Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity?
2019-01-16ShiruLiuWinsonCheung
Shiru L Liu, Winson Y Cheung
Abstract Colorectal cancer (CRC) is a prevalent disease and represents a major cause of morbidity and mortality in the developed world. Intensive post-treatment surveillance is routinely recommended by major expert groups for early stage (II and III) CRC survivors because previous meta-analyses showed a modest, but significant survival benefit. This practice has been recently challenged based on data emerging from several large phase III randomized trials that demonstrated a lack of survival benefit from intensive surveillance strategies. In addition,findings from cost-effectiveness analyses of such an approach are inconsistent.Data on real-world practice, specifically adherence to these follow-up guidelines,are also limited. The debate is especially controversial in resected stage IV patients where there are currently no clear guidelines for follow-up. In an era of personalized medicine, there may be a shift towards a more risk-adapted approach to better define the optimal follow-up strategy. In this article, we review the evidence and highlight the role of surveillance in CRC survivors.
Key words: Surveillance; Imaging; Endoscopy; Colorectal cancer; Follow-up
INTRODUCTION
Colorectal cancer (CRC) remains one of the most common cancers in Western countries, and ranks second in North America[1]. Over one million cases of CRC are diagnosed annually in the world[2]. It is also one of the most common causes of cancerrelated mortality. Approximately 80% of all CRC cases are diagnosed at an early stage at which point curative intent surgery is the standard of care. Despite improvements in surgical techniques and adjuvant treatments, including chemotherapy and radiation, approximately 40% of patients with localized disease will experience disease recurrence after completing potentially curative treatment. Half of these recurrences are locoregional, and 90% occur within the first 3 to 5 years of treatment[3,4]. Median survival following recurrence is estimated at one year, based on findings from the ACCENT database in 2008[5]. There have been improvements in survival over the last decade due to an increase in the rates of metastatic resections.For example, 5-year survival rates can be over 40% for select patients who undergo hepatectomy for liver-limited metastasis[6]. In addition, locoregional recurrences may also be considered for resection, with some being potentially cured.
The goal of postoperative surveillance in CRC is to identify potentially resectable recurrences because this may improve survival outcomes. Surveillance may also lead to the early identification and removal of precancerous polyps, thereby preventing secondary metachronous CRC[7]. Standard CRC postoperative surveillance guidelines have been published by various cancer societies and expert groups, and they are continuously being updated[8-10]. The majority of these guidelines incorporate a combination of clinic visits with history and physical examinations, carcinoembryonic antigen (CEA), computed tomography (CT) scans, and endoscopies at regular intervals. The frequency of these different modalities has been subject to debate.Recently, several studies showed that less intensive approaches to surveillance may not be inferior to more intensive strategies[11,12]. Conversely, there is also some degree of evidence from the literature that demonstrates improvements in survival with an intensive follow-up approach[13]. It is important to recognize that postoperative surveillance is not without clinical and financial risks, which are important to consider in the era of personalized medicine as well as within the context of healthcare systems that are facing increasing fiscal constraints. In this review, we describe the CRC surveillance guidelines published by major societies and highlight findings from several large clinical trials that question the value of intensive followup.
CURRENT SURVEILLANCE GUIDELINES
Several recommendations regarding post-treatment surveillance for resected CRC have been published and endorsed by professional societies. The most recent ones are shown in Table 1. In general, a history and physical examination along with a CEA measurement is recommended every 3-6 mo for 5 years, a CT scan of the chest/abdomen/pelvis is recommended every 6-12 mo for 3 to 5 years, and a colonoscopy is recommended at 1 and 3 years. Subsequent endoscopies are guided by findings in the initial colonoscopy. For example, if the first colonoscopy is normal, a repeat endoscopy would not be needed until 5 years later. The frequency of these investigations appears to be the major difference across guidelines. For CEA and clinic visits, for instance, a 3-6 mo frequency is recommended for 5 years by American society of clinical oncology (ASCO), whereas European society for medical oncology(ESMO) only recommends this for the first 3 years followed by a frequency of every 6-12 mo for the last 2 years. Similarly, for CT scans, while national comprehensive cancer network (NCCN) recommends CT imaging every 6-12 mo for up to 5 years,ASCO recommends CT imaging only annually for 3 to 5 years[7,14-17].
For surveillance colonoscopy, however, the recommendations are more variable and largely guided by pathologic findings. In 2016, the United States Multi-SocietyTask Force (USMSTF) published updated recommendations on the role of surveillance endoscopies after resected CRC[7]. Compared to the previous set of recommendations published in 2006[18], the frequency of colonoscopies after surgical resection has not changed, with the need for a colonoscopy within the first year after surgery, followed by a repeat procedure in three years if negative, and another repeat procedure in five years (or nine years after initial resection) if negative. However, if polyps are found, endoscopies would be more frequently performed, as per polypectomy surveillance guidelines. This more intensive approach is based on evidence and is cost-effective[19].
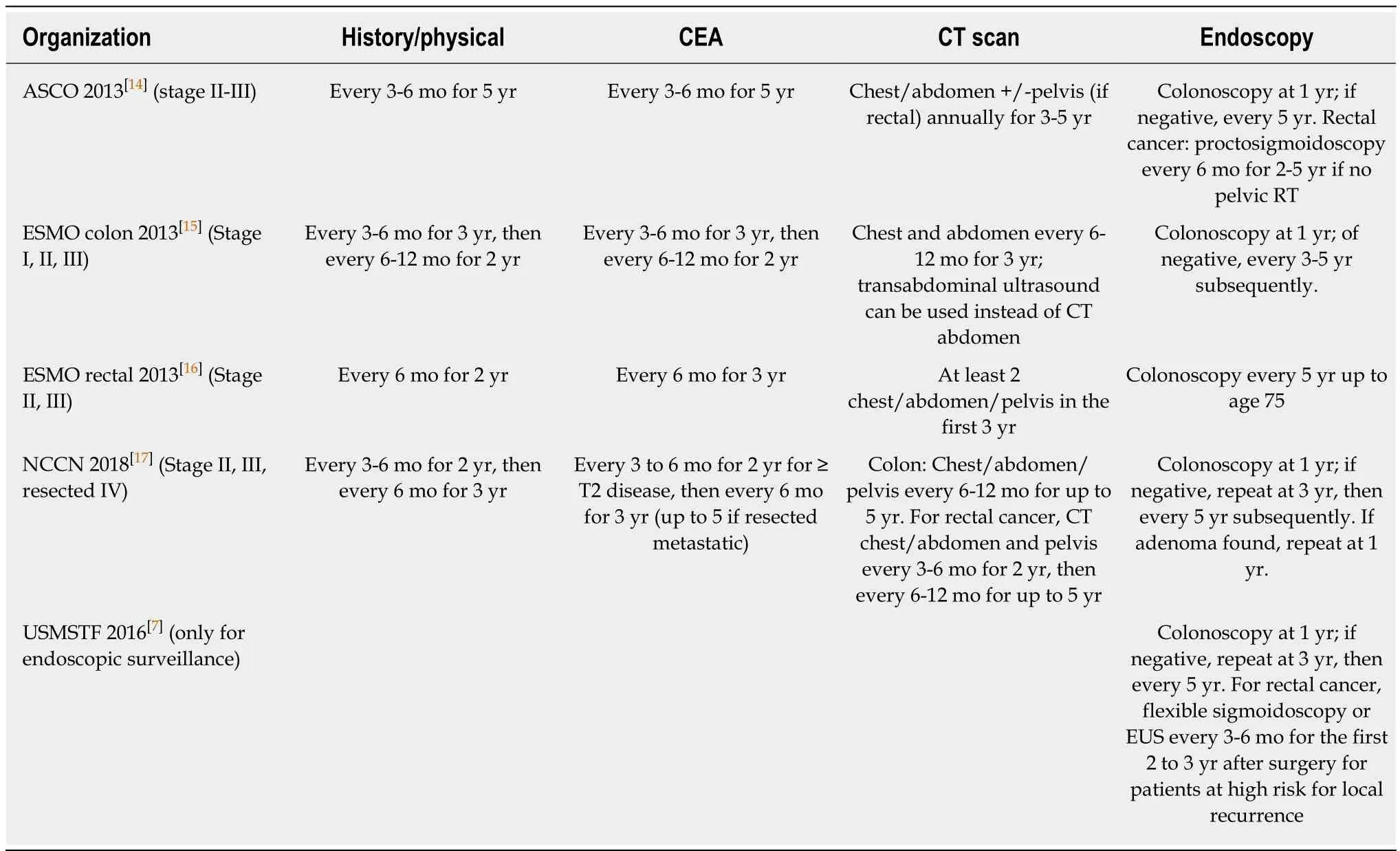
Table 1 Summary of postoperative surveillance recommendations for colorectal cancer by different professional societies
What’s new in the literature?
Over the past two decades, several literature reviews suggested a survival benefit from intensive surveillance strategies. A recent systematic review published by Pita-Fernandez et al[13]evaluated 11 studies (n = 4055 patients) and showed a modest but significant improvement in overall survival (HR: 0.75, 95%CI: 0.66-0.86), a higher probability of detection of asymptomatic recurrences (RR: 2.59, 95%CI: 1.66-4.06), a higher rate of curative surgeries attempted at recurrences (RR: 1.98, 95%CI: 1.51-2.60),and better overall survival after recurrences (RR: 2.59, 95%CI: 1.24-3.69) that favored the use of intensive follow-up strategies. However, there was no significant difference in cancer-specific survival when compared to less intensive strategies. Prior to this,two additional meta-analyses by Tjandra et al[20]and Jeffery et al[21]from 2007 showed similar results regarding intensive surveillance, with an improvement in overall survival (HR: 0.74, 95%CI: 0.59-0.93 and HR: 0.73, 95%CI: 0.59-0.91), but no improvement in cancer-specific survival. Taken together, this body of evidence appears to support the notion that earlier detection of asymptomatic recurrences may improve survival, and likely contributed to many of the recommendations adopted by professional guidelines.
Since that time, new randomized trials and prospective studies have not consistently offered support towards the findings from these prior meta-analyses. The most recently completed studies are shown in Table 2. In the COLOFOL study published in JAMA in 2018[11], investigators examined 2509 patients with stage II or III CRC treated at 24 centers in Sweden, Denmark, and Uruguay from 2006 to 2010.Patients were followed until 2015. Specifically, patients were randomized to either follow-up testing with CT and CEA every 6 mo after surgery for 3 years (high frequency group) or follow-up testing with CT and CEA at 12 mo and 36 mo after surgery (low frequency group). At the end of the follow-up period, they found no statistically significant differences in 5-year overall mortality (risk difference 1.1%,95%CI: -1.6% to 3.8%, P = 0.43), 5-year CRC-specific mortality (risk difference 0.8%,95%CI: -1.7% to 3.3%, P = 0.52), and CRC-specific recurrence (risk difference 2.2%,95%CI -1.0% to 5.4%, P = 0.15) between the high frequency and low frequency groups.Likewise, another randomized trial performed by the GILDA group in Italy published updated results in 2016[22]. Authors randomized 1228 patients with resected Duke B2-C CRC from 1998 to 2006 to either intensive or minimal surveillance. They found no statistically significant difference in overall survival between the two strategies and no difference in health-related quality of life scores. Finally, the FACS trial published in 2014 evaluated the effect of 3 to 5 years of scheduled CEA and CT follow-up in detecting CRC recurrences[23]. A total of 1202 patients from the United Kingdom participated between 2003 and 2009. These subjects had undergone curative surgery for primary CRC and they were subsequently assigned to 4 different follow-up groups: CEA only, CT only, CEA + CT, or minimal follow-up where patients received follow-up only if symptoms occurred. The results showed that the use of imaging or CEA measurements resulted in an increased rate of curative resection at the time of recurrence when compared to minimal follow-up. However, there was no additional benefit seen by combining CEA and CT (adjusted OR: 3.1, 95%CI: 1.1-8.71).Furthermore, the number of deaths was not significantly different between the group that underwent CEA and CT and the group that underwent minimal, symptom-based follow-up (difference 2.3%, 95%CT: -2.6% to 7.1%). These three large clinical trials showed highly consistent results. Collectively, they seem to indicate that intensive surveillance strategies are not associated with a survival advantage. There is one ongoing phase III trial called the PRODIGE 13 study, which is based in France. In this particular trial, investigators randomized 1750 patients with stage II or III resected CRC to an intensive group consisting of clinic visits, CEA measurements,colonoscopies, and CT imaging studies, or to a control group consisting of only abdominal ultrasounds and chest X-rays. Results from this study is anticipated in 2021 and may provide further clarity regarding the role of intensive follow-up[24].
An updated systematic review published in 2016 appears to corroborate the findings from the recent clinical trials and seems to suggest that there is no overall survival benefit for intensive post-operative follow-up[25]. This systematic review represents a second update from the Cochrane Collaboration Group, which contrasts the first one published in 2007 that demonstrated a survival advantage[21]. With 5403 participants enrolled in 15 studies, a statistically significant advantage with intensive follow-up was not detected for overall survival (HR: 0.90, 95%CI: 0.78-1.02, high quality evidence), cancer-specific survival (HR: 0.93, 95%CI: 0.78-1.12, moderate quality evidence), or relapse-free survival (HR: 1.03, 95%CI: 1.53-2.56, high quality evidence). Harms from colonoscopies also did not differ with intensive follow-up (RR:2.08, 95%CI: 0.11-40.17). Finally, a large retrospective cohort study of patient data from the National Cancer Database was published recently in 2018[12]. With a random sample of 8529 patients with resected stage I, II, or III CRC from 1175 facilities who underwent follow-up, there was no significant association between imaging and CEA surveillance intensity and detection of cancer recurrence.
Continuing controversy
There is increasing evidence that intensive surveillance strategies, whether they pertain to the type and number of tests or their frequency interval, are not associated with improved cancer survival. However, most guidelines still recommend relatively intensive approaches. Because many of the endpoints in the clinical trials that examined surveillance were different and the control groups for comparison were also not consistent, consensus regarding the best approach has been difficult to reach.From an endoscopy perspective, there appears to be relatively good quality evidence to support colonoscopy at 1 year after surgery, which is then followed by the same procedure at 3- and 5-years if findings are benign. This is currently recommended by the updated USMSTF. While there is no cancer specific survival advantage demonstrated in any of the studies, the standard use of post-operative endoscopic surveillance is endorsed by all major societies. Evidence from cost-effectiveness data also supports this practice. Similarly, data from the most recent meta-analysis did not reveal a significant harm from such an approach.
The use of CEA and CT imaging is more controversial. From a CEA perspective,there have been a number of studies evaluating its utility as both a screening and a surveillance test. Due to its low sensitivity and specificity[26], CEA is not viewed as a useful screening tool. However, it has a more established role in informing prognosis and disease burden. For example, an elevated preoperative CEA should normalize after surgery such that a persistently high level following resection may represent thepresence of residual disease[27]. Based on a pooled analysis in a Cochrane review[26], an elevation in postoperative CEA was associated with a high probability of disease recurrence, but a normal postoperative CEA was associated with a high false negative rate since this alone is not always useful for excluding disease recurrence. In fact, 30 to 40% of all CRC recurrences do not have an accompanying elevation in tumor markers,such as CEA[28]. There are also no clear data that confirm a consistent survival benefit with the use of CEA testing[29], and its cost-effectiveness continues to be unclear. Of interest, one meta-analysis[20]showed that CEA testing was the only investigation that was associated with a higher probability of detecting asymptomatic recurrences. In addition, a cost-analysis from the Eastern Collaborative Oncology Group database showed that CEA represented the most cost-effective method for detecting potentially curable recurrences[30]. For CT imaging, there is also evidence to support its value in detecting asymptomatic distant recurrences which may still be resected curatively[31,32].CT scans are particularly helpful given the high false negative rate of CEA assays along with the inability of endoscopies to detect asymptomatic distant recurrences.However, the optimal frequency of CT imaging is not well established. With the most recent clinical trials, we have new evidence that the frequent use of CEA and CT does
not seem to be superior to less frequent use (COLOFOL), and that the combination of CEA and CT does not seem to be superior to either alone (FACS). These recent findings have not yet been incorporated into the expert guidelines from ASCO,ESMO, or NCCN.
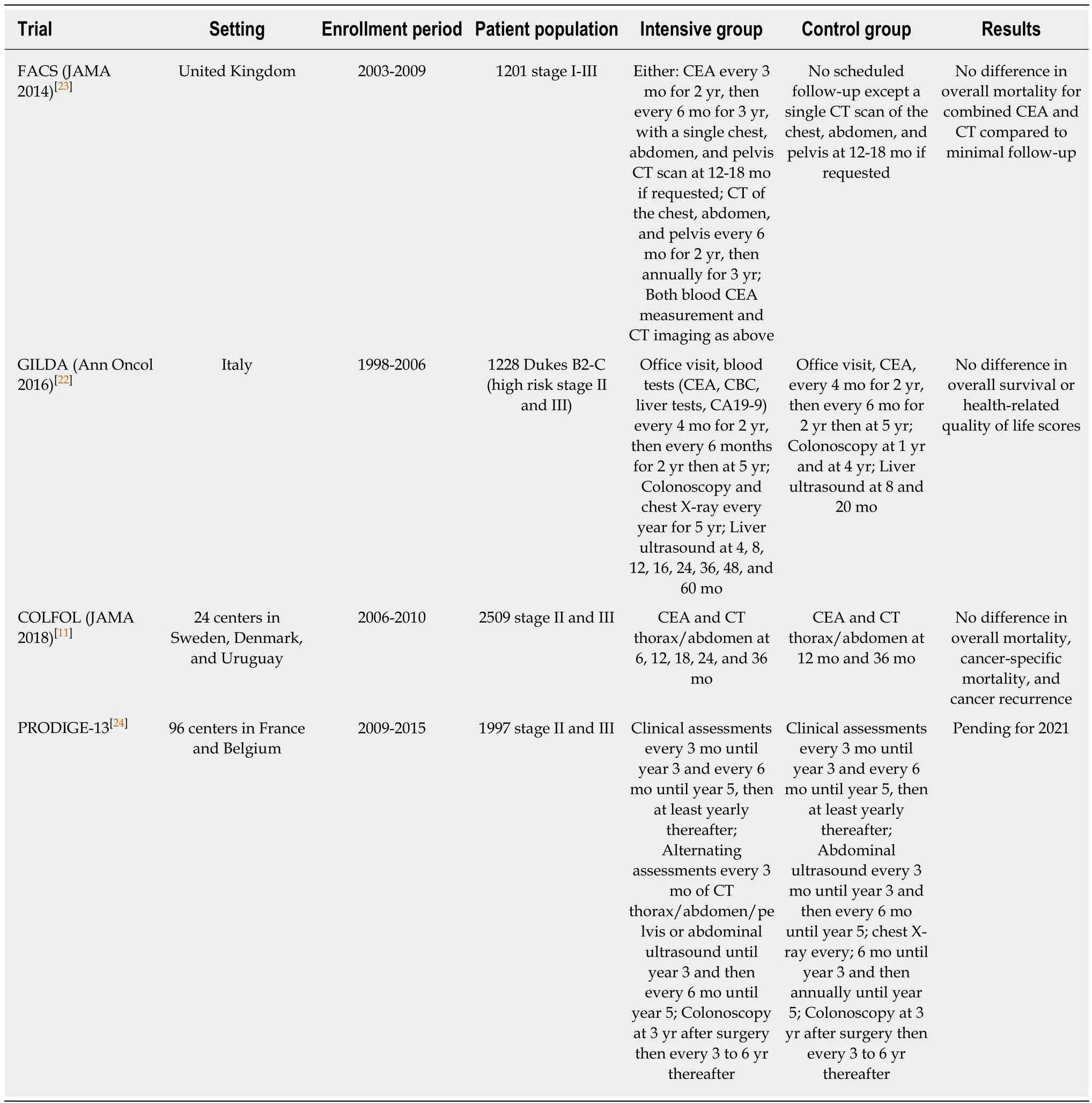
Table 2 Summary of recent randomized control trials evaluating intensive vs less intensive surveillance strategies
Very few studies have evaluated the cost-effectiveness of different post-operative surveillance strategies. One study used decision analysis to assess the costeffectiveness of surveillance colonoscopy at 1 year after cancer resection[19]. This study compared a 1-year endoscopic surveillance strategy with a “no early” endoscopy approach. They found the incremental cost-effectiveness ratio to be $40313 per lifeyear gained. The number needed to treat to detect one CRC and to prevent one CRC-related death was 143 and 926, respectively. They concluded that conducting a colonoscopy at 1 year following CRC resection is cost-effective and clinically effective for both cancer detection and cancer-specific death prevention. An older study from 2004 published in the British Medical Journal[33]compared cost-effectiveness of intensive vs conventional follow-up after curative resection for CRC; this also concluded that intensive follow-up using CT and CEA was economically justified based on an adjusted cost of life saved of $5884 USD. A more recent study published in Cancer in 2016[34]analyzed cost-effectiveness of the USMSTF guideline regarding colonoscopy surveillance postoperatively. The results showed that the US guideline is not cost-effective, with an incremental cost-effectiveness ratio as high as $140000 per life year gained. Given the paucity and inconsistency of data, along with variability in the cost impact under different health care systems, more research is warranted to explore the benefits, harms, and economic implications of different practices.
Risks with surveillance
Importantly, any extra investigations pose associated risks. Although rare, endoscopy is associated with the potential for bleeding and perforation, and the risk increases with more frequent use[35]. In addition, lack of a proper bowel cleaning regimen prior to endoscopy may result in an inadequate procedure that would lead to repeat testing. As with most procedures, the use of local anesthetics may also be associated with side effects. For CEA assays, the levels may be falsely elevated in the context of cigarette smoking[36]and adjuvant 5-FU treatment[37], which can lead to unnecessary imaging and anxiety. Finally, routine CT imaging is associated with radiation exposure and a small but real risk of second malignancies, which is of particular concern in younger individuals undergoing surveillance. As such, alternative imaging modalities such as chest X-rays and liver ultrasound may be employed, although the evidence to support the use of these modalities is poor, and none of the prior metaanalyses addressed thoracic imaging specifically.
Real-world practice
Few population-based studies have evaluated real-world practice patterns with respect to CRC follow-up. One Canadian population-based study evaluated adherence to guidelines on CRC surveillance and outcomes for patients enrolled in an innovative and intensive follow-up program at the Cross Cancer Institute in Edmonton. With 408 patients, the investigators found 14%, 33%, and 24% nonadherent rates to annual CT imaging, colonoscopy, and CEA testing. Less than half had complete adherence to all 3 components. The recurrence rate after a median of 1.6 years was 17%, most of which were diagnosed via surveillance, and almost half were considered potentially resectable[38]. Another Canadian study assessed adherence to ASCO CRC surveillance guidelines and compared patterns between a community and an academic cancer center. The authors observed significant inconsistencies between practices, with an academic institution using more intensive surveillance strategies,consisting of more frequent imaging studies, than the community cancer center. There were no significant differences in the use of CEA monitoring and surveillance colonoscopies. Of note, the researchers also found that surveillance was associated with a higher proportion of resectable tumor recurrences[39]. In contrast, another large population-based cohort study from the National Cancer Database compared high vs low intensity imaging or CEA testing for CRC surveillance and detected no significant association between intensity of surveillance and survival outcomes[12].
Special considerations
While the guidelines for surveillance apply to most survivors of CRC, there are specific populations that are not explicitly addressed. Older patients, for example,form a significant proportion of the survivorship population, but the existing guidelines do not specifically indicate the age at which continued surveillance is unlikely to provide meaningful benefit. The updated 2016 USMSTF states that“postoperative colonoscopic surveillance in CRC patients is indicated long term, or until the benefit is outweighed by decreased life expectancy due to age and/or competing comorbidity”[7]. Very few studies have evaluated outcomes of surveillance in the elderly population. One retrospective study evaluated 4834 elderly patients over the age of 75 years who were undergoing surveillance and found that the incidence of CRC among older adults was significantly lower than in younger individuals (0.24 vs 3.61 per 1000 person-years), and that advanced age was an independent factor associated with post-endoscopic hospitalization after adjusting for other factors (adjusted OR: 2.54, 95%CI: 2.06-3.14)[40]. It should be noted that these current guidelines also do not apply to patients with hereditary syndromes, such as Lynch syndrome, as these patients need more frequent endoscopic screening and surveillance, as per USMSTF consensus guidelines for Lynch syndrome patients[41].
Further, the expert guidelines do not consistently consider stage I and resected stage IV patients. There are significant variations in their recommendations due to a lack of robust data. Because over 95% of stage I patients are cured with surgery alone,adjuvant chemotherapy is not recommended, nor is intensive surveillance. The only exception is that postoperative colonoscopy is endorsed at the same frequency and interval as for stage II and III patients. Data from the COST trial[42]suggest, however,that stage I patients likely benefit equally from postoperative surveillance. The authors analyzed over 500 patients with stage I, II, and III resected colon cancer undergoing surveillance with CEA every 3-6 mo, chest X-ray every 6-12 months, and colonoscopy as per USMSTF guidelines. The investigators noted higher recurrence rates at 5 years with more advanced stages of disease, but there were similar salvage rates and sites of recurrences across all stages. Thus, they concluded that implementation of similar surveillance guidelines for all early stages of resected colon cancer patients is appropriate. This has not been routinely endorsed by expert guidelines from ASCO and NCCN. However, ESMO consensus guidelines on surveillance of early stage colon cancer includes stages I to III[15], while the same guidelines for rectal cancer are not clear whether these recommendations would apply to stage I patients[16].
Resected stage IV patients face similar uncertainty. There are no data for surveillance in this population, and decisions are often individualized based on patient factors and institutional practices. The rate of curative metastatic resections is increasing[43,44]. For liver limited metastasis, surgical resection is associated with the highest likelihood of cure, with 5-year survival rates of over 40%[45]. There is also medical advancement in many other domains, such as stereotactic radiotherapy,which can provide an alternative option for achieving potential cure in the setting of metastatic disease. Because of this, many have adopted the standard surveillance strategies for early stage CRC in otherwise fit stage IV patients who may be candidates for further curative treatments. Currently, the NCCN recommends routine surveillance for resected stage IV patients, including CEA every 3-6 mo for 2 years then every 6 mo for 3 years, CT of chest/abdomen/pelvis every 3-6 mo for 2 years then every 3-6 mo for up to 5 years, and colonoscopy at 1 year and then every 5 years subsequently, if normal[17]. In one study that evaluated outcomes of intensive surveillance after resection of hepatic metastases, 5-year survival rates were significantly higher in patients managed with hepatic resection compared to those managed palliatively[46]. In addition, intensive surveillance with 3-monthly CT for the first two years along with CEA at each clinic visit resulted in a relatively high rate of early detection of recurrences (444/705 patients). The authors also analyzed cost per life-year gained with this intensive strategy and found this to be reasonable within the British health care system. Therefore, they concluded that intensive 3-monthly surveillance CT after hepatic resection is reasonable, cost-effective, and can detect a considerable number of recurrent patients to improve outcomes.
Future directions
There is a heterogeneous group of patients that may benefit from CRC surveillance.Given this scenario, there is significant interest in a more risk-adapted surveillance strategy, where follow-up investigations and intervals are tailored based on the individual’s risk profile for cancer recurrence. This risk would be based on pathological and molecular biomarkers. In an era of personalized medicine, such tools are increasingly needed, but few studies have evaluated risk-adapted surveillance strategies in CRC. An older study randomized patients to either a risk-adapted follow-up protocol or a minimal follow-up schedule based on their risk status (high vs low), which was predefined prior to randomization[47]. The research group observed significantly improved 5-year overall survival for the risk-adapted follow-up protocol group regardless of risk status. However, the choice and definition of the risk factors were not well validated. Other similar studies in this area have largely evaluated prognostic and predictive biomarkers for survival outcomes and responses to chemotherapy. With a better understanding of CRC through these molecular studies,we may eventually be able to implement these into a standardized recurrence risk calculator where we can guide personalized planning of post-treatment surveillance.Such tools already exist to assist with decision regarding adjuvant chemotherapy for stage II patients, such as the Oncotype DX, but similar tools to guide surveillance is lacking.
Another emerging instrument in the field of oncology is the use of circulating tumor DNA (ctDNA) to detect the presence of tumor cells in a more reliable and less invasive way. ctDNA is a portion of tumor DNA that is shed into the patient’s bloodstream, which can be detected via blood analysis without imaging or biopsy.Many studies evaluating ctDNA have found it to be a sensitive test for assessing disease recurrence, often times much earlier than standard testing[48]. One study evaluated ctDNA postoperatively in 27 CRC patients who underwent surgery.Remarkably, the investigators detected ctDNA to be present in all 14 patients who relapsed but absent in the other patients. In addition, ctDNA detected recurrences much earlier than either CEA or CT scan[49]. Unfortunately, these data have not been consistently replicated. At present, the routine use of ctDNA for monitoring disease recurrence should not be widely implemented[50].
CONCLUSION
In summary, the current state of surveillance for resected stage II and III CRC is controversial. Although standard guidelines from professional societies recommend relatively intensive strategies for disease monitoring, new data suggest that less intensive approaches may not be inferior. With the emergence of precision medicine and a better understanding of CRC, the future of surveillance may be moving towards a more risk-adapted, personalized approach that accounts for both patient and disease factors, as well as cost. More research is needed to clarify the role of surveillance for the growing population of resected stage IV patients who have undergone successful metastatic resections.
杂志排行
World Journal of Gastroenterology的其它文章
- Endoscopic foregut surgery and interventions: The future is now.The state-of-the-art and my personal journey
- Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma
- Initial management for acute lower gastrointestinal bleeding
- Endoscopic trans-esophageal submucosal tunneling surgery: A new therapeutic approach for diseases located around the aorta ventralis
- Autonomic functions and gastric motility in children with functional abdominal pain disorders
- Usefulness of urinary trypsinogen-2 and trypsinogen activation peptide in acute pancreatitis: A multicenter study in Japan
