictive and liberal transfusion strategies in the acute UGIB setting, restrictive transfusion of red blood cells (Hb threshold, 7-8 g/dL) was associated with a lower risk of all-cause mortality [relative risk (RR): 0.65, 95% confidence interval (CI) 0.44-0.97] and rebleeding (RR: 0.58,95%CI: 0.40-0.84) than liberal transfusion (Hb threshold 9-10 g/dL)[34]. The LGIB guideline applied this result to recommendations for LGIB[13].
However, it should be noted that a previous RCT and a meta-analysis indicated that for patients with cardiovascular disease which limits myocardial oxygen delivery, rates of mortality and cardiovascular events were higher in a restrictive
transfusion group than in a liberal transfusion group[35,36]. LGIB guideline recommended that liberal transfusion be considered in patients with massive bleeding or cardiovascular disease[13].
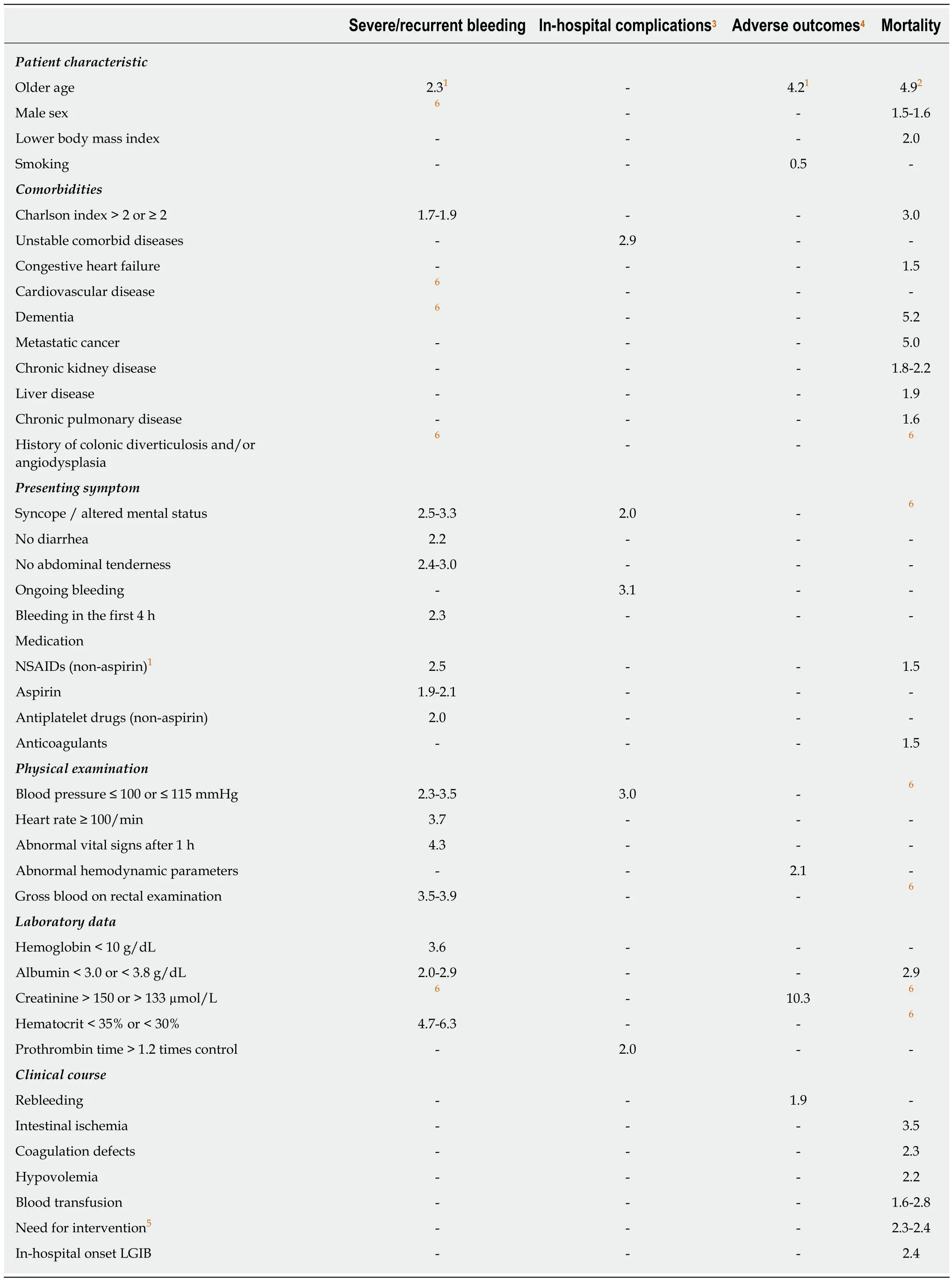
Table 1 Risk factors and odds ratios for various outcomes according to 11 studies[7,8,20-28]
With respect to platelet transfusion, a systematic review concluded that there are no data to inform optimal therapeutic platelet count targets in the acute gastrointestinal bleeding (GIB) setting[37]. Based on expert opinion and the standard in the hematology literature, a platelet count of 50 × 109/L is proposed as the LGIB guideline threshold[13,38].
DIAGNOSIS AND TREATMENT
Colonoscopy
Colonoscopy is the initial procedure for nearly all patients presenting with acute LGIB, because it has both diagnostic and therapeutic utility[13]. Common causes of acute LGIB include diverticular bleeding, ischemic colitis, angioectasia, and postpolypectomy bleeding. Other, less common causes include rectal ulcers, infectious colitis, inflammatory bowel disease, colorectal polyps/neoplasms, radiation proctitis,and hemorrhoids[13]. One of the most important issues in diagnostic colonoscopy for acute LGIB is identifying stigmata of recent hemorrhage (SRH), including active bleeding, a non-bleeding visible vessel, and an adherent clot[39,40]. SRH is regarded as an indication for endoscopic hemostasis because a prospective study showed that the rebleeding rate within 30 d in patients with SRH was 66% when endoscopic therapy was not performed, whereas patients without SRH had no rebleeding[40].
When to perform colonoscopy
The optimal timing of colonoscopy remains controversial. The definition of early colonoscopy used in most studies was within 24 h of presentation, and the definition in a few prospective trials was within 6-12 h[41-45]. Two RCTs and three meta-analyses examined the utility of early colonoscopy compared with elective colonoscopy in the acute LGIB setting (Table 3). These studies showed that early colonoscopy had the possibility of improving identification of the bleeding source, and the rate of endoscopic intervention, compared with elective colonoscopy. However, there is no clear evidence that early colonoscopy reduces important clinical outcomes, such as rebleeding or mortality.
The limitations of past studies may have affected these results. Previous RCTs were single-center studies and were terminated during enrollment because of difficulties in achieving the originally planned sample size. To address these issues, we are now conducting a multicenter RCT to examine the superiority of early colonoscopy over elective colonoscopy in patients with acute LGIB[46]. The primary outcome measure is identification of SRH. Secondary outcomes include 30-d rebleeding, the need for transfusion, and 30 d mortality. This trial will provide high-quality evidence of the benefits of early colonoscopy.
The safety of early colonoscopy in the acute LGIB setting has been reported. The rate of complications associated with bowel preparation was not significantly different between early colonoscopy (1.8%) and elective colonoscopy (1.2%) in a study based on a propensity score-matching analysis[47]. A literature review showed that the rate of complications associated with colonoscopic procedures is low for both early colonoscopy (0.6%) and elective colonoscopy (0.3%)[48].
For whom and how to perform early colonoscopy
The LGIB guideline recommends that patients with high-risk clinical features and signs of ongoing bleeding should undergo early colonoscopy, i.e., within 24 h of presentation[13]. One of the signs of ongoing bleeding is extravasation on a CT scan,which should lead to early colonoscopy. However, clinical factors remain uncertain which can be easily obtained at the presentation and can suggest the indication for early colonoscopy. Although the NOBLADS score, one of the predictive score for severe LGIB, indicated the need for intervention in a derivation cohort (P = 0.001 for trend)[24], the score was not a significant predictor of the need for intervention in an externally validated cohort (P = 0.060 for trend; area under the curve, 0.54)[49]. In a recent LGIB study, all seven previous models for predicting severe GIB were not useful for distinguishing patients who required therapeutic intervention[27]. No existing model directly predicts the need for intervention in the LGIB setting; thus,appropriate models and novel strategies are required.
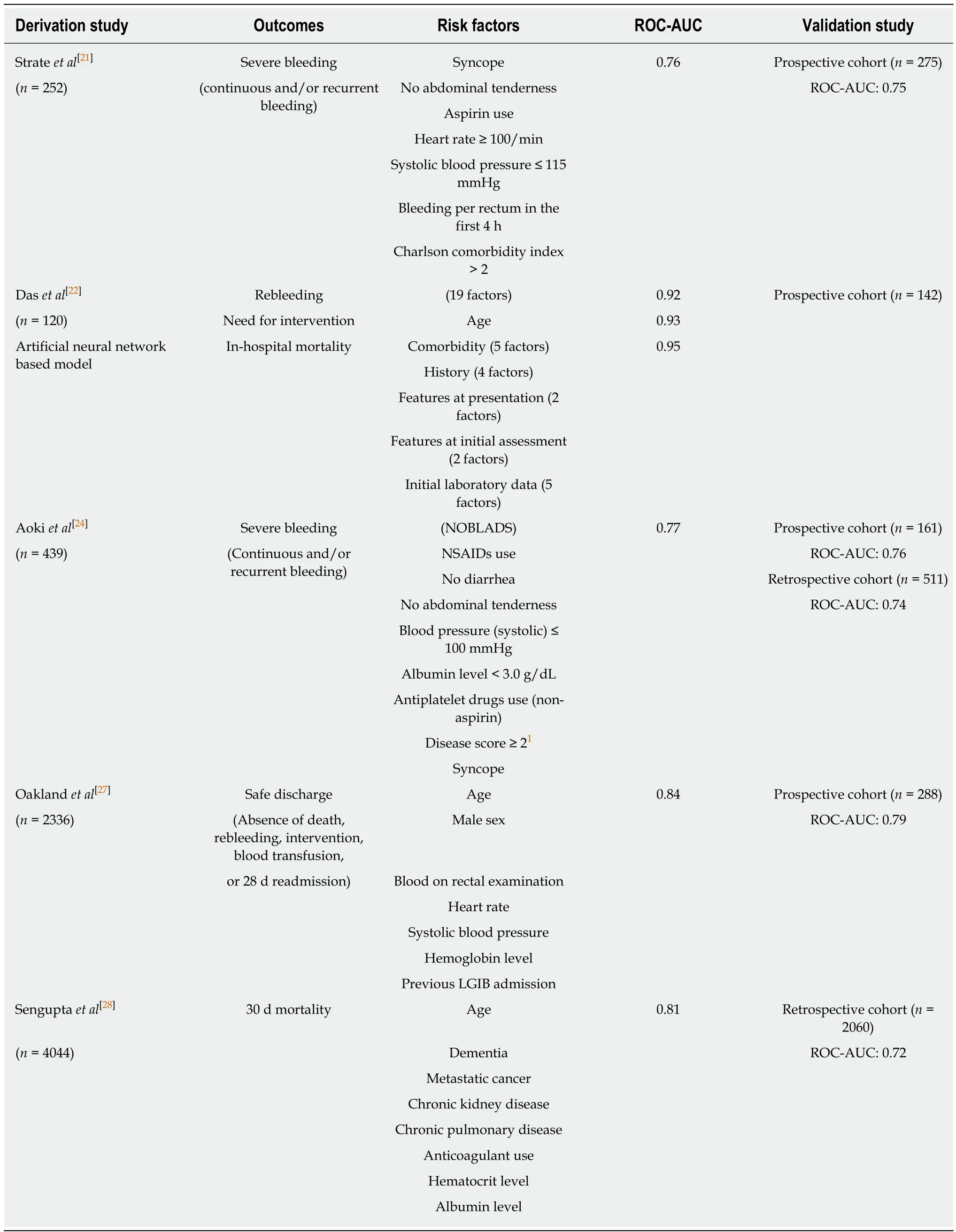
Table 2 Risk scoring systems for severe acute lower gastrointestinal bleeding which have been validated
Based on evidence to date, the primary purpose of early colonoscopy is to identify the bleeding site and perform endoscopic hemostatic therapy. In addition to earlier colonoscopy, adequate colon preparation, an expert colonoscopist, use of a cap, and use of a water-jet scope have been suggested to improve the rate of SRH identificationin patients with diverticular bleeding[39,50]. Because more than half of SRH has been reported to locate in the right colon (71%)[51], cecal intubation with adequate colon preparation is required, even for early colonoscopy. A nasogastric tube can be placed for bowel preparation when patients with LGIB are unable to tolerate rapid colon preparation[39,41].
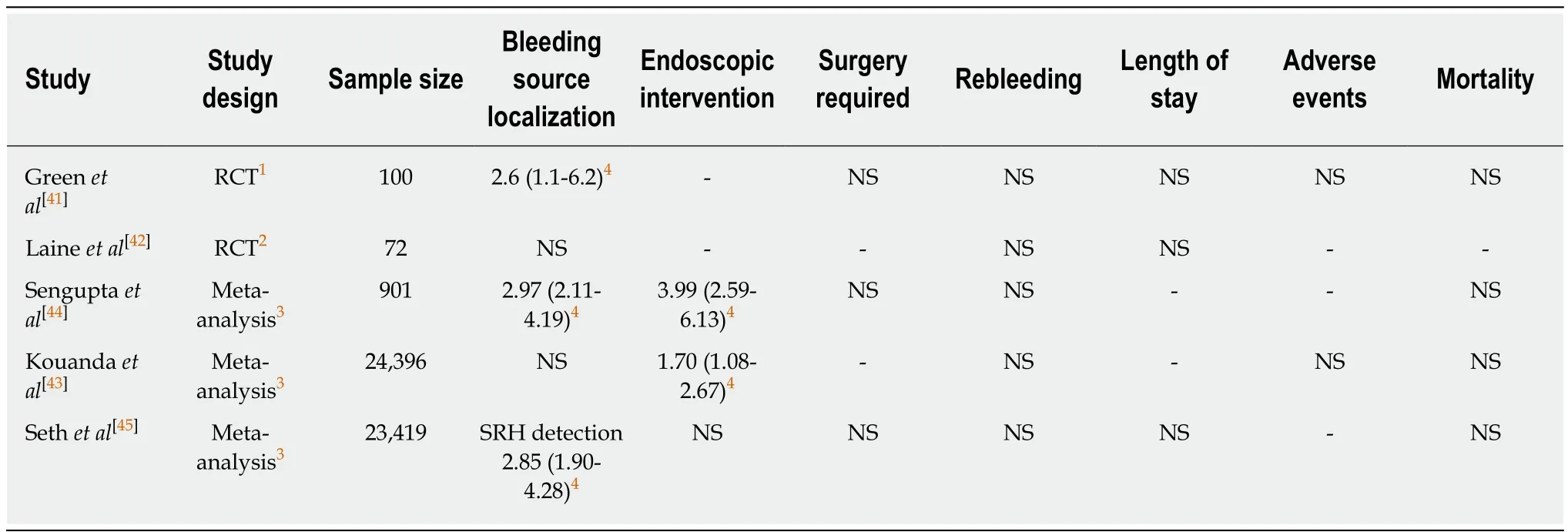
Table 3 Utility of early colonoscopy compared with elective colonoscopy according to randomized controlled trials and meta-analyses
Computed tomography
A systematic review showed high sensitivity (85.2%) and high specificity (92.1%) of CT angiography for diagnosing acute GIB[52]. The American College of Gastroenterology guideline suggest that CT angiography should be considered to localize the bleeding site before angiography or surgery, when the hemodynamics do not permit endoscopic evaluation and/or when patients are unable to tolerate the bowel preparation[13].
The clinical significance of performing CE-CT before colonoscopy has been examined in recent years. Our retrospective study of acute LGIB reported that the detection rate for vascular lesions was higher for colonoscopy following CT than for colonoscopy alone (35.7% vs 20.6%, P = 0.01), leading to more endoscopic therapies(34.9% vs 13.4%, P < 0.01)[53].
Furthermore, several studies have focused on the association between extravasation on CT and definitive diverticular bleeding on colonoscopy (Table 4). The colonoscopic detection rate of bleeding diverticula is significantly higher in patients with extravasation on CT than in those without (60%-76% vs 18%-31%)[54-56], suggesting that extravasation on CT is a reasonable indication for urgent colonoscopy to detect SRH.However, CT is not recommended for all cases due to the low rate of positive extravasation (15%-25%) documented in prospective studies of diverticular bleeding[56,57]. The intermittent nature of diverticular bleeding can reduce the sensitivity of CT for diagnosing diverticular bleeding. A prospective multicenter study suggested that patients who can be examined within 4 h of the last hematochezia would be candidates for urgent CT, because sensitivity is higher in this group than in those examined after 4 h (64.7% vs 33.3%, P < 0.01)[56].
Angiography and embolization
The major advantage of angiography and embolization is that it can control severe bleeding without bowel preparation. A systematic review reported that superselective angiographic embolization achieves immediate hemostasis in 40%-100% of diverticular bleeding with occasional rebleeding (15%)[58]. The disadvantages of angiography and embolization include the requirement for active bleeding and the risk of bowel ischemia and contrast-induced nephropathic complications. The rate of bowel ischemia following embolization was 1%-4% in recent studies[59,60]. The LGIB guideline recommends that this intervention should be reserved for patients with very brisk, ongoing bleeding who do not respond adequately to hemodynamic resuscitation efforts and are unlikely to tolerate bowel preparation and early colonoscopy[13].
Angiography localizes the LGIB source in 24%-70% of cases[59,61]. Angiography requires blood loss rates > 0.5 mL/min to localize a bleeding site[62].Transfusion of > 5units of red blood cells or 4 units of fresh frozen plasma within 24 h, hemodynamic instability at the time of angiography, and older age are predictors of a positive angiography[63,64]. In addition, CT angiography may be useful as a noninvasive diagnostic tool before angiography, because it is more sensitive than transcatheter angiography and identifies bleeding at rates of 0.3 mL/min[65].
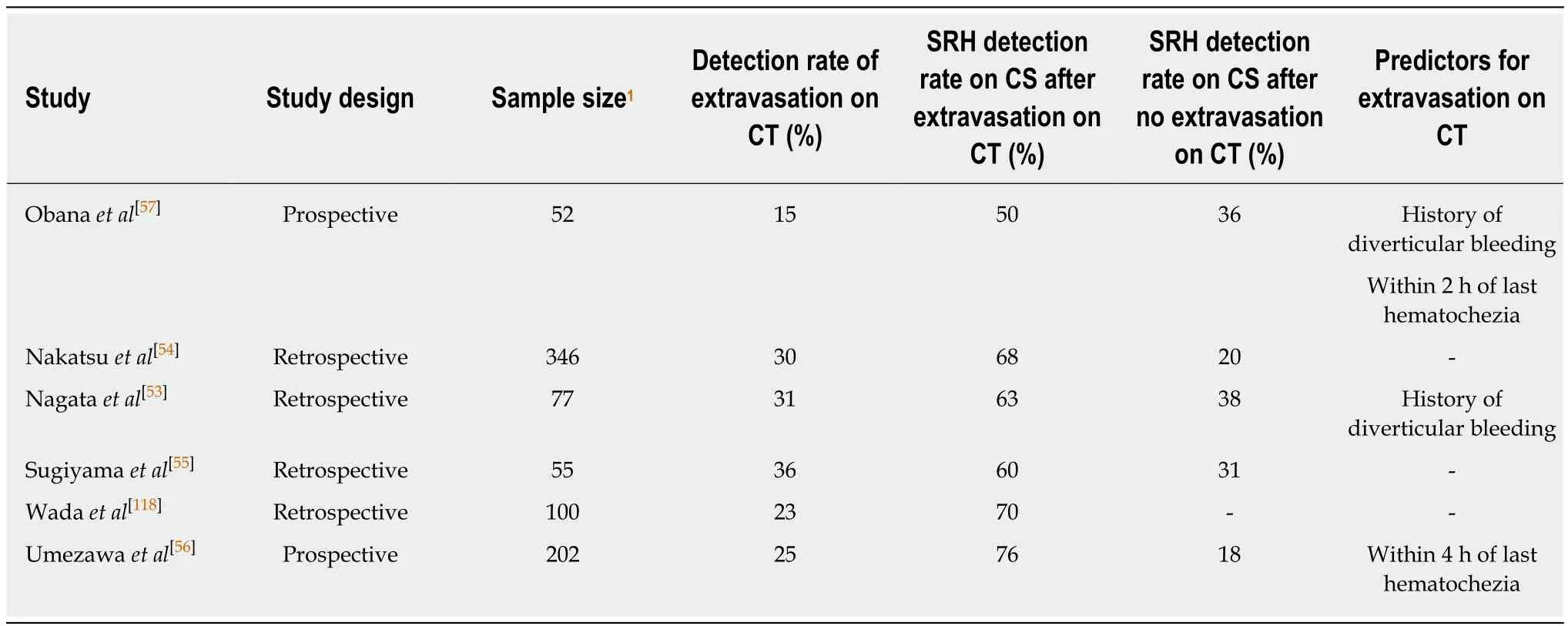
Table 4 Clinical significance of performing contrast-enhanced computed tomography before colonoscopy for colonic diverticular bleeding
In a retrospective study of colonic diverticular bleeding with SRH on colonoscopy,the rate of interventional radiology and/or surgery due to failure of repeated colonoscopic hemostasis was higher for bleeding from the ascending colon (19%) than from other parts of the colon (0)[51]. Therefore, patients with bleeding from the ascending colon have a higher risk of being transferred for interventional radiology after colonoscopy.
Surgery
Studies on surgery for acute LGIB have recently decreased, probably because of advances in endoscopic hemostasis and interventional radiology. The complication and mortality rates of surgery for acute LGIB are as high as 60% and 16%,respectively[66]. Given these high rates, surgery should be reserved for patients with brisk, ongoing LGIB. Indications for emergency surgery for severe LGIB include (1)the bleeding source has been clearly identified but non-surgical interventions have failed, and ii) continued bleeding (6 units of red blood cells transfused) and the lack of a diagnosis despite a thorough work-up using endoscopic and radiographic modalities[48,67].
Localizing the bleeding lesion before surgical resection is important to prevent rebleeding after surgery from an unresected culprit lesion, and to prevent excess mortality after a blind total colectomy. In previous studies of surgical management for acute LGIB[68-71], the rebleeding rate was higher after a limited colonic resection (4%-18%) than after a total colonic resection (0-4%). In most of these studies[68-70], the mortality rate was lower after limited colonic resection (7%-22%) than after total colonic resection (20%-40%).
Therapeutic barium enema for diverticular bleeding
High-dose barium impaction therapy using concentrated (200%) barium sulphate for diverticular bleeding has been reported. Evidence of the effectiveness of initial hemostasis is poor, due to the studies being case reports or case series. Nevertheless,these reports suggest that this therapy may have advantages for hemostasis in patients with uncontrolled or recurrent presumptive diverticular bleeding[72-75]. Novel barium impaction therapy using an enteroscopic overtube with a balloon has been reported for diverticular bleeding in the right-sided colon, and can be used to apply sufficient barium pressure to the deep colon[76].
The effectiveness of barium impaction therapy with respect to long-term prevention of rebleeding was demonstrated in an RCT[77]. The hazard ratio (HR) of rebleeding in the barium group, comparing barium therapy to conservative therapy after spontaneous cessation of diverticular bleeding, was 0.34 (95%CI: 0.12-0.98).
MEDICATION MANAGEMENT
The management of medication use in the LGIB setting requires considering the risks of ongoing/recurrent bleeding and thromboembolic events (Figure 1). Cessation of these agents can be considered in patients on antithrombotic agents with lifethreatening or serious bleeding. Although there are few data to guide the timing of the resumption of antithrombotic agents, current guidelines recommend resumption as soon as hemostasis is achieved[13,14]. A multidisciplinary approach involving cardiology, neurology, hematology, and gastroenterology is necessary, particularly for managing patients taking dual antiplatelet agents or anticoagulants.
Non-aspirin NSAIDs
Previous studies have indicated that NSAIDs increase the risk of both event and recurrence of LGIB[11,78-80]. In a retrospective cohort study of 342 patients with LGIB, the HR of NSAID use for recurrence was 2.0 (95%CI: 1.2-3.3)[11]. In a prospective study of 132 patients with diverticular bleeding, the recurrence rate at 12 mo was significantly higher in patients who continued NSAID use (77%) than in those who discontinued use (9%)[81]. Therefore, non-aspirin NSAIDs should be discontinued after acute LGIB,particularly in cases of diverticular bleeding. Unlike UGIB, changing from a nonselective NSAID to a cyclooxygenase-2 (COX-2) selective NSAID might be ineffective for preventing recurrence, because COX-2-selective and -non-selective agents increase the risk of LGIB[79,82].
Antiplatelet agents
Antiplatelet agents increase the risk of both event and recurrence of LGIB[11,79,80,83]. The risk of LGIB with antiplatelet agents use is approximately three times that for UGIB[84,85], probably because LGIB lacks prophylactic measures, such as H. pylori eradication and PPIs.
Available data on the influence of discontinuing aspirin in the GIB setting are as follows. A retrospective cohort study of patients with LGIB showed that the rate of cardiovascular events was significantly higher in those who discontinued aspirin(37%) than in those who continued the drug (23%), while the rate of recurrent LGIB was lower in the former cohort (7%) than in the latter cohort (19%) within 5 years[86].In an RCT of peptic-ulcer bleeding, 60 d mortality was significantly higher in patients who discontinued aspirin after endoscopic therapy than in those who continued; the rate of rebleeding was not different between the groups[87]. Based on this evidence,aspirin for secondary prophylaxis in patients with established cardiovascular disease should not be interrupted to prevent thrombotic events in the LGIB setting. However,aspirin as the primary prophylaxis for patients who are not at high risk of cardiovascular events had little effect (0.07% absolute risk reduction)[88]and should be discontinued after LGIB.
The influence of short-term drug interruption in single antiplatelet users (aspirin or other antiplatelet agents) has not been determined. No difference in in-hospital rebleeding was observed in a retrospective study comparing patients who had their antiplatelet drug stopped for < 5 d with those who continued it throughout their admission[83]. In that study, cardiovascular events were too few to allow meaningful comparison.
There are some data that can guide the management of dual antiplatelet therapy.The risk of myocardial infarction and death after discontinuing dual antiplatelet therapy is high during the first 30 d following coronary stenting and during the first 90 d following acute coronary syndrome[89]. Such patients are advised to continue dual therapy. In contrast, discontinuing the second non-aspirin antiplatelet agent for up to 7 d is allowed for patients with more distant coronary stenting or coronary syndrome,because it seems to carry a relatively low risk as long as aspirin is continued[90].
Anticoagulants
Anticoagulants are classified into warfarin and DOACs. Two types of DOACs are currently available: thrombin inhibitors (dabigatran) and coagulation factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Current endoscopic and LGIB guidelines do not discuss the role of a heparin bridge sufficiently, nor management of DOACs in the acute GIB setting[13,14]. Evidence is mainly based on studies of UGIB or all types of GIB.
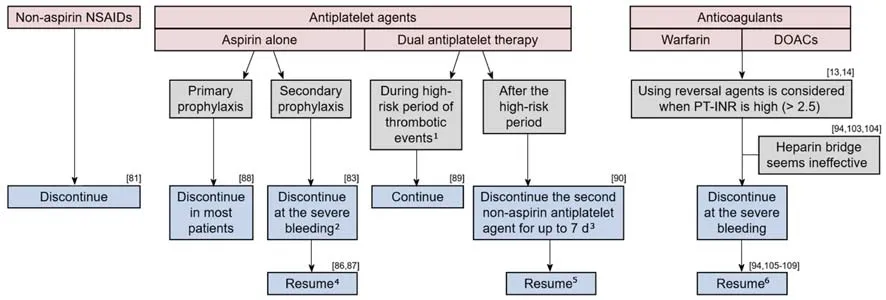
Figure 1 Recommendation for the management of medication based on current studies.1During the first 30 d following coronary stenting and during the first 90 d following acute coronary syndrome; 2The influence of short-term discontinuation has not been determined; 3Aspirin should be continued; 4Resumption reduces cardiovascular events but may increase rebleeding; 5The influence of long-term discontinuation has not been determined; 6Changing to apixaban, or reducing the dose of dabigatran to 110 mg b.i.d may reduce rebleeding in GIB patients taking warfarin, dabigatran (150 mg b.i.d) or rivaroxaban. NSAIDs: Nonsteroidal antiinflammatory drug; DOAC: Direct-acting oral anticoagulant; PT-INR: Prothrombin time-international normalized ratio.
Prothrombin time-international normalized ratio and the reverse method:Guidelines[13,14]recommend INR < 2.5 as being reasonable for endoscopy in the acute GIB setting, based on reports that a moderate elevation in INR does not increase the risk of rebleeding following endoscopic therapy for nonvariceal UGIB[91-93]. Guidelines also recommend using reversal agents before endoscopy for patients with an INR >2.5, but the evidence for this is not well-established. Indeed, some retrospective studies found that a higher INR does not increase the rebleeding rate in LGIB[94]or all types of GIB[95]. Thus, an elevated INR appears not to carry a risk of rebleeding.However, an elevated INR at onset has been reported to be a predictor of thromboembolism within 90 d of endoscopy for all GIB (INR > 2.5, OR: 7.9)[94], and of mortality for nonvariceal UGIB (INR > 1.5, OR: 5.6)[96]. This is presumably because INR is an indicator of underlying comorbid diseases. In a study of all types of GIB,other factors related to anticoagulant management, such as the difference in onset and pre-endoscopic INR, reversal agent use, and anticoagulant interruption, were associated with thromboembolism[94]. Therefore, it might be unnecessary to actively reduce the INR. Rather, early endoscopy without using a reversal agent or interrupting anticoagulant therapy may be warranted for acute GIB.
Reversal of the anticoagulant effect should be considered for ongoing severe bleeding via intravenous vitamin K, fresh frozen plasma, or prothrombin complex concentrate (PCC) for warfarin users[97,98], and via oral charcoal, hemodialysis,idarucizumab, or PCC for DOAC users[99-102]. Oral charcoal is considered if a DOAC was taken within 2 h. Hemodialysis or idarucizumab is considered for dabigatran users. The effect of PCC on bleeding of DOAC users has not been established.
Heparin Bridge: Previous reports suggest that a heparin bridge might be ineffective in the acute GIB setting. A heparin bridge did not significantly alter the risk of rebleeding or thromboembolism in a recent retrospective study of patients with GIB[94]. In an RCT of warfarin users undergoing invasive procedures, the heparin bridge group suffered from more major bleeding than the non-bridged group,without a difference in the thromboembolism rate during the periprocedural period[103]. Furthermore, a similar result was found in a prospective observational study of DOAC users undergoing interventional procedures[104].
Resumption of anticoagulants: A meta-analysis concluded that resuming anticoagulants reduces the rate of thrombotic events in patients with disrupted use of anticoagulants due to GIB (HR: 0.68, 95%CI: 0.52-0.88), and mortality (HR: 0.76,95%CI: 0.66-0.88), without significantly increasing the rebleeding rate (HR: 1.20,95%CI: 0.97-1.48)[105]. This result was consistent with other reports[106-108]. Studies that compared warfarin and DOAC users reported that the rate of thrombotic events was similar between the two groups, during the 90 d after GIB[94]and during the anticoagulant-interrupted period[109]. A retrospective cohort study on DOAC users reported that the rate of thromboembolism within 90 d of GIB did not differ between those who resumed DOAC and those who did not[110]. In that study, a history of venous thromboembolism was associated with thromboembolism events (HR: 3.30,95%CI: 1.29-7.38).
The optimal duration before restarting anticoagulants after an episode of GIB remains uncertain. In a retrospective cohort study, the HRs of rebleeding,thromboembolism, and mortality in patients who resumed warfarin within 7 d were 3.27 (95%CI: 1.82-5.91), 0.76 (95%CI: 0.37-1.59), and 0.56 (95%CI: 0.33-0.93),respectively, compared with patients who resumed warfarin after 1 mo[107].
The bleeding risk of individual anticoagulants should be considered, when resuming anticoagulants in patients with high-risk GIB. Changing to apixaban, or reducing the dose of dabigatran to 110 mg b.i.d may reduce rebleeding in GIB patients taking warfarin, dabigatran (150 mg b.i.d) or rivaroxaban[111-115]. The HAS-BLED is a scoring system to evaluate bleeding risk among anticoagulants users[116]. However, the main outcome of the score is composite bleeding events, including intracerebral hemorrhage and GIB. One study focused specifically on the risk of acute GIB in anticoagulant users, and developed a new scoring model for acute GIB risk based on five factors (no PPI use, chronic kidney disease, chronic obstructive pulmonary disease, history of peptic ulcer disease, and liver cirrhosis). The c-statistic of the new score (0.65) was superior to that of the HAS-BLED score (0.57) for predicting acute GIB[117,118]. The utility of these scoring systems for predicting re-bleeding, and the strategy of changing anticoagulants would be important topics of study.
CONCLUSION
This literature review has summarized evidence for the initial management of acute LGIB. Assessing various clinical factors, including comorbidities, medication use,presenting symptoms, vital signs, and laboratory data is useful for risk stratification of severe LGIB. Early timing of colonoscopy could improve identification of the bleeding source and the rate of endoscopic intervention. CE-CT before colonoscopy may support identification, particularly for patients who can be examined immediately after the last hematochezia. How to deal with antithrombotic agents after hemostasis should be carefully considered. Further investigations are required to predict the need for early colonoscopy and hemostatic intervention in patients with LGIB.
杂志排行
World Journal of Gastroenterology的其它文章
- Endoscopic foregut surgery and interventions: The future is now.The state-of-the-art and my personal journey
- Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma
- Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity?
- Endoscopic trans-esophageal submucosal tunneling surgery: A new therapeutic approach for diseases located around the aorta ventralis
- Autonomic functions and gastric motility in children with functional abdominal pain disorders
- Usefulness of urinary trypsinogen-2 and trypsinogen activation peptide in acute pancreatitis: A multicenter study in Japan
