Molecular bases of Sorcin-dependent resistance to chemotherapeutic agents
2018-12-19IlariaGenoveseAndreaIlariTheoBattistaValerioChiariniFrancescoFaziAnnaritaFiorilloGianniColotti
Ilaria Genovese, Andrea Ilari, Theo Battista, Valerio Chiarini, Francesco Fazi, Annarita Fiorillo,Gianni Colotti
1Department of Radiology, Oncology and Pathology, Sapienza University of Rome, Rome 00161, Italy.
2IBPM-CNR, Institute of Molecular Biology and Pathology, Italian National Research Council, Rome 00185, Italy.
3Department of Biochemical Sciences, Sapienza University of Rome, Rome 00185, Italy.
4Institute of Biotechnology, University of Helsinki, Helsinki 00014, Finland.
5Department of Anatomical, Histological, Forensic Medicine & Orthopedic Sciences, Sapienza University of Rome, laboratory affiliated to Istituto Pasteur Italia-Fondazione Cenci Bolognetti, Rome 00161, Italy.
Abstract Soluble resistance-related calcium binding protein (Sorcin) is a protein initially labelled “resistance-related”, since it is co-amplified with ABCB1 in multidrug (MD)-resistant cells. While for years Sorcin overproduction was believed to be a by-product of the co-amplification of its gene with the P-glycoprotein gene, many recent studies view Sorcin as an oncoprotein, playing an important role in MD resistance (MDR). Sorcin is one of the most highly expressed calciumbinding proteins, which is overexpressed in many human tumors and MD resistant cancers, and represents a novel MDR marker. Sorcin expression in tumors inversely correlates with patients’ response to chemotherapies and overall prognosis. Sorcin is highly expressed in MDR cell lines over their parent cells. Sorcin overexpression by gene transfection increases drug resistance to a variety of chemotherapeutic drugs in many cancer lines. On the other hand, Sorcin silencing leads to reversal of drug resistance in many cell lines. This review describes: (1) the roles of Sorcin in the cell;(2) the studies showing Sorcin overexpression in tumors and cancer cells; (3) the studies showing the effects of Sorcin overexpression and silencing; (4) the molecular effects of Sorcin overexpression; and (5) the structural and genetic bases of Sorcin-dependent MDR.
Keywords: Sorcin, calcium, cancer, multidrug resistance in cancer, doxorubicin, endoplasmic reticulum, heart, brain,ryanodine receptors, ER stress
SORCIN STRUCTURE AND ACTIVATION
Soluble resistance-related calcium binding protein (Sorcin) was named “resistance-related” since it was found to be co-amplified with ABCB1 in multidrug (MD)-resistant cells[1]. The gene coding for Sorcin (SRI)is located in the chromosome region 7q21 and is about 21.9 kb-long. At least four alternative Sorcin isoforms are transcribed, i.e., isoforms A (a transcript of 15 kb with eight exons and seven introns, translated into a 198-residues long, 22-kDa Sorcin), B, C and D, translated into shorter 19-kDa isoforms, where part of the N-terminal domain and/or of the last aminoacids of the C-terminal domain are missing. Most literature refers to isoform A, although a few studies deal about 19-kDa form of Sorcin. The pseudogene Sorcin-like(SRIL) is in the chromosomal region 4q12[2].
Sorcin is present in vertebrates, and more generally in metazoans. Its sequence is highly conserved among species: protein sequences of mouse and human Sorcin show only eight differences, concentrated in the second half of the protein, and six of them regard possibly phosphorylatable serine and threonine residues,indicating that species-specific phosphorylation-dependent regulation of Sorcin may occur.
Usually calcium-binding proteins contain an even number of EF-hands, paired both structurally and functionally. Sorcin belongs to the small penta-EF-hand (PEF) family, containing an odd (5) number of EF-hands. Sorcin is a homodimer, although heterodimerization with another PEF protein, grancalcin, has been observed[3,4]. Sorcin monomers are formed by two domains, i.e., a rather short, fl exible, glycine- and prolinerich N-terminal domain and a C-terminal domain Sorcin calcium-binding domain (SCBD), globular and composed by eight alpha-helices, which formfive EF-hands (EF1-5) [Figure 1].
EF-hands couple through short β-sheets: EF1 pairs with EF2; EF3 pairs with EF4; the odd EF5 pairs with another EF5, belonging to the other monomer, forming part of the dimeric interface. The dimer containsfive EF pairs and can be considered the structural unit of Sorcin. The long and rigid D- and G-helices connect different EF-hands pairs, with the D-helix belonging to both EF2 and EF3, and the G-helix connecting EF4 and EF5. The SCBD can be divided in two subdomains: the EF1-3 subdomain (residues 33-134) is composed by the three EF-hands that bind calcium with high affinity, while the EF4-5 subdomain (residues 135-198)mediates dimerization, contains many potential phosphorylation sites, but does not bind Ca2+with high affinity. Calcium binding to EF hands determines the transition from a “closed” structure to an “open”structure[5,6]. Binding of calcium to EF3, the highest-affinity calcium-binding motif, EF2 and EF1 sites activate Sorcin: Ca2+binding to EF3 alters the conformation of the loop containing Glu124, and this change is transmitted to EF2 via a movement of the long D-helix connecting EF3 with EF2. Overall, calcium binding to the EF1-3 hands promotes a large conformational change in Sorcin structure, involving a movement of the long D-helix and the opening of EF1, with the two subdomains moving away of 21° [Figure 1]. This movement determines the exposure to solvent of hydrophobic residues of the D-helix, of the EF loop and of the G-helix, with a consequent dramatic decrease of solubility, thus allowing Sorcin to translocate from cytosol to membranes, and to bind and regulate a series of target proteins[6-9]. The hydrophobic pocket can accommodate in Ca2+-bound Sorcin a portion of the N-terminal domain displaying the consensus binding motif identified by phage display experiments [Figure 2].
THE ROLES OF SORCIN IN THE CELL: SORCIN LOCALIZATION, CELL CYCLE, AND FUNCTIONS IN THE CELL
Sorcin is one of the most expressed human calcium binding proteins; it is expressed in most human tissues,at high levels in bone, heart, brain, B- and T-lymphocytes, monocytes, kidney, breast and skin (sources:MOPED, PaxDb and MaxQB databases)[2]. In addition, Sorcin is overexpressed in many cancer types,and especially in MD-resistant cells (see below). Cell localization of Sorcin is dynamic. During interphase Sorcin is localized in the nucleus, in the cytosol, in the plasma membranes, at the endoplasmic reticulum(ER) and in ER-derived vesicles along the microtubules, containing ryanodine receptors (RyRs), ER Ca2+ATPase (SERCA), calreticulin and Rab10[10]. The shorter 19-kDa Sorcin variant was found localized at the mitochondrion[11]. During mitosis, Sorcin localizes in the cleavage furrow during late telophase, and at the midbody before cytokinesis[10].
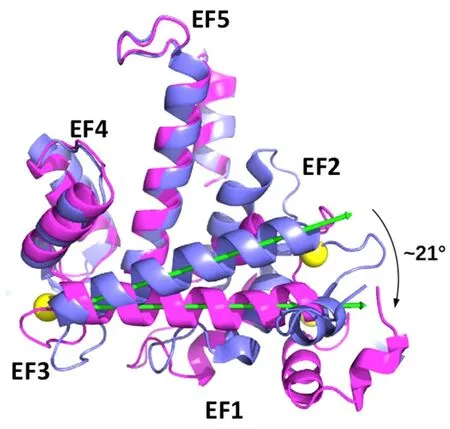
Figure 1. X-ray crystal structure of human sorcin in the apo (blue) and calcium-bound (magenta) forms[5]

Figure 2. A: In human sorcin, upon calcium binding, the conformational change determines exposure of hydrophobic residues, that are able to bind ligands; B: in the X-ray crystal structure of sorcin, part of the hydrophobic N-terminal domain (brown) results bound to residues of the D-helix
In the cytosol, Ca2+concentration is maintained below 100 nm since on one side it is actively pumped from the cytosol to the extracellular space, into ER and into mitochondria, and on the other many proteins bind Ca2+, contributing to calcium buffering[12]. Sorcin participates in the regulation of Ca2+homeostasis by many mechanisms. Sorcin is able to bind Ca2+in the micromolar range, thereby participating in cation buffering.More importantly, Ca2+-bound Sorcin can interact with RyR and ER Ca2+ATPase (SERCA), located in the ER,with the L-type calcium channel Cav1 and Na+-Ca2+exchangers (NCX), located in the plasma membrane,and regulates them: Sorcin increases ER Ca2+storage by activating SERCA and by inhibiting RyR [Figure 3],increases size and Ca2+load of ER-derived vesicles, and increases mitochondrial Ca2+concentration[7,8,10,11,13-19].

Figure 3. Sorcin inhibits the ryanodine receptor and up-regulates ER Ca2+ ATPase (SERCA) and Na+-Ca2+ exchangers (NCX), increasing Ca2+ load of the endoplasmic reticulum (ER) (and possibly of mitochondria) and decreasing ER stress
High Sorcin expression increases ER Ca2+concentration, can prevent ER stress and the unfolded protein response, and increases escape from apoptosis[10]. Sorcin is necessary for normal glucose tolerance and protects against lipotoxicity in vivo, is able to induce ATP-evoked Ca2+release from intracellular stores and induces glucose-stimulated insulin secretion[20,21]. Sorcin represses the glucose-6-phosphatase catalytic subunit-2 promoter activity through NFAT activation, activates ATF6 transcriptional activity while repressing ER stress markers as CHOP and Grp78/BiP[20]. Conversely, Sorcin silencing activates apoptotic proteases as caspase-3 and caspase-12, Grp78/BiP, Bcl-2, Bax, c-jun, c-fos and release of cytochrome c, results in major mitosis and cytokinesis defects, blocks cell cycle progression in mitosis, increases the formation of rounded, polynuclear cells and induces apoptosis[10,18,22]. Sorcin increases basal and caffeine-stimulated mitochondrial Ca2+concentration[18]; the folding and expression of the mitochondrial 19-kDa Sorcin isoform is regulated by TRAP1[11].
Sorcin contains several potential phosphorylation sites, and also interacts in a Ca2+-dependent fashion with many serine-threonine kinases, which participate in the regulation of mitosis progression, such as Akt2,Csnk2A1, Csnk2A2, polo-like kinase 1 (Plk1), Aurora A and Aurora B. Sorcin is phosphorylated by Plk1,induces Plk1 autophosphorylation, and participates in Plk1 regulation[10]; Ca2+-calmodulin dependent kinase II (CaMKII) and cAMP-dependent protein kinase (PKA) phosphorylate Sorcin, thereby regulating Sorcin binding to RyRs and SERCA and Ca2+homeostasis[6,23].
Sorcin has been identified in other types of vesicles, other than the ER-dependent ones. Sorcin is present in nanovesicles released in a Ca2+-dependent fashion from the erythrocytes and containing Annexin A7[24]; Sorcin was found to interact with Annexins A7 and A11[7,8,10,15,25], Ca2+-dependent phospholipid-binding proteins, the latter being required for midbody formation and completion of the terminal phase of cytokinesis[26].
Sorcin has been identified in many types of exosomes from different sources, such as B-cells, mesenchymal stem cells, human urine, platelets and from many types of tumor cells, as colorectal cancer, ovarian cancer,prostate cancer cells, squamous carcinoma and neuroblastoma[27-35].
The microRNA miR-1 targets Sorcin specifically and non-redundantly; miR-1 decreases Sorcin levels,while the antagomiR-1 increases Sorcin expression[36]; treatment with miR-1 determines Ca2+signaling dysregulation, especially at cardiac level.
Sorcin contributes to regulate Ca2+homeostasis and cardiac excitation-contraction-relaxation processes.Excitation-contraction-relaxation is a fast (800 ms) process, started by the electrical excitation of cardiomyocytes by a wave of depolarization that opens voltage-dependent Na+channels located in the T-tubules, determining a rapid membrane depolarization and Ca2+in fl ux via voltage-operated Ca2+channels(mainly Cav1 L-type channels). Cav1 channels (with four subunits Cav1.1, Cav1.2, Cav1.3, Cav1.4) are juxtaposed to ryanodine receptors (RyRs), i.e., sarcoplasmic reticulum (SR) or ER Ca2+release channels.Ca2+entry via Cav1 increases Ca2+concentration near RyRs, triggering Ca2+release from the SR. This fl ux further raises the free intracellular Ca2+concentration [Ca2+]Iof the cardiomyocyte: Ca2+binds to troponin C and triggers contraction. For relaxation to take place, RyR is closed and Ca2+is pumped out of the cytosol,mainly via the SR Ca2+-ATPase (SERCA), which pumps Ca2+back into the SR, and the sarcolemmal (and mitochondrial) Na+/Ca2+exchanger (NCX)[37]: [Ca2+]idecreases rapidly and Ca2+dissociation from the myofilaments can occur.
Sorcin is able to interact with all the channels, exchangers and pumps responsible for Ca2+fl uxes during the excitation-contraction-relaxation process, and to regulate them. Sorcin modulates Cav1, by interacting with its Cav1.2 (CACNA1C) subunit with its C-terminal domain, slowing its Ca2+-dependent inactivation and stimulating voltage-dependent inactivation of Cav1-dependent Ca2+currents[13,14].
Sorcin also Ca2+-dependently interacts with RyR2, the most expressed cardiac RyR, and strongly inhibits it, thereby reducing Ca2+eラux from SR/ER by decreasing RyR mean open time and frequency of open event[8,15,17]. Sorcin is also able to interact with SERCA and to activate it[16], and to activate NCX via Ca2+-dependent interaction of the respective C-terminal, calcium binding domains; Sorcin overexpression in cardiomyocytes has also been associated with increased NCX activity[19,38]. Fast rates of association and of dissociation between Sorcin and NCX allows NCX regulation on a “beat to beat” basis.
Overall, Sorcin regulates the excitation-contraction-relaxation processes in the heart and muscle: Sorcin terminates the excitation-contraction processes, by inhibiting Ca2+-induced Ca2+release by the SR, helps[Ca2+] decreasing and induces relaxation by inhibiting RyR2-dependent calcium release from the SR, by activating SERCA2-dependent calcium pumping from cytosol to the SR and by increasing NCX-dependent Ca2+eラux through the sarcolemma (and possibly into the mitochondrion) [Figure 3].
Sorcin phosphorylation alters its Ca2+-dependent activation, its translocation to the SR and the ability to regulate RyR2 activity. Sorcin is hyperphosphorylated in the failing heart; this increases Sorcin translocation to the SR membrane and the SR Ca2+content and possibly results in the reduction of SR stress, in the reduction of basal [Ca2+]iand in improvement of cardiac relaxation[16,23,39].
The mutation F112L, located in the EF3 hand and associated with a familiar form of hypertrophic cardiomyopathy and hypertension, as other mutations in the EF3 and/or in the D-helix of Sorcin decreases the capacity of Sorcin to interact with RyR and to modulate SR Ca2+release, and determines complex cardiac alterations[15,40]. In failing heart, levels of SERCA2a and of RyR2 are often decreased, and consequent altered cytosolic Ca2+transients lead to abnormal contraction. Sorcin overexpression in mice increases cardiac contractility in the normal heart and determines a rescue of the abnormal contractile function of the diabetic heart, possibly due to improved Ca2+transients[40-43], while Sorcin KO mice present ventricular arrhythmia and sudden death when challenged by acute stress[44].
Sorcin has a very high expression level in the brain, 5-10 times higher than in the heart. Sorcin is one of the most expressed Ca2+binding proteins in the prefrontal cortex, in the amygdala, in the hypothalamus and in many brain tumors (GeneAtlas). The extent of Sorcin expression and its ability to regulate Ca2+homeostasis make Sorcin a potentially important protein in brain function and dysfunction. According to the so-called“Calcium Hypothesis”, deregulation of Ca2+-mediated signaling, perturbed ER Ca2+homeostasis and ER stress are at the basis of the abnormal accumulation and aggregation of specific proteins, which are deposited in intracellular inclusions or extracellular aggregates during brain aging and in many neurodegenerative diseases, as Alzheimer's disease (AD) and Parkinson’s disease (PD)[45]. PD is characterized by progressively distributed Lewy pathology and neurodegeneration, linked to the degeneration of dopaminergic neurons in the substantia nigra pars compacta. Ca2+entry through Cav1 channels, mitochondrial oxidant stress and PD pathogenesis are strictly linked[46]. Alterations in RyR expression and function are observed in cells bearing familial mutations in the genes of the β-amyloid precursor protein (βAPP) and of presenilins (the catalytic core of γ-secretase complexes that cleave the βAPP to generate amyloid β-peptides), in the brain of transgenic AD mice models and in AD-affected human brains. RyR participates in the control of βAPP processing and of Aβ peptide production: RyR alteration or mysfunction alters neuronal death, synaptic function and memory and learning abilities[47], and its regulation by Sorcin can be important in maintaining ER Ca2+load in ER and possibly decreasing ER stress and unfolded protein production in the brain. Further,Sorcin is able to directly interact as a function of Ca2+concentration (in vitro, in cultured cells and in human brain) with alpha-synuclein (AS) and presenilin 2 (PS2), important in PD and AD pathogenesis,respectively[48,49]. Sorcin binds to the C-terminal part of PS2 that forms low-conductance Ca2+channels in planar lipid bilayers[50], interacts with RyR in a Ca2+-dependent fashion, and regulates Ca2+homeostasis[51].Sequestration of Sorcin by aberrant forms of tau compromises its function, impairing calcium homeostasis and cellular resistance by ER stress, and contributing to the progression of AD[52]. Sorcin is overexpressed in a PD cell model induced by 1-methyl-4-phenylpyridinium ion (MPP+) in SH-SY5Y cells[53], which is one of the most differentially expressed proteins in PD vs. normal human substantia nigra[54]and in PD vs. human brain samples[55], and is overexpressed in AD brain samples[56-58], in particular in sporadic AD[59]and in AD brains with severe cerebral amyloid angiopathy[60], as well as in frontal cortical tissues from postmortem cases of frontotemporal dementia[61]. Further, Sorcin is overexpressed in 7 different Huntington’s disease human and mice models, where it has been associated with the unfolded protein response[62]. Sorcin also interacts with the ionotropic glutamate receptor NMDAR1 Ca2+channel, important in several cascade pathways and in synaptic plasticity, in caudate-putamen nucleus[63], and with annexins A7 and A11, involved in Ca2+homeostasis in astrocytes[64].
SRI OVEREXPRESSION IN MD-RESISTANT CANCERS, SORCIN ROLES IN MD RESISTANCE
Sorcin was isolated in 1981 as a soluble protein expressed in hamster lung tumor cells resistant to vincristine,and named according to its main characteristics as soluble, resistance-related, calcium binding protein(Sorcin)[65]. Sorcin is overexpressed in different tumors, from many tissues, mostly with an ABCB1-dependent MD-resistant phenotype. The SRI gene resides in the same amplicon of ABCB1, in chromosome 7q21.12, and was identified as a resistance-related gene because often its gene is co-amplified with ABCB1 in MD-resistant cancer cells[1]. For two decades, Sorcin overexpression was believed to be an accidental by-product of this genomic co-amplification process[66]; in the last 15 years many studies have defined Sorcin as an oncoprotein,characterized both as a marker and a cause of MD resistance (MDR).
Sorcin is overexpressed in many human cancers, including adenocarcinoma, breast cancer, colorectal cancer,gastric cancer, lung cancer, nasopharyngeal cancer, hepatocellular carcinoma and ovarian cancer, lymphoma,leukemia and myeloma, and particularly in cancers with ABCB1-dependent MD-resistant phenotype[67-81].The level of expression of Sorcin is one of the main markers of poor outcome in embryonal tumors of central nervous system[82]; Sorcin is a histological marker for malignant glioma, and is overexpressed in anaplastic astrocytoma, glioblastoma and oligodendroglioma[83-86].
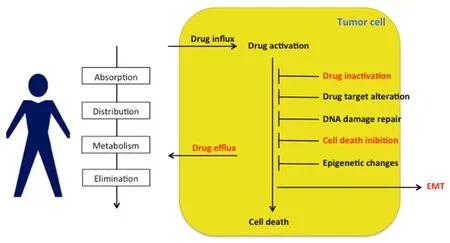
Figure 4. Multidrug-resistance (MDR) depends on a series of factors, including absorption, distribution, metabolism, elimination (ADME),drug influx and efflux, drug activation/inactivation, drug target alteration, DNA damage repair, cell death inhibition, epigenetic alteration and epithelial-to-mesenchymal transition (EMT). Sorcin contributions to the onset of a MD-resistant phenotype are indicated in red (see text)
Sorcin is an oncoprotein with multifaceted activity in both tumorigenesis and the onset of a MD-resistant phenotype [Figure 4]. Sorcin, by loading calcium in ER and mitochondria, prevents ER stress and possibly the unfolded protein response, and increases cell escape from apoptosis[10,11,78]: in MD-resistant tumor cells overexpressing Sorcin, the equilibrium between cell life and death is shifted towards proliferation.
In doxorubicin-resistant leukemia cell lines, Sorcin is overexpressed with respect to the drug-sensitive parental cell line. In leukemia patients, Sorcin expression level correlates with low-response to chemotherapies and poor prognosis; Sorcin overexpression (with gene transfection techniques) increases MDR to doxorubicin,vincristine, etoposide and homoharringtonine in leukemia K562 cells and in lung tumors, to doxorubicin,vincristine, paclitaxel and 5- fl uorouracil in SGC7901 cells, ovarian and breast cancer[22,69,72,74,76,87]. Conversely,Sorcin silencing reverses MDR in many tumor cell lines, as MD-resistant leukemia and Sorcin-transfected leukemia cells, breast cancer, HeLa, colorectal cancer and nasopharyngeal carcinoma cells[2,22,74-78,88-94]. In a lung cancer cell line, Sorcin silencing decreases ABCB1 protein levels and ABCB1 activity, thereby decreasing rhodamine123 eラux[87][Table 1].
Sorcin is also able to bind directly and with high affinity several chemotherapeutic drugs, as doxorubicin,paclitaxel, vinblastine and cisplatin; Sorcin acts as a cytosolic drug scavenger, thereby reducing doxorubicin nuclear uptake, and allowing an increase of drug resistance and of cell survival[87]. Sorcin binds doxorubicin with high affinity in a site at the EF5 hand, which is involved in Sorcin dimerization and does not bind calcium with high affinity [Figure 5], and changes cell localization upon doxorubicin treatment, and presumably upon drug binding[87].
Sorcin overexpression increases migration, invasion and metastasis in vitro, while Sorcin silencing inhibits the epithelial-to-mesenchymal (EMT) transition in a human breast cancer cell line, possibly via E-cadherin and VEGF expression, and reduces breast cancer metastasis[94]. Sorcin induces migration and invasion also in gastric cancer cells, while inhibition of Sorcin expression down-regulates the expression of markers of invasion, migration and proliferation as MMP2, MMP9, CTSZ and p-STAT3, followed by suppression of tumor growth and metastasis[95]. Sorcin interacts with STAT3 and increases its phosphorylation, thereby negatively regulating NF-κB signaling[96].

Figure 5. Sorcin is able to bind with high affinity doxorubicin and other drugs: the crystal structure of the sorcin-doxorubicin complex shows that the drug binds to the EF5 hand[87]

Table 1. Selected studies on the effects of sorcin overexpression and/or silencing in tumor cell lines
Sorcin expression is upregulated in hepatocellular carcinoma, with respect to adjacent non-tumor liver tissues and to normal liver tissues. Sorcin expression correlates with hepatocellular carcinoma metastasis:patients with high Sorcin expression have shorter survival and higher recurrence compared with patients with low Sorcin expression. Sorcin increases proliferation, migration, and invasion in vitro in hepatocellular carcinoma and colorectal cancer cell, and facilitates cancer growth, metastasization and EMT, via activation of ERK and/or PI3K/Akt signaling pathways[79,97]. Sorcin is also involved in the regulation of Ca2+-mediated angiogenesis, via the VEGF/PI3K/Akt pathway in endometrial cells and plays a crucial role in preparing the endometrium for implantation[98].
Sorcin induces the expression of ABCB1 via a cAMP response element (CRE), located between -716 and-709 base pairs upstream the ABCB1 gene: Sorcin overexpression increases CRE-binding protein (CREB)phosphorylation and its binding to the CRE site in the ABCB1 promoter induces ABCB1 expression[72].
In the mitochondrion, Sorcin interacts with TRAP1, a mitochondrial chaperone with antioxidant and antiapoptotic protein, upregulated in several human tumors. TRAP1 modulates apoptosis by exerting a quality control on the short (19-kDa) mitochondrial Sorcin isoform. TRAP1 silencing in colorectal carcinoma cells decreases mitochondrial Sorcin expression, and Sorcin silencing increases TRAP1 degradation, while overexpression of a TRAP1 mutant localized in the ER increases the expression of mitochondrial Sorcin and protects from paclitaxel-dependent apoptosis[11]. Sorcin overexpression increases ER and mitochondrial calcium levels, while Sorcin silencing activates caspase-3, caspase-12 and GRP78/BiP, increases mitotic defects, blocks cell cycle progression in G2/M, increases the number of rounded polynucleated cells and induces apoptosis and cell death[10,22,78]. Sorcin overexpression in leukemia cells increases expression of Bcl-2 and decreases Bax levels[22].
Sorcin is an interesting oncoprotein and MDR marker, expressed in many cancer cell types, and whose overexpression results in the MDR phenotype. Sorcin has an important role in calcium homeostasis, and is able to interact/regulate several targets and to contribute to the onset of a MD-resistant phenotype in different ways.
Sorcin has no enzymatic activity, and targeting its activity may prove difficult. The modulation of Sorcin expression and activity for overcoming tumorigenesis, cancer-related EMT and MDR is a possible target:small molecules as dihydromyricetin have been used to target Sorcin, and administration of extracts from plants as Tegillarca granosa extract Haishengsu is another strategy used[76,99-101]. Sorcin-specific miR-1 or antagomiR-1 are possibly the most specific ways to target Sorcin[36], together with approaches of Sorcin silencing or the use of Crispr-Cas9.
THE SRI GENE RESIDES IN THE ABCB1 AMPLICON: GENE COAMPLIFICATION AND PROTEIN COEXPRESSION IN MDR TUMORS
Sorcin was identified as “resistance-related”, because it’s encoded by a gene co-amplified with ABCB1, i.e.,the most important broad substrate specificity ATP-dependent eラux pump, able to pump xenobiotics (such as toxins or drugs) out of cells[65].
The human ABCB1 gene, in the chromosomal region 7q21.1[102], confers MDR when overexpressed or amplified[102-106]; increased ABCB1 expression upon treatment with chemotherapeutic drug has been largely reported in the last thirty years[107-115].
In many types of cancers, amplification of the chromosome 7q21 region containing genes of ABCB1 and Sorcin, has been reported in MD-resistant cell lines as neuroblastoma[116], lung cancer cells[117]and leukemia cells[118]. Often, genomic instability and chromosomal rearrangements result in genomic amplification, yielding an increase in the ABCB1 gene copy number and transactivation of ABCB1 overexpression[119-124].
Several genes surrounding ABCB1 have recently been identified as contributors to the MD-resistant phenotype when overexpressed or amplified together with ABCB1 or suppressed in resistance-induced cancer cell lines. Many studies describe a genomic amplification of chromosome 7q21.12 region, where ABCB1 and other MDR-related genes reside [Figure 6], in MD-resistant tumors; the MD-resistant phenotype depends at least in part on the amplification and/or overexpression of these genes[1,66,106,116-118,125-136]. The ABCB1 amplicon in the chromosomal region 7q21.12 is formed by the SRI, ADAM22, DBF4, SLC25A40,RUNDC3B (RPIP9), ABCB1, ABCB4, CROT, TP53TG1 lncRNA, TMEM243 (MGC4175) and DMTF1(DMP1) genes [Figure 6][137], that have been associated with tumorigenesis and MDR; DBF4 and Sorcin,among these genes, are possibly the most important contributors to the MD-resistant phenotype, together with the eラux pumps ABCB1 and ABCB4 (MDR3): DBF4 and Sorcin are both important markers of poor prognosis and drivers of MDR in several types of cancers, acting on different (although partially overlapping) mechanisms with respect to ABCB1[137].

Figure 6. The ABCB1 amplicon is formed by the genes SRI, ADAM22, DBF4, SLC25A40, RUNDC3B (RPIP9), ABCB1, ABCB4, CROT,TP53TG1 lncRNA, TMEM243 (MGC4175) and DMTF1 (DMP1)
In lung cancer cells with acquired paclitaxel resistance, the 7.q21.12 region is amplified, but ABCB1 expression is increased up to 1000-fold, showing a discrepancy between expression level and gene copy number[133]. A regional activation on chromosome 7q21.11-13, of about 22 co-expressed genes over an area of 8 Mb, was identified in taxane-induced MD-resistant ovarian tumor cells; the amplified region (with increase in genes copy number) included ABCB1, MGC4175 (TMEM243), DMTF1, CROT, ABCB1, ABCB4,ADAM22, RUNDC3B, DBF4 and SRI[138]. A gene copy number gain on chromosome 7q21.12, including the genes ABCB1, ABCB4, SRI, DMTF1, SLC25A40 and CROT, was observed in taxane-resistant breast tumor cell lines[129]. On the other hand, MD-resistant ovarian tumor cells containing a translocation generating fused ABCB1 and SLC25A40 genes, determining the overexpression of ABCB1, were found only in MD-resistant cancers, while no evidence of this event occurs in drug-sensitive cancer cells[131]. Deletions in the ABCB1 genes locus in breast cancer patients determine a 2-8-fold decreased expression of these MDR locus-related genes; cancer patients harboring these deletions display a better response to neoadjuvant chemotherapy[130].
In addition to genomic rearrangements and presence of high copy, other mechanisms may contribute to increased gene expression, such as transcriptional upregulation, mRNA stabilization, post-transcriptional regulation and epigenetic modifications. Non-coding RNAs, such as miRNAs and long non-coding RNAs,can possibly exert post-transcriptionally regulate oncogene functions on cancer cells, resulting in metastatic or drug-resistant phenotypes: the long non-coding RNA TP53TG1, present in the chromosomal region 7.q21.12, is down-regulated in A549 cisplatin-resistant lung cancer cells[139]; miR-1, specifically targeting Sorcin[36], is frequently downregulated in various types of cancer, such as lung cancer, colon cancer,genitourinary cancer, head and neck tumor, thyroid cancer and sarcoma; low miR-1 levels have been shown to be associated with chemosensitivity[140,141].
CONCLUSION
Sorcin has an important role as a modulator of cellular calcium homeostasis, acting on Ca2+channels,exchangers and pumps, thereby regulating ER and mitochondrial Ca2+levels, reducing ER stress and mitochondrial dysfunction, and protecting the cell from apoptosis.
Sorcin is highly expressed in the heart and in the brain, it is overexpressed in cancers and other pathological conditions, and plays important roles in the onset of cancer, cardiac diseases and possibly neurodegenerative diseases.
Sorcin is overexpressed in MD-resistant cancer cells, co-amplified with ABCB1, ABCB4 and other proteins involved in the resistance to chemotherapy in cancer cells. Sorcin overexpression induces resistance to many different chemotherapeutic drugs, while Sorcin silencing reverts drug resistance, by acting on many pathways.
Sorcin is therefore an important MDR marker and may represent a therapeutic target for reversing MDR.
DECLARATIONS
Acknowledgments
We acknowledge “Quality methods for Design of Experiments in Scientific Research”, in the FaReBio di Qualità Project: Quality and Project Management OpenLab: qPMO CNR; the Flagship Project Nanomax:“NADINE: Nanotechnology-based Diagnostics In Neurological diseases and Experimental oncology”; PRIN 20154JRJPP MIUR; Progetto Ricerca Finalizzata Min. Salute RF-2016-02364123 RAREST-JHD.
Authors’ contributions
Conception of the work: Ilari A, Colotti G
Draft and revision of the work: Genovese I, Ilari A, Battista T, Chiarini V, Fazi F, Fiorillo A, Colotti G
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no con fl icts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
杂志排行
Cancer Drug Resistance的其它文章
- Linking tyrosine kinase inhibitor-mediated inflammation with normal epithelial cell homeostasis and tumor therapeutic responses
- Glutamine metabolism in cancer therapy
- Hitting a moving target: inhibition of the nuclear export receptor XPO1/CRM1 as a therapeutic approach in cancer
- Sphingolipid metabolism and drug resistance in ovarian cancer