CHRM3基因与孤独症谱系障碍*
2018-12-10巨兴达
巨兴达 宋 伟 徐 婧
基因与孤独症谱系障碍*
巨兴达1宋 伟1徐 婧2
(1东北师范大学心理学院, 长春 130024) (2长春中医药大学临床医学院, 长春 130117)
孤独症谱系障碍是一类具有遗传基础的儿童发展障碍疾病。近些年, 研究者们从分子病理学层面发现中枢胆碱能神经系统异常与孤独症患者认知和行为异常存在相关性。尸检研究、临床案例、动物模型研究均发现毒蕈碱型(M型)乙酰胆碱受体异常和孤独症的发生有着密切的关系。在以小鼠为模型的行为学研究中, 编码毒蕈碱型乙酰胆碱受体Ⅲ亚型的基因突变会导致小鼠出现认知障碍、刻板行为等孤独症样表现。深入了解基因的功能将能够帮助研究者进一步解释孤独症的相关行为特征, 为孤独症儿童教育方案的制定提供新的思路和方法。
孤独症谱系障碍;基因; 临床特征; 动物模型
1 引言
孤独症谱系障碍(Autism Spectrum Disorders, ASD), 简称孤独症, 是一种发病于婴幼儿时期的、常见的社会性发展障碍, 与大脑的神经化学机制异常有着密切的关系。美国精神疾病手册第五版(Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-V)指出孤独症患者的核心症状表现为:持续性的社会交流和社会互动能力缺失, 以及兴趣狭窄和重复刻板的行为方式。美国疾病控制与预防中心(Christensen et al., 2012)最新调查结果显示, 儿童孤独症患病率已达14.4‰, 即每68名8岁以下儿童中就有一名孤独症患儿, 与2000年相比, 患病比率增长了2.18倍。因此, 探究孤独症的发病原因已经成为医学、生物学界的重要议题之一。
生物遗传学研究表明, 大约10%~30%孤独症发病是由基因异常导致的(Huguet, Ey, & Bourgeron, 2013; Gaugler et al., 2014; Sanders et al., 2015), 即基因异常影响了其编码的蛋白质的结构和功能, 进而改变了脑的特定功能, 最终表现为患者的认知和行为异常。双生子研究也证明遗传因素在孤独症发病中起着非常重要的作用, 同卵双生的孤独症共患率大约为77%~95%, 显著高于异卵双生子31% (Ronald, Happe, & Plomin, 2005; Taniai, Nishiyama, Miyachi, Imaeda, & Sumi, 2008; Rosenberg et al., 2009)。家族聚集性研究显示, 同胞患孤独症的几率为10%~20%, 大约是家庭中出现新生孤独症概率的20倍(Ozonoff et al., 2011; Wood et al., 2015), 据此推测父母某一方患孤独症其子代患病风险大概为10%~15%, 且男婴患病率高于女婴(Vorstman et al., 2017)。根据同卵双生、异卵双生共患的差异以及患者同胞再患的危险度推断, 孤独症的遗传几率可达91%~93% (Bailey et al., 1995)。借助基因二代测序技术, 已发现多个染色体区域上的拷贝数变异(Copy Number Variants, CNV)会增加孤独症患病风险。到目前为止, 有4%~20% 的孤独症患者携带疾病相关的CNV (Schaaf & Zoghbi, 2011; Pinto et al., 2014), 已发现的包含CNV的染色体片段达2223个, 遍及所有染色体。除此之外, 基因新生突变(de novo mutations)也被认为是孤独症发生的一个重要原因。SFARI (Simons Foundation Autism Research Initiative)目前已经收录了990个孤独症相关基因, 包括和等(Michaelson et al., 2012; Pinto et al., 2011; Roohi et al., 2008; Neale et al., 2012; Bernier et al., 2014)。其中部分已经实验验证为孤独症易感基因, 如基因突变影响神经元突触发育过程, 导致该基因缺失小鼠表现出多项典型的孤独症行为特征(Durand et al., 2007)。无义突变使转录过程提前终止, 导致编码产物缩短, 破坏了蛋白质原有功能, 影响神经元增殖、树突发育和突触形成过程, 被认为是导致孤独症发病的重要风险因素(Bernier et al., 2014; O’Roak, Vives, Fu, et al., 2012; Neale et al., 2012)。基因突变改变了对组蛋白H2A的化学修饰, 使得小鼠出现孤独症类似行为(Gao et al., 2014)。此类研究均证实了基因功能异常是孤独症发生的重要原因。
目前已发现的孤独症易感基因多与神经系统发育有关, 涉及神经细胞的运动与增殖、神经元的轴突投射、树突棘可塑性、突触形成和维持等, 与核染色质重组、基因转录调控、酶的活性调控、细胞骨架调控、蛋白化学修饰等过程密切相关(Pinto et al., 2010; Sanders et al., 2012; Sakai et al., 2011; O’Roak, Vives, Fu, et al., 2012; King et al., 2013; Donato, Chowdhury, Lahr, & Caroni, 2015), 所涉及的分子信号通路包括Wnt信号通路(O’Roak, Vives, Girirajan, et al., 2012; Mine, Yuskaitis, King, Beurel, & Jope, 2010; Okerlund & Cheyette, 2011)、钙离子信号通路(Yun & Trommer, 2011; Moretti et al., 2006)、神经生长因子(nerve growth factor, NGF)信号通路(Riikonen & Vanhala, 1999; Nelson et al., 2001)、以及G蛋白偶联受体(G Protein-Coupled Receptor, GPCR)信号通路等(Zhang & Alger, 2010; Maccarrone et al., 2010; Chen et al., 2011; Silverman et al., 2012)。由此可见, 基因异常影响了关键的神经细胞信号转导, 因此被视作孤独症发生的高风险因素之一。近年来以基因为靶点开展孤独症研究已成为了相关领域研究者关注的重点。
长期以来, 人们对孤独症的认识多是从异常行为入手。有学者指出, 孤独症患者个体之间存在巨大差异, 且不同基因突变可导致不同孤独症行为特征(Happe, Ronald, & Plomin, 2006), 一些针对刻板行为和交流障碍的研究已证实了该现象(Cuccaro et al., 2003; Buxbaum et al., 2001)。所以将基因功能和行为研究联系起来, 不但能揭示孤独症发病机制, 更能促进孤独症治疗和康复(State & Sestan, 2012)。
2 毒蕈碱型乙酰胆碱受体Ⅲ亚型(cholinergic receptor, muscarinic 3, CHRM3)
作为一种神经递质, 乙酰胆碱(acetylcholine, ACh)在信号传递中扮演着重要角色, 可调节神经系统发育和神经元兴奋性变化。胆碱能神经元广泛分布于全脑, 涉及学习记忆、认知调节、情绪控制以及社会交往等过程(Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003; Dani & Bertrand, 2007; Karva & Kimchi, 2014), 胆碱能信号通路异常与多种精神类疾病的发生有关(Bowen, Smith, White, & Davison, 1976; Whitehouse et al., 1982; Deng, & Reiner., 2016)。动物模型研究发现胆碱能相关基因突变会导致小鼠出现孤独症症状(Zhang et al., 2016), 基因功能异常影响脑内胆碱能信号通路的信号传递以及胆碱能相关因子的表达水平, 进而引发孤独症。同时, 还有研究发现孤独症患者脑内灰质和颞叶脑区胆碱能信号通路异常(Perry et al., 2001; Lee et al., 2002; Martin-Ruiz et al., 2004; Ray et al., 2005; Friedman et al., 2006; Deutsch, Urbano, Neumann, Burket, & Katz, 2010; Petersen et al., 2013), 药物学研究中利用VPA (valproic acid)大鼠模型发现, 给孕期大鼠注射VPA能够导致大鼠及其子代的胆碱能神经系统紊乱, 增加患孤独症的风险, 而使用ACh酯酶抑制剂药物对缓解其出现的社交障碍、认知障碍和重复刻板行为问题十分有效(Kim er al., 2014)。目前美国食品药物管理局(Food and Drug Administration, FDA)已批准使用ACh酯酶抑制剂缓解孤独症症状(Dineley, Pandya, & Yakel, 2015), 因此, 胆碱能相关通路应在孤独症研究和治疗中受到更多关注, 检测其正常与否在未来也许可以成为研究、诊断和治疗孤独症或是区分孤独症不同亚型的一个重要参考指标。
毒蕈碱型乙酰胆碱受体Ⅲ亚型(cholinergic receptor, muscarinic 3, CHRM3)是介导ACh信号传递的受体之一, 是毒蕈碱型乙酰胆碱受体(muscarinic acetylcholine receptor, mAChR)家族一员, 广泛分布于前脑、海马以及下丘脑等区域, 在脑内神经信号传导和行为调节中具有重要作用(Levey, Edmunds, Heilman, Desmond, & Frey, 1994)。CHRM3属于G蛋白偶联受体, 是一种大量分布在神经系统中的突触后膜促代谢型受体。在正常生理状况下, CHRM3接收到乙酰胆碱信号刺激后通过Gq蛋白激活磷脂酶C (PLC, phospholipase C), 进而作用于第二信使二酰甘油(DAG, diacylglycerol)和三磷酸肌醇(IP3, inositol 1, 4, 5-triphosphate), 调控细胞的增殖、代谢、细胞骨架和突触可塑性(Matsui et al., 1999)。由于CHRM3分布广泛, 对个体高级神经活动的发生有着关键性的作用, 因此基因突变会对神经系统生长发育产生重要的影响, 可能导致癫痫(Koeleman, 2018)、精神分裂症(Devor et al., 2017)、阿尔茨海默症(Tsang et al., 2008)等多种神经系统疾病。近年来, 越来越多的研究者开始关注GPCRs以及Gq-PLC信号通路异常与孤独症的关系(Chen et al., 2011; Silverman et al., 2012; O'Connor, Bariselli, & Bellone, 2014)。遗传学研究证实, 位于Gq-PLC信号通路下游的基因是孤独症易感基因(Spinelli, Black, Berg, Eickholt, & Leslie, 2015; Cupolillo et al., 2015)。药物研究发现给孤独症模型小鼠BTBR T~(+)tf/J注射mGlu5R拮抗剂对于改善小鼠的刻板行为和社交行为有明显的效果(Silverman et al., 2012)。值得注意的是, mGlu5R与CHRM3同为G蛋白偶联受体, 均通过与Gq蛋白偶联激活PLC。这一系列研究暗示CHRM3及Gq-PLC信号通路可能对孤独症发生发展有重要影响。
临床报道与基因检测结果均表明基因所在的1q43染色体区域缺陷与孤独症相关(见表1, Perrone et al., 2012; Petersen et al., 2013; Soueid et al., 2016)。该基因突变患者会表现出不同程度的行为异常、认知障碍、言语障碍以及运动发育迟缓等问题(Silipigni et al., 2017; Luukkonen et al., 2017)。Gai等人在(2012)年通过单核苷酸多态性微阵列(SNP microarray)技术对1224名孤独症患者的染色体进行分析, 结果显示有患者的编码区内存在CNV (Gai et al., 2012)。此外, 利用全基因组关联分析等方法, 多项研究都提出基因可能是孤独症易感基因(Hussman et al., 2011; De Rubeis et al., 2014; Butler, Rafi, & Manzardo, 2015; Ch'ng, Kwok, Rogic, & Pavlidis, 2015; Li et al., 2017), 从统计学角度证实了基因突变会提高孤独症患病风险。同时研究者在动物模型研究中也发现, 抑制或过度激活CHRM3都将会导致小鼠出现不同程度的孤独症样异常行为(Alexander et al., 2009; Wang & McGinty, 1997; Amodeo, Sweeney, & Ragozzino, 2014)。上述结果说明基因与孤独症发生之间存在密切联系。
3 CHRM3基因异常的孤独症患者临床研究
近期已有两例与基因异常密切相关的典型孤独症病例被相继报道。
患者一:Perrone等人(2012)报道了一名7岁的意大利男性孤独症患者。该患者为非近亲生独子, 足月分娩出生。出生体重3.4 kg, 身高34 cm, 哺乳时吸入困难, 同时伴有运动功能发育迟缓(12月龄独坐, 4岁独走)、智力低下、隐睾、身体矮小, 生长发育迟缓以及孤独症行为等特征。查体显示枕骨周围有脱发斑点, 出现脱发迹象; 脚趾拇指和第五指先天性趾侧弯; 有内斜视和咬手的问题特征; 在喂养方面由于患者有咀嚼困难的问题, 因此只能吃混合食物。基因检测结果显示患者1号染色体丢失91172 bp, 为新生突变, 该缺失区域包含基因、基因、基因。其中,为假基因, 即在基因组上的非功能性基因组DNA拷贝, 一般情况下不被转录, 没有明确的生理意义。基因和基因均与中枢神经系统发育有关, 是潜在的致病基因。患者MRI (Magnetic Resonance Imaging)、心电图和腹部超声检查正常。

表1 孤独症家系研究中的CHRM3突变

图1 CHRM3信号传导模式图。CHRM3可能通过“Gq-PLC-第二信使”信号通路调控神经细胞的增殖、运动、分化、突起生长和兴奋性
患者二:Petersen等人(2013)报道的是一名3岁7个月的男性患者, 患者系G3P1A1 (怀孕3次; 分娩1次; 流产1次)母亲足月生胎儿, 出生体重3.3 kg。4个月时常规查体和MRI检查发现患者表现出斜视和颅神经麻痹的症状, 12个月左右被发现语言发育迟缓, 3岁7个月时经ADOS (Autism Diagnostic Observation Schedule)诊断为孤独症。患者表现出多动、易怒、注意力差、自伤行为倾向、对触觉/视觉刺激异常敏感、行为刻板、社交能力严重受损等行为缺陷。基因检测结果显示患者1号染色体丢失473 kb, 为新生突变, 丢失区域内只含有基因。此外, 患者母亲报告在产前曾出现子痫前期的症状。
将两名基因缺失的孤独症患者的临床表现进行对比, 发现患者均表现出认知功能受损、发育迟缓、进食困难的特征(表2)。此外, 在目前报道的其他基因缺失的临床案例中, 患者还出现了癫痫、中风、发育迟缓以及注意力缺陷等与神经系统功能异常有关的特征(Shimojima et al., 2012; Luukkonen et al., 2017)。
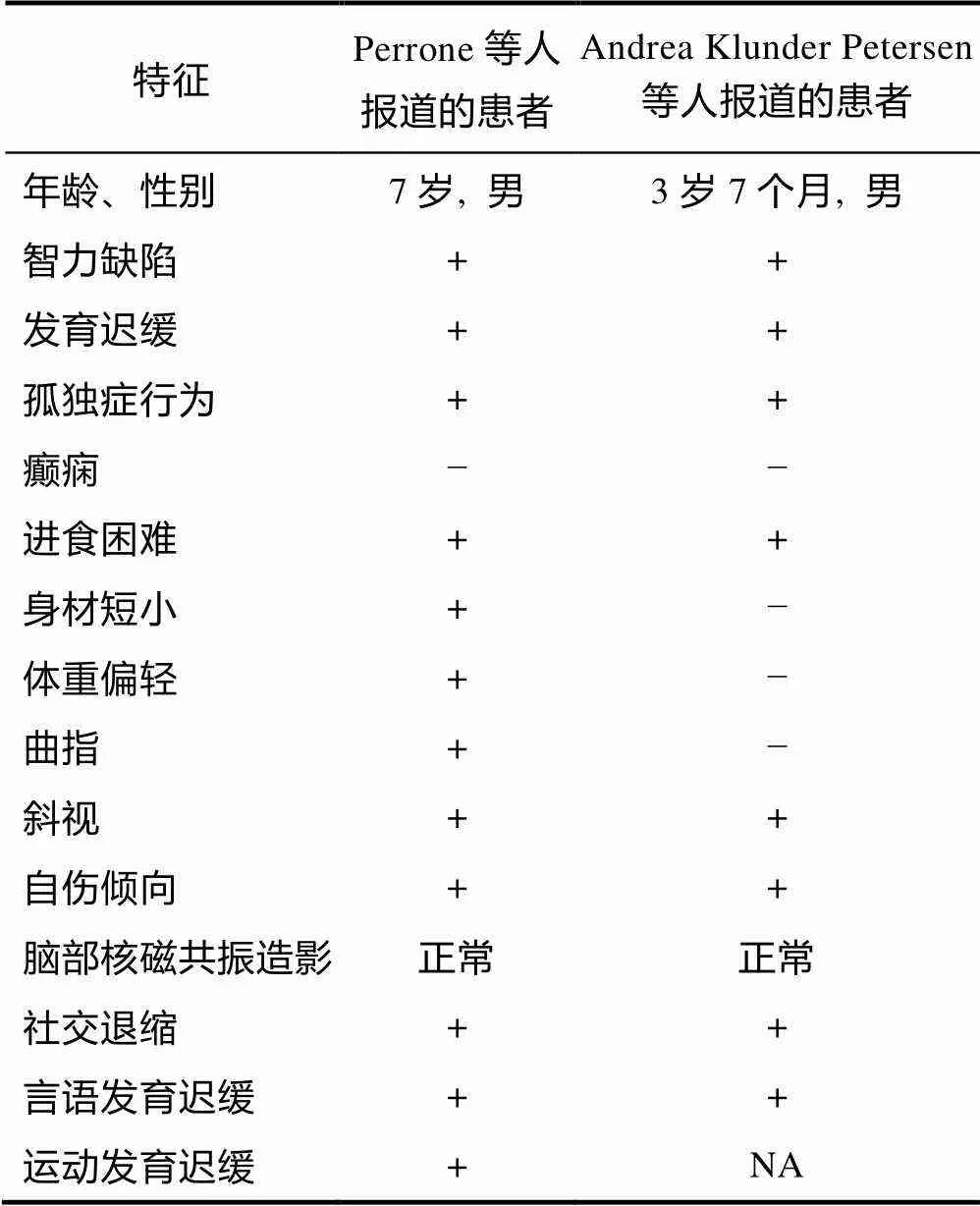
表2 两名CHRM3基因缺失的孤独症患者的临床表现对比
注:NA, date not available
4 CHRM3相关动物模型研究
4.1 CHRM3异常与孤独症刻板行为
重复刻板行为是孤独症诊断中的一项重要标准。在《精神疾病诊断与统计手册第五版》(DSM-V)中, 刻板行为被定义为:一种重复性、限制性的行为、兴趣或活动。其主要表现为自我刺激行为, 如尖叫、转圈等和自伤行为, 还包括一些仪式性、规则性的行为, 具体表现为每天在固定的时间完成某项任务, 或者固定地以某种方式进行某项活动等。刻板行为会严重影响患者的正常生活, 对患者的社交和学习造成阻碍。
Petersen等人(2013)报道的基因异常的患者表现出刻板行为:经常抓自己的头发、用头撞墙, 只吃固定的食物; 同时患者也出现咬手的自伤行为。因此孤独症患者的刻板行为可能与基因异常有关。在孤独症的动物模型研究中, 改变基因的功能不仅会影响孤独症小鼠的刻板行为, 还会影响正常小鼠是否会出现孤独症样行为特征。
BTBR T~(+)tf/J (简称BTBR)小鼠是一种近交系小鼠, 即不同个体间98%以上的基因座为纯和状态的小鼠品系, 因此具有稳定的基因型。该品系小鼠能在不同代子代中稳定地表现出社会交往交流障碍和重复刻板的行为、兴趣等孤独症样行为, 以及与孤独症患者类似的脑发育异常、免疫生化指标异常的问题特征(Yang et al., 2007; Bolivar, Walters, & Phoenix, 2007), 是一种良好的孤独症研究动物模型。研究发现BTBR小鼠脑内乙酰胆碱水平显著低于野生型小鼠(McTighe, Neal, Lin, Hughes, & Smith, 2013), 给小鼠注射M型受体激动剂氧化震颤素(Oxotremorine)可以显著减少小鼠的自我理毛和埋珠子等刻板行为(Amodeo et al., 2014)。另外在临床药理学研究中也曾发现, 当给孤独症患者使用拮抗M型乙酰胆碱受体的精神类药物后, 患者重复刻板问题行为显著增加(Martin, Koenig, Scahill, & Bregman, 1999; Hardan, Jou, & Handen, 2005)。但是以上有关研究只是发现改变M型受体的信号转导功能会影响孤独症的重复刻板行为出现, 并没有详细探究这种异常是否是由于CHRM3功能异常所致。
Alexander等人(2009)的研究证明, 改变CHRM3功能将会影响小鼠出现重复刻板的孤独症样行为。研究者使改造后的人(human M3 muscarinic DREADD receptor coupled to Gq, hM3Dq)基因在小鼠前脑中正常表达, 由于hM3Dq无法接受内源性乙酰胆碱的信号刺激, 因此注射叠氮平-N-氧化物(clozapine-N-oxide, CNO)可以诱导激活CHRM3下游信号通路, 起到过度激活CHRM3的效果。研究者发现当不给hM3Dq小鼠注射外源性配体CNO时, hM3Dq小鼠与野生型小鼠的各项行为指标均无显著差异。当给小鼠注射较高浓度CNO后, CHRM3被过度激活, hM3Dq小鼠的刻板行为显著增加, 多动行为增多且出现癫痫症状。上述研究不仅揭示了CHRM3功能与孤独症刻板行为间的关系, 也为孤独症患者的行为干预提出了新的思路和方法。
4.2 CHRM3异常与认知功能受损
认知功能受损并非孤独症诊断标准中的核心症状, 但是绝大多数孤独症患者都伴有不同程度的认知功能受损问题(Wing, 1981; Crane, Pring, Jukes, & Goddard, 2012)。美国疾病控制与预防中心(CDCP 2012)的调查结果显示42%~60%的孤独症患者表现出认知功能受损的特征, 具体体现为患者在基本概念认知、记忆力、注意力等方面的表现低于正常儿童。缺乏正常的认知能力导致孤独症儿童无法对图形符号或语言指令做出正确的识别、理解和应答, 且由于孤独症患者均存在不同程度的语言沟通困难, 进而也无法与老师或家长进行沟通, 患者的学习过程受到了极大的阻碍。因此提高孤独症患者的认知能力有利于提高患者的生活技能、适应人际交往活动。脑发育过程中在大脑皮层和海马等区域大量表达(Levey, Edmunds, Koliatsos, Wiley, & Heilman, 1995),意味着基因可能与认知功能有关。Perrone和Petersen等人报道的两例基因变异的孤独症患者也都出现了智力发育落后、注意力缺陷等认知功能受损的问题。
Poulin等人(2010)在研究中发现,基因敲除小鼠在恐惧性条件反射(fear conditioning)实验中依赖海马的环境联系性记忆能力均显著低于野生型小鼠。由于小鼠的痛觉和焦虑反应与野生型小鼠没有显著差异, 因此研究者推测小鼠表现出来的这种认知功能受损可能源于海马CHRM3功能异常。通过对基因突变小鼠的研究, Poulin等人认为突变小鼠的认知功能受损是由CHRM3不能正常磷酸化导致的。CHRM3受体磷酸化发生在第384号丝氨酸位点上, 当编码该位点氨基酸的基因突变后, CHRM3无法正常磷酸化, 影响了β-arrestin与CHRM3的结合过程, 导致受体内在化过程受阻, 最终阻断了神经信号通路的信号传递过程, 小鼠表现出认知能力受损的特征。为了进一步了解CHRM3如何影响小鼠的学习记忆能力, 研究者测定了小鼠海马神经元中基因的表达水平。在环境联系性学习过程中, 突触后神经元兴奋产生长时程增强(long term potentiation, LTP)激活基因。基因编码的磷酸蛋白可作为转录因子与DNA结合, 促进或抑制相关基因的表达, 从而把由外界刺激所诱发的短暂的细胞内信息与由基因改变所产生的突触可塑性过程偶联起来, 一旦再次接受该环境刺激时,基因的表达水平会迅速增加, 因此诱导mRNA的表达可能是形成长时记忆的必要条件(Beck & Fibiger, 1995; Tischmeyer, Kaczmarek, Strauss, Jork, & Matthies, 1990)。Poulin等人的结果显示突变小鼠海马和齿状回内基因表达水平显著低于野生型小鼠。Rosethorne、Nahorski和Challiss (2008)也曾发现CHRM3对表达起着调节作用:CHRM3可以促进CREB (cAMP response-element binding protein)磷酸化, 而CREB磷酸化能够诱导基因表达, 因此激活CHRM3可以提高的表达水平。值得注意的是, CREB在神经元发育、突触可塑性建立、学习记忆过程中起着重要的调节功能(Silva, Kogan, Frankland, & Kida, 1998; Lonze & Ginty, 2002; Carlezon, Duman, & Nestler, 2005)。综合以上研究推测,突变小鼠学习记忆能力较低的原因可能是由于学习记忆相关神经元内依赖Gq-PLC的钙离子信号通路信号传递受阻抑制了CREB磷酸化, 进而抑制了基因启动应对环境刺激反应的下游基因的表达, 因此无法激活与学习记忆相关神经元, 特定脑区功能受损, 最终表现为个体学习记忆能力较低, 无法在短时间内习得应对环境刺激的反应。除此之外, 在Karvat和Kimchi (2014)的研究中还发现, 向BTBR小鼠背内侧纹状体注射乙酰胆碱酯酶抑制剂后可以有效改善小鼠的学习能力缺陷的问题(Karvat & Kimchi, 2014)。由此可见, 在后续研究中可以通过向BTBR小鼠的海马或背内侧纹状体注射CHRM3特异性激动剂, 观察小鼠是否表现出学习记忆能力变化, 并测定表达量来进一步探究基因在孤独症患者认知活动中的作用。
当前关于认知功能机制的研究大多集中于边缘系统,突变的孤独症患者的认知功能受损主要被认为与海马功能异常有关, 但对此也有不同的观点, 有研究者认为CHRM3介导的信号传递过程可能是小脑浦肯野细胞突触形成的主要机制(Rinaldo & Hansel, 2013), 因此突变的孤独症患者的认知障碍或许是由小脑功能异常所致, 这还需要在今后的研究中进一步探讨。
4.3 CHRM3异常与孤独症生长发育迟缓
研究发现, 孤独症患者中出现生长发育迟缓问题的比例较高(Haglund & Kallen, 2010), 因此有学者提出生长发育迟缓可能是导致孤独症发生的中介因素之一(Haglund & Kallen, 2010)。在已报道的基因异常的临床案例中, 患者均出现了发育迟缓的症状。
动物模型研究发现敲除小鼠会出现体重减轻, 摄食量减少、血清内瘦素和胰岛素水平显著降低等一系列生长发育迟缓的特征(Yamada et al., 2001; Matsui et al., 2000; Meyer, Zhu, Miller, & Roghair, 2014), 这与Perrone等人(2012)和Shimojima等人(2012)报道的患者的临床表现相似。研究人员发现在野生型小鼠脑内, CHRM3主要分布在下丘脑, 而敲除小鼠下丘脑内CHRM3数量与野生型小鼠相比下降了近50%, 同时免疫组化研究显示小鼠下丘脑内黑色素聚集激素(melanin- concentrating hormone, MCH)的表达水平也显著低于野生型小鼠(Yamada et al., 2001)。已有研究证实MCH对于调控摄食和体重变化具有重要作用(Qu et al., 1996), 且CHRM3与MCH被证实在外侧下丘脑细胞内共表达, 因此Ymada等人推测在有关饮食调节的信号通路中, 瘦素和胰岛素作为上游的信号因子刺激下丘脑弓形核, 激活MCH细胞, 从而激活了下丘脑信号通路, 开启信号转导过程。在该信号通路下游的外侧下丘脑内, CHRM3通过控制MCH细胞分泌MCH从而调控个体的摄食行为, 即当外侧下丘脑内的MCH细胞接收到乙酰胆碱信号刺激后, CHRM3被激活, MCH释放量迅速提高, 个体出现摄食行为。因此在敲除小鼠体内, CHRM3缺失导致MCH细胞无法被激活释放MCH, 小鼠摄食量下降, 进而表现出体重减轻等发育迟缓的问题症状。
由于瘦素是激活下丘脑饮食调节信号通路的主要因子, 因此瘦素含量降低也会导致个体出现生长发育迟缓的症状(Meyer et al.,2014)。研究发现, 婴儿期瘦素缺失将导致发育迟缓的小鼠在成年期出现运动能力降低、社交兴趣丧失、认知能力受损、以及杏仁核体积增大等孤独症样的异常特征(Meyer et al., 2014)。因此婴儿期个体瘦素水平降低可能与孤独症的发生有关。结合在Ymada等人的研究中敲除小鼠血清内瘦素含量显著降低这一结果, 推测瘦素含量下降与基因表达水平降低有关, 早期营养不足可能是后期行为问题出现的原因之一, 即缺失会降低个体的摄食行为, 在一定程度上影响身体生长和脑的发育过程, 最终导致问题行为出现。
另外, 免疫组化研究证实小鼠唾液腺上2/3的M型受体为CHRM3受体, 说明CHRM3对于调控唾液分泌也具有重要作用(Matsui et al., 2000; Bymaster et al., 2003), 因此突变的生长发育迟缓小鼠出现进食障碍有可能是由于唾液分泌过程异常引起的食物消化功能受损所致。以上研究表明CHRM3与生长发育之间有着紧密的联系, 一方面CHRM3可以通过调节摄食行为来影响生长发育, 另一方面可以通过调节消化能力影响生长发育。
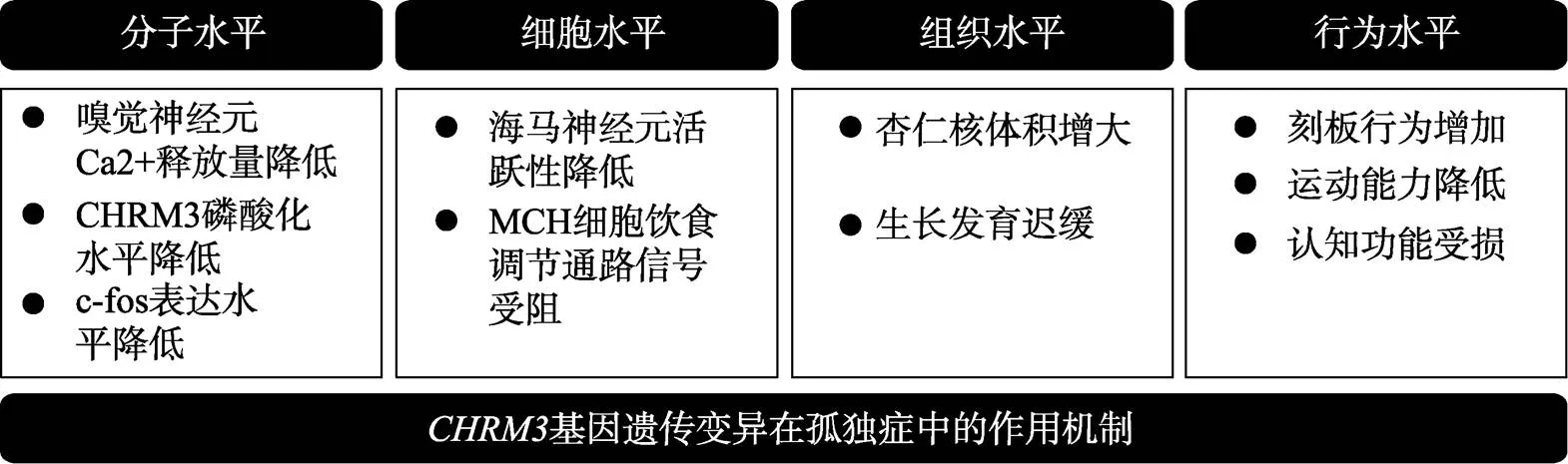
图2 CHRM3异常在脑与个体不同水平上的影响
5 总结与展望
作为G蛋白偶联受体家族一员, CHRM3介导Gq-PLC信号通路参与突触信号传递, 对于调控细胞增殖、代谢、细胞骨架建立和突触可塑性形成具有重要作用。由于突触依赖性的神经元信号传导是学习、记忆等高级心理活动的生理基础, 因此CHRM3可能与人的认知能力发展以及社会化等发育过程密切相关。
临床案例和动物模型研究均发现改变CHRM3功能会引发动物出现认知缺陷以及刻板行为等孤独症特征(见图2)。抑制基因的表达将会影响受体磷酸化过程, 降低海马、杏仁核、嗅球等组织中神经元的活跃水平, 进而导致一系列异常行为特征出现。而过表达会导致海马内兴奋性神经元被过度激活, 也会影响孤独症样行为出现, 因此无论CHRM3所介导的神经信号通路被抑制或是增强, 一旦神经系统内环境稳态被破坏都有可能引发孤独症的发生。鉴于此, 控制Gq-PLC信号通路活动水平适中对于特定行为的发展有重要作用。但选择哪一项指标作为衡定信号通路适中的标准, 尚有待今后的深入研究。除此之外, 当孤独症高风险基因发生突变时,的表达也会受到影响(Forrest, Waite, Martin-Rendon, & Blake, 2013; Chan et al., 2015)。另外在对孤独症患者家系全基因组检测中, 发现了一个CHRM3下游分子PLC家族成员(磷酯酶)的编码基因存在新生突变, 这暗示CHRM3及其所调控信号通路对孤独症发生发展有重要影响。但是目前有关基因突变在孤独症发生发展中的作用以及在脑发育过程中的机制还有待进一步探讨。
在接下来的研究中, 可以在建立小鼠动物模型的基础上, 通过检测基因分子水平变化、细胞组织器官发育分化、形态差异以及分析行为特征来研究基因在神经系统发育中的作用, 及其对孤独症发生的影响。另外, 关注CHRM3所介导的Gq-PLC信号通路在孤独症发生中的作用, 可为孤独症的基因靶向干预提供新的思路和方法, 为教育方案的制定提供科学的帮助和指导。
Alexander, G. M., Rogan, S. C., Abbas, A. I., Armbruster, B. N., Pei, Y., Allen, J. A., … Roth, B. L. (2009). Remote control of neuronal activity in transgenic mice expressing evolved g protein-coupled receptors.,(1), 27−39.
Amodeo, D. A., Yi, J., Sweeney, J. A., & Ragozzino, M. E. (2014). Oxotremorine treatment reduces repetitive behaviors in btbr t+ tf/j mice.,(17), 1−8
Bailey, A., Le Couteur, A., Gottesman, I., Bolton, P., Simonoff, E., Yuzda, E., & Rutter, M. (1995). Autism as a strongly genetic disorder: Evidence from a British twin study.(1), 63−77.
Beck, C. H., & Fibiger, H. C. (1995). Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: With and without diazepam pretreatment.(1), 709−720.
Bentley, P., Vuilleumier, P., Thiel, C. M., Driver, J., & Dolan, R. J. (2003). Cholinergic enhancement modulates neural correlates of selective attention and emotional processing.(1), 58−70.
Bernier, R., Golzio, C., Xiong, B., Stessman, H. A., Coe, B. P., Penn, O., ... Eichler, E. E. (2014). Disruptive CHD8 mutations define a subtype of autism early in development.(2), 263−276.
Bolivar, V. J., Walters, S. R., & Phoenix, J. L. (2007). Assessing autism-like behavior in mice: Variations in social interactions among inbred strains.(1), 21−26.
Bowen, D. M., Smith, C. B., White, P., & Davison, A. N. (1976). Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies.(3), 459−496.
Butler, M. G., Rafi, S. K., & Manzardo, A. M. (2015). High-resolution chromosome ideogram representation of currently recognized genes for autism spectrum disorders.(3), 6464−6495.
Buxbaum, J. D., Silverman, J. M., Smith, C. J., Kilifarski, M., Reichert, J., Hollander, E., ... Davis, K. L. (2001). Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity.(6), 1514−1520.
Bymaster, F. P., Carter, P. A., Yamada, M., Gomeza, J., Wess, J., Hamilton, S. E., ... Felder, C. C. (2003). Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine- induced seizure activity.(7), 1403−1410.
Carlezon Jr, W. A., Duman, R. S., & Nestler, E. J. (2005). The many faces of CREB.(8), 436−445.
Chan, S. F., Huang, X., McKercher, S. R., Zaidi, R., Okamoto, S. I., Nakanishi, N., & Lipton, S. A. (2015). Transcriptional profiling of MEF2-regulated genes in human neural progenitor cells derived from embryonic stem cells.3(C), 24−27.
Chen, M., Wan, Y., Ade, K., Ting, J., Feng, G., & Calakos, N. (2011). Sapap3 deletion anomalously activates short-term endocannabinoid-mediated synaptic plasticity.(26), 9563−9573.
Ch'ng, C., Kwok, W., Rogic, S., & Pavlidis, P. (2015). Meta-analysis of gene expression in autism spectrum disorder.(5), 593−608.
Christensen, D. L., Baio, J., Van Naarden Braun, K., Bilder, D., Charles, J., Constantino, J. N., ... Yeargin-Allsopp, M. (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years--Autism and developmental disabilities monitoring network, 11 sites, United States, 2012.(3), 1−23.
Crane, L., Pring, L., Jukes, K., & Goddard, L. (2012). Patterns of autobiographical memory in adults with autism spectrum disorder.(10), 2100−2112.
Cuccaro, M. L., Shao, Y., Grubber, J., Slifer, M., Wolpert, C. M., & Donnelly, S. L., et al. (2003). Factor analysis of restricted and repetitive behaviors in autism using the autism diagnostic interview-r.,(1), 3−17.
Cupolillo, D., Hoxha, E., & Faralli, A., De Luca, A., Rossi, F., Tempia, F., & Carulli, D., (2015). Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock- out mice.41(6), 1457−1466.
Dani, J. A., & Bertrand, D. (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system., 699−729.
De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., Cicek, A. E., ... Buxbaum, J. D. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism.(7526), 209−215.
Deng, Y. P., & Reiner, A. (2016). Cholinergic interneurons in the Q140 knockin mouse model of Huntington's disease: Reductions in dendritic branching and thalamostriatal input.(17), 3518−3529.
Deutsch, S. I., Urbano, M. R., Neumann, S. A., Burket, J. A., & Katz, E. (2010). Cholinergic abnormalities in autism: Is there a rationale for selective nicotinic agonist interventions?(3), 114−120.
Devor, A., Andreassen, O. A., Wang, Y., Mäki-Marttunen, T., Smeland, O. B., Fan, C. C., ... Dale, A. M. (2017). Genetic evidence for role of integration of fast and slow neurotransmission in schizophrenia.(6), 792−801.
Dineley, K. T., Pandya, A. A., & Yakel, J. L. (2015). Nicotinic ACh receptors as therapeutic targets in CNS disorders.(2), 96−108.
Donato, F., Chowdhury, A., Lahr, M., & Caroni, P. (2015). Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning.(4), 770−786.
Durand, C. M., Betancur, C., Boeckers, T. M., Bockmann, J., Chaste, P., Fauchereau, F., ... Bourgeron, T. (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders.(1), 25−27.
Forrest, M. P., Waite, A. J., Martin-Rendon, E., & Blake, D. J. (2013). Knockdown of human TCF4 affects multiple signaling pathways involved in cell survival, epithelial to mesenchymal transition and neuronal differentiation.(8), e73169.
Friedman, S. D., Shaw, D. W. W., Artru, A. A., Dawson, G., Petropoulos, H., & Dager, S. R. (2006). Gray and white matter brain chemistry in young children with autism.(7), 786−794.
Gai, X., Xie, H. M., Perin, J. C., Takahashi, N., Murphy, K., Wenocur, A. S., ... White, P. S. (2012). Rare structural variation of synapse and neurotransmission genes in autism.(4), 402−411.
Gao, Z., Lee, P., Stafford, J. M., von Schimmelmann, M., Schaefer, A., & Reinberg, D. (2014). An AUTS2-Polycomb complex activates gene expression in the CNS.(7531), 349−354.
Gaugler, T., Klei, L., Sanders, S. J., Bodea, C. A., Goldberg, A. P., Lee, A. B., ... Buxbaum, J. D. (2014). Most genetic risk for autism resides with common variation.(8), 881−885.
Haglund, N. G. S., & Kallen, K. B. M. (2011). Risk factors for autism and Asperger syndrome. Perinatal factors and migration.(2), 163−183.
Happe, F., Ronald, A., & Plomin, R. (2006). Time to give up on a single explanation for autism.(10), 1218−1220.
Hardan, A. Y., Jou, R. J., & Handen, B. L. (2005). Retrospective study of quetiapine in children and adolescents with pervasive developmental disorders.(3), 387−391.
Huguet, G., Ey, E., & Bourgeron, T. (2013). The genetic landscapes of autism spectrum disorders.(1), 191−213.
Hussman, J. P., Chung, R. H., Griswold, A. J., Jaworski, J. M., Salyakina, D., Ma, D., ... Pericak-Vance, M. A. (2011). A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism.(1), 1−16
Karvat, G., & Kimchi, T. (2014). Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism.(4), 831−840.
Kim, J. W., Seung, H., Kwon, K. J., Ko, M. J., Lee, E. J., Oh, H. A., ... Bahn, G. H. (2014). Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism.(8), e104927.
King, I. F., Yandava, C. N., Mabb, A. M., Hsiao, J. S., Huang, H. S., Pearson, B. L., ... Zylka, M. J. (2013). Topoisomerases facilitate transcription of long genes linked to autism.(7465), 58−62.
Koeleman, B. P. C. (2018). What do genetic studies tell us about the heritable basis of common epilepsy? Polygenic or complex epilepsy?, 10−16.
Lee, M., Martin-Ruiz, C., Graham, A., Court, J., Jaros, E., Perry, R., ... Perry, E. (2002). Nicotinic receptor abnormalities in the cerebellar cortex in autism.(Pt 7), 1483−1495.
Levey, A. I., Edmunds, S. M., Heilman, C. J., Desmond, T. J., & Frey, K. A. (1994). Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain.(1), 207−221.
Levey, A. I., Edmunds, S. M., Koliatsos, V., Wiley, R. G., & Heilman, C. J. (1995). Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation.(5), 4077−4092.
Li, J., Wang, L., Guo, H., Shi, L., Zhang, K., Tang, M., ... Xia, K. (2017). Targeted sequencing and functional analysis reveal brain-size-related genes and their networks in autism spectrum disorders.(9), 1282−1290.
Lonze, B. E., & Ginty, D. D. (2002). Function and regulation of CREB family transcription factors in the nervous system.(4), 605−623.
Luukkonen, T. M., Mehrjouy, M. M., Pöyhönen, M., Anttonen, A. K., Lahermo, P., Ellonen, P., ... Varilo, T. (2017). Breakpoint mapping and haplotype analysis of translocation t(1;12) (q43;q21.1) in two apparently independent families with vascular phenotypes.(1), 56−68.
Maccarrone, M., Rossi, S., Bari, M., De Chiara, V., Rapino, C., Musella, A., ... Centonze, D. (2010). Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA.(7), 1500−1509.
Martin, A., Koenig, K., Scahill, L., & Bregman, J. (1999). Open-label quetiapine in the treatment of children and adolescents with autistic disorder.,(2), 99−107.
Martin-Ruiz, C. M., Lee, M., Perry, R. H., Baumann, M., Court, J. A., & Perry, E. K. (2004). Molecular analysis of nicotinic receptor expression in autism.(1-2), 81−90.
Matsui, M., Araki, Y., Karasawa, H., Matsubara, N., Taketo, M. M., & Seldin, M. F. (1999). Mapping of five subtype genes for muscarinic acetylcholine receptor to mouse chromosomes.(1), 15−21.
Matsui, M., Motomura, D., Karasawa, H., Fujikawa, T., Jiang, J., Komiya, Y., ... Taketo, M. M. (2000). Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype.(17), 9579−9584.
McTighe, S. M., Neal, S. J., Lin, Q., Hughes, Z. A., & Smith, D. G. (2013). The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex.(4), e62189.
Meyer, L. R., Zhu, V., Miller, A., & Roghair, R. D. (2014). Growth restriction, leptin, and the programming of adult behavior in mice., 131−135.
Michaelson, J. J., Shi, Y. J., Gujral, M., Zheng, H. C., Malhotra, D., Jin, X., ... Sebat, J. (2012). Whole-genome sequencing in autism identifies hot spots for de novo germline mutation.(7), 1431−1442.
Mines, M. A., Yuskaitis, C. J., King, M. K., Beurel, E., & Jope, R. S. (2010). GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism.(3), e9706.
Moretti, P., Levenson, J. M., Battaglia, F., Atkinson, R., Teague, R., Antalffy, B., ... Zoghbi, H. Y. (2006). Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome.(1), 319−327.
Neale, B. M., Kou, Y., Liu, L., Ma'ayan, A., Samocha, K. E., Sabo, A., ... Daly, M. J. (2012). Patterns and rates of exonic de novo mutations in autism spectrum disorders.(7397), 242−245.
Nelson, K. B., Grether, J. K., Croen, L. A., Dambrosia, J. M., Dickens, B. F., Jelliffe, L. L., ... Phillips, T. M. (2001). Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation.(5), 597−606.
O'Connor, E. C., Bariselli, S., & Bellone, C. (2014). Synapticbasis of social dysfunction: A focus on postsynaptic proteins linking group-I mGluRs with AMPARs and NMDARs.(7), 1114−1129.
Okerlund, N. D., & Cheyette, B. N. R. (2011). Synaptic Wnt signaling-a contributor to major psychiatric disorders?(2), 162−174.
O'Roak, B. J., Vives, L., Fu, W., Egertson, J. D., Stanaway, I. B., Phelps, I. G., ... Shendure, J. (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders.(6114), 1619−1622.
O'Roak, B. J., Vives, L., Girirajan, S., Karakoc, E., Krumm, N., Coe, B. P., ... Eichler, E. E. (2012). Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations.(7397), 246−250.
Ozonoff, S., Young, G. S., Carter, A., Messinger, D., Yirmiya, N., Zwaigenbaum, L., ... Stone, W. L. (2011). Recurrence risk for autism spectrum disorders: A baby siblings research consortium study.(3), e488−e495.
Perrone, M. D., Rocca, M. S., Bruno, I., Faletra, F., Pecile, V., & Gasparini, P. (2012). De novo 911 Kb interstitial deletion on chromosome 1q43 in a boy with mental retardation and short stature.(2), 117−119.
Perry, E. K., Lee, M. L. W., Martin-Ruiz, C. M., Court, J. A., Volsen, S. G., Merrit, J., ... Wenk, G. L. (2001). Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain.(7), 1058−1066.
Petersen, A. K., Ahmad, A., Shafiq, M., Brown-Kipphut, B., Fong, C. T., & Anwar Iqbal, M. (2013). Deletion 1q43 encompassing only CHRM3 in a patient with autistic disorder.(2), 118−122.
Pinto, D., Delaby, E., Merico, D., Barbosa, M., Merikangas, A., Klei, L., ... Scherer, S. W. (2014). Convergence of genes and cellular pathways dysregulated in autism spectrum disorders.(5), 677−694.
Pinto, D., Pagnamenta, A. T., Klei, L., Regan, R., Conroy, J., Casey, J., ... Ennis, S. (2011). Functional impact of global rare copy number variation in autism spectrum disorders(7304), 368−372.
Poulin, B., Butcher, A., McWilliams, P., Bourgognon, J. M., Pawlak, R., Kong, K. C., ... Tobin, A. B. (2010). The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner.(20), 9440−9445.
Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., ... Maratos-Flier, E. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour.(6571), 243−247.
Ray, M. A., Graham, A. J., Lee, M., Perry, R. H., Court, J. A., & Perry, E. K. (2005). Neuronal nicotinic acetylcholine receptor subunits in autism: An immunohistochemical investigation in the thalamus.(3), 366−377.
Riikonen, R., & Vanhala, R. (1999). Levels of cerebrospinal fluid nerve-growth factor differ in infantile autism and Rett syndrome.(3), 148−152.
Rinaldo, L., & Hansel, C. (2013). Muscarinic acetylcholine receptor activation blocks long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses via cannabinoid signaling.(27), 11181−11186.
Ronald, A., Happe, F., & Plomin, R. (2005). The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism.(5), 444−458.
Roohi, J., Tegay, D. H., Pomeroy, J. C., Burkett, S., Stone, G., Stanyon, R., & Hatchwell, E. (2008). A de novo apparently balanced translocation [46,XY,t(2;9) (p13;p24)] interrupting RAB11FIP5 identifies a potential candidate gene for autism spectrum disorder.4), 411−417.
Rosenberg, R. E., Law, J. K., Yenokyan, G., McGready, J., Kaufmann, W. E., & Law, P. A. (2009). Characteristics and concordance of autism spectrum disorders among 277 twin pairs.(10), 907−914.
Rosethorne, E. M., Nahorski, S. R., & Challiss, R. A. J. (2008). Regulation of cyclic AMP response-element binding-protein (CREB) by Gq/11-protein-coupled receptors in human SH-SY5Y neuroblastoma cells.(4), 942−955.
Sakai, Y., Shaw, C. A., Dawson, B. C., Dugas, D. V., Al-Mohtaseb, Z., Hill, D. E., & Zoghbi, H. Y. (2011). Protein interactome reveals converging molecular pathways among autism disorders.(86), 86ra49.
Sanders, S. J., He, X., Willsey, A. J., Ercan-Sencicek, A. G., Samocha, K. E., Cicek, A. E., ... State, M. W. (2015). Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci.(6), 1215−1233.
Sanders, S. J., Murtha, M. T., Gupta, A. R., Murdoch, J. D., Raubeson, M. J., Willsey, A. J., ... State, M. W. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism.(7397), 237−241.
Schaaf, C. P., & Zoghbi, H. Y. (2011). Solving the autism puzzle a few pieces at a time.(5), 806−808.
Cuccaro, M. L., Shao, Y., Grubber, J., Slifer, M., Wolpert, C. M., & Donnelly, S. L., et al. (2003). Factor analysis of restricted and repetitive behaviors in autism using the autism diagnostic interview-r., 34(1), 3-17.
Shimojima, K., Okamoto, N., Suzuki, Y., Saito, M., Mori, M., Yamagata, T., ... Yamamoto, T. (2012). Subtelomeric deletions of 1q43q44 and severe brain impairment associated with delayed myelination.(9), 593−600.
Silipigni, R., Monfrini, E., Baccarin, M., Giangiobbe, S., Lalatta, F., Guerneri, S., & Bedeschi, M. F. (2017). Familial duplication/deletion of 1q42.13q43 as meiotic consequence of an intrachromosomal insertion in chromosome 1.(2), 73−80.
Silva, A. J., Kogan, J. H., Frankland, P. W., & Kida, S. (1998). CREB and memory.(1), 127−148.
Silverman, J. L., Smith, D. G., Rizzo, S. J., Karras, M. N., Turner, S. M., Tolu, S. S., ... Crawley, J. N. (2012). Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism.(131), 131ra151.
Soueid, J., Kourtian, S., Makhoul, N. J., Makoukji, J., Haddad, S., Ghanem, S. S., ... Boustany, R. M. (2016). RYR2, PTDSS1 and AREG genes are implicated in a Lebanese population- based study of copy number variation in autism.6(2) 19088, 1-11
Spinelli, L., Black, F. M., Berg, J. N., Eickholt, B. J., & Leslie, N. R. (2015). Functionally distinct groups of inherited PTEN mutations in autism and tumour syndromes.,(2), 128−134.
State, M. W., & Šestan, N. (2012). The emerging biology of autism spectrum disorders.(6100), 1301− 1303.
Taniai, H., Nishiyama, T., Miyachi, T., Imaeda, M., & Sumi, S. (2008). Genetic influences on the broad spectrum of autism: Study of proband-ascertained twins.6), 844−849.
Tischmeyer, W., Kaczmarek, L., Strauss, M., Jork, R., & Matthies, H. (1990). Accumulation of c-fos mRNA in rat hippocampus during acquisition of a brightness discrimination.(2), 165−171.
Tsang, S. W., Francis, P. T., Esiri, M. M., Wong, P. T., Chen, C. P., & Lai, M. K. (2008). Loss of [3h]4-damp binding to muscarinic receptors in the orbitofrontal cortex of alzheimer's disease patients with psychosis.,(2), 251.
Vorstman, J. A. S., Parr, J. R., Moreno-De-Luca, D., Anney, R. J. L., Nurnberger, J. I., Jr., & Hallmayer, J. F. (2017). Autism genetics: Opportunities and challenges for clinical translation.(6), 362−376.
Wang, J. Q., & McGinty, J. F. (1997). Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats.(1-2), 62−70.
Whitehouse, P. J., Price, D. L., Struble, R. G., Clark, A. W., Coyle, J. T., & Delon, M. R. (1982). Alzheimer's disease and senile dementia: Loss of neurons in the basal forebrain.(4537), 1237−1239.
Wing, L. (1981). Language, social, and cognitive impairments in autism and severe mental retardation.(1), 31−44.
Wood, C. L., Warnell, F., Johnson, M., Hames, A., Pearce, M. S., McConachie, H., & Parr, J. R. (2015). Evidence for ASD recurrence rates and reproductive stoppage from large UK ASD research family databases.(1), 73−81.
Yamada, M., Miyakawa, T., Duttaroy, A., Yamanaka, A., Moriguchi, T., Makita, R., ... Wess, J. (2001). Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean.(6825), 207−212.
Yang, M., Scattoni, M. L., Zhodzishsky, V., Chen, T., Caldwell, H., Young, W. S., ... Crawley, J. N. (2007). Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice.(1).
Yun, S. H., & Trommer, B. L. (2011). Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus.(2), 176−182.
Zhang, L., & Alger, B. E. (2010). Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome.(16), 5724−5729.
Zhang, Y., Cao, S. X., Sun, P., He, H. Y., Yang, C. H., Chen, X. J., ... Li, X. M. (2016). Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via alpha7 receptor in hippocampus.(6), 728−742.
gene and autism spectrum disorder
JU Xingda1; SONG Wei1; XU Jing2
(1School of Psychology, Northeast Normal University, Changchun 130024, China) (2School of Clinical Medicine, Changchun University of Chinese Medicine, Changchun 130117, China)
Autism Spectrum Disorder is one of the most complex developmental disorders with a strong genetic impact. In recent years, researchers have increasingly linked effects of central cholinergic system dysfunction to autism-related cognitive and behavioral abnormalities at the molecular pathological level. Results from autopsy studies, clinical cases and animal experiments revealed that aberrant muscarinic acetylcholine receptors have a strong relationship with autism. In behavioral studies using mouse models, the variations ofgene, which encodes the muscarinic acetylcholine receptor subtype III receptor, can cause autistic phenotypes such as cognitive impairment and stereotypic behavior. Accordingly, in-depth functional understanding ofgene may have important implications to further explain the characteristics and mechanisms of autistic behavior and may potentially provide new ideas and methods for the development of educational programs for autistic children.
autism spectrum disorder;gene; clinical features; animal models
2018-05-19
* 全国教育科学“十二五”规划教育部青年专项课题“儿童孤独症的基因靶向教育策略研究”(EBA140364)资助。巨兴达、宋伟为共同第一作者
巨兴达, E-mail: juxd513@nenu.edu.cn 徐婧, E-mail: xuj391@nenu.edu.cn
B845
10.3724/SP.J.1042.2018.02141
