POSS功能化镧系稀土配合物杂化材料的制备与表征
2018-11-25胡皓李颖
胡皓 李颖
摘要:
选用三羟基多面体低聚倍半硅氧烷(POSS-OH)为基质,三乙氧基硅基丙基异氰酸酯(TEPIC)为偶联剂,通过化学键合的方法将POSS与β-二酮类有机配体乙酰丙酮相结合,再以配位的形式引入稀土铽离子,制备了一种新型POSS功能化的稀土有机-无机杂化材料Tb(POSS-ACAC)3。利用红外光谱和紫外光谱确定了Tb(POSS-ACAC)3的结构,并通过与稀土小分子配合物Tb(ACAC)3的热重分析对比发现,POSS基团的引入能够提高材料的热稳定性。进一步对材料的荧光性能进行分析,结果表明,Tb(POSS-ACAC)3的发光纯度和荧光强度较纯配合物都有明显提高,同时解决了荧光淬灭问题。
关键词:
乙酰丙酮功能化POSS; 稀土荧光; 有机-无机杂化材料
中图分类号: TQ 324.2 文献标志码: A
Synthesis and Characteration of POSS Functionalized Lanthanide Rear Earth Complexes Hybrid Material
HU Hao, LI Ying
(School of Materials Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China)
Abstract:
POSS functionalized rare-earth organic-inorganic hybrid material Tb(POSS-ACAC)3 was prepared by linking the Tb3+ complexes to the functionalized hydroxy polyhedral oligomeric silsesquioxane(POSS-OH) with the triethoxysilyl propyl isocyanate(TEPIC) as coupling agent through the process of chemical bonding between POSS and the β-diketone ligand ACAC.The structure of Tb(POSS-ACAC)3 was well characterized by IR and UV spectra.Compared with pure Tb(ACAC)3 complexes,it was showed that the introduction of POSS group could improve the thermal stability of the material.Furthermore,the luminescence properties of the resulting material was characterized in detail,and the results revealed that the luminescence intensity and purity of Tb(POSS-ACAC)3 were significantly improved.In addition,the problem of fluorescence quenching was solved.
Keywords:
ACAC functionalized POSS; rear-earth luminescence; organic-inorganic hybrid material
有机β-二酮配体中的亚甲基非常活泼,由于受到双重羰基吸引电子的氧原子的影响,容易发生各种反应。稀土β-二酮配合物中存在着从具有高吸收系数的β-二酮配体到Eu3+,Tb3+等离子的高效能量传递,而具有极高的发光效率。它们与镧系离子形成稳定的六元环,直接吸收激发光并有效地传递能量。为了进一步提高该类配合物的性能,將稀土有机β-二酮类配合物与固体基质进行复合以获得稀土有机杂化发光材料,此类研究引起了广泛的关注[1-3]。稀土有机杂化发光材料是一类以稀土发光配合物为客体,凝胶[4-5]、多孔框架[6]、纳米颗粒[7]、高分子[8-9]和离子液[10-11]等为主体基质,通过主客体之间的相互作用进行组装构筑的新型功能杂化材料。这类材料的荧光性能优良,光谱呈窄带发射、单色性好,有较强的紫外线吸收能力和较长的荧光寿命。
多面体低聚倍半硅氧烷(POSS)是一种新型的纳米功能性分子,由于其具有高度对称的SiOSi笼型骨架结构,自身的热稳定性较好并具有多个反应活性点,具有无机材料的热稳定性和优异的力学性能,同时兼具有机材料的结构可调控的优点[12-13]。以POSS骨架作为基质与稀土配合物进行复合可以有效地提高材料的热稳定性,从而在一定程度上解决小分子稀土配合物光热稳定性差的问题。本文通过化学键将POSS与β-二酮类有机配体乙酰丙酮(ACAC)进行键合,再以配位的形式引入稀土铽离子(Tb3+),制备了一种新型POSS功能化的稀土有机-无机杂化材料Tb(POSS-ACAC)3,对材料的结构进行了分析,并进一步研究了杂化材料的热稳定性和荧光性能。
1 试验部分
1.1 试验材料
乙酰丙酮(ACAC)(纯度为99%,质量分数,下同),三乙氧基硅基丙基异氰酸酯(TEPIC)(纯度为97%),三羟基多面体低聚倍半硅氧烷(POSS-OH),氧化铽(纯度为99.99%),过氧化氢(分析纯),四氢呋喃(分析纯),氢化钠(纯度为95%),浓盐酸(质量分数37%),三氯甲烷(分析纯),无水乙醇(分析纯AR)。
1.2 试验步骤
1.2.1 制备稀土配合物Tb(ACAC)3
将TbCl3(0.33 mmol)溶于15 mL去离子水中,再加入ACAC(1 mmol),用NaOH(0.1 mol/L)水溶液调节pH至中性,60 ℃加热搅拌4 h,旋转蒸发除去溶剂,得到淡黄色固体Tb(ACAC)3。
1.2.2 合成前驱体ACAC-Si
在氮气气氛保护下,将ACAC(1 mmol)溶于20 mL四氢呋喃中,然后加入氢化钠(2 mmol),65 ℃加热回流,反应2 h后,缓慢滴加TEPIC(2 mmol),反应6 h后,旋转蒸发去除溶剂,得到黄色油状产物ACAC-Si。
1.2.3 合成ACAC功能化的POSS(POSS-ACAC)
将POSS-OH与ACAC-Si加入三氯甲烷(25 mL)与四氢呋喃(5 mL)的混合溶剂中,60 ℃加热搅拌4 h,旋转蒸发后得到黄色黏稠油状产物POSS-ACAC。
1.2.4 合成含Tb3+的POSS基杂化材料Tb(POSS-ACAC)3
将上述制备的POSS-ACAC溶于无水乙醇中,再滴加0.1 mol/L氯化铽的乙醇溶液(3.3 mL),70 ℃加热回流,旋转蒸发除去溶剂,得到产物为白色粉末,70 ℃干燥。
1.3 表 征
采用SPECTRUM 100 Perkin Elmer型红外光谱仪,通过溴化钾压片在4 000~400 cm-1测量红外光谱;采用Lambda 750型紫外-可见光光谱仪,以乙醇作溶剂测量紫外光谱;采用Pyris1型热重-差热分析仪,在初始质量3~8 mg,升温速率10 ℃/min,氮气氛围下测量热失重图谱;采用RF-5301型分光光度计,以氙灯作为发射光源(波长分辨率为0.5 nm)进行荧光激发和发射光谱的测量。
2 结果与讨论
2.1 红外光谱
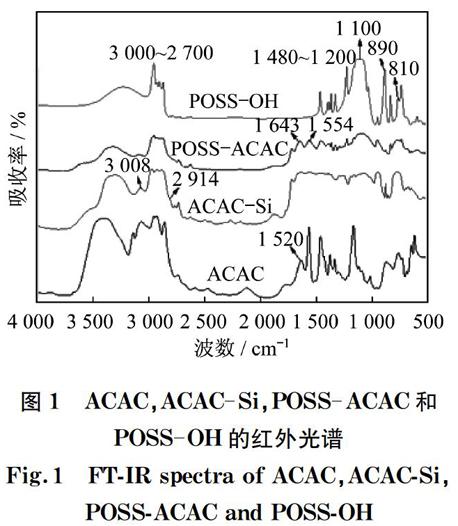
图1为ACAC,ACAC-Si,POSS-ACAC和POSS-OH的红外光谱。从ACAC的图谱中可以观察到,位于1 620 cm-1处的吸收峰归属于β-二酮结构中的CO的伸缩振动。在ACAC-Si和POSS-ACAC的图谱中出现了同样位置的对应吸收峰,说明ACAC与POSS成功进行了嫁接[14-15]。此外,从POSS-ACAC的图谱中可以观察到,由SiO伸缩振动引起的1 100 cm-1左右处的吸收峰,以及位于1 643 cm-1和1 554 cm-1处的由CO伸缩振动引起的吸收峰,同样证实了POSS与ACAC之间发生了键合作用。
图1 ACAC,ACAC-Si,POSS-ACAC和POSS-OH的红外光谱
Fig.1 FT-IR spectra of ACAC,ACAC-Si,POSS-ACAC and POSS-OH
2.2 紫外光谱
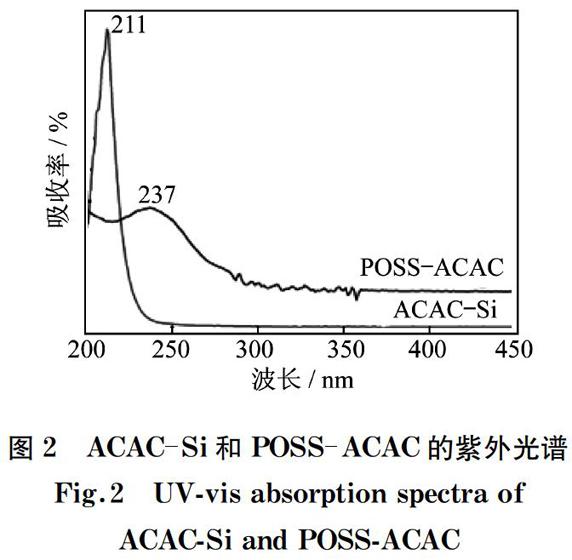
POSS-ACAC和ACAC-Si的紫外光谱如图2所示,其中ACAC-Si的吸收光谱在200~250 nm处出现宽的吸收峰,且最高峰在211 nm处,这是由β-二酮的烯醇基团的π-π*跃迁所引起的[16]。对比ACAC-Si和POSS-ACAC,从它们的紫外吸收光谱图上能够观察到从211到237 nm处π-π*电子跃迁的红移现象。结果表明,TEPIC及POSS骨架中SiO的介入,不同程度地引起了配体结构的改变,从而证实了POSS与ACAC的成功嫁接。
图2 ACAC-Si和POSS-ACAC的紫外光谱
Fig.2 UV-vis absorption spectra of ACAC-Si and POSS-ACAC
2.3 热稳定性
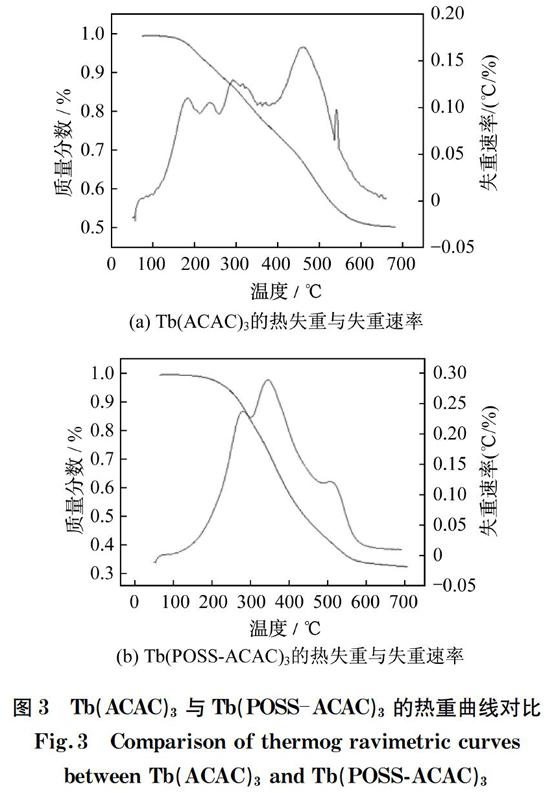
Tb(ACAC)3和Tb(POSS-ACAC)3的热重分析如图3所示。从图3(a)中可以观察到,Tb(ACAC)3在150 ℃开始分解,这是由小分子水和有机溶剂蒸发所引起的。从失重速率图可以看出,其分解速率在300和480 ℃处不断升高,这主要是由ACAC的分解所引起的[17]。从图3(b)中可以看出,Tb(POSS-ACAC)3从200 ℃时开始分解,在280 ℃左右達到第1个分解速率顶点,随后在350 ℃达到最高分解速率,这主要归因于POSS的分解。与Tb(ACAC)3相比,杂化材料Tb(POSS-ACAC)3的初始分解温度有所升高,这表明POSS骨架的引入能够提高材料的热稳定性。
图3 Tb(ACAC)3与Tb(POSS-ACAC)3的热重曲线对比
Fig.3 Comparison of thermog ravimetric curves between Tb(ACAC)3 and Tb(POSS-ACAC)3
2.4 荧光性能
以Tb3+的最高发射波长545 nm为监测波长,测得Tb(ACAC)3和Tb(POSS-ACAC)3的荧光激发光谱(EX)和发射光谱(EM),如图4所示。从图4中可以观察到,Tb(ACAC)3和Tb(POSS-ACAC)3的激发光谱在250~450 nm处都出现了较宽的激发峰,且最佳激发波长分别位于328和343 nm处,这归因于有机配体ACAC引起的π-π*跃迁,从Tb(POSS-ACAC)3的荧光发射光谱可以观察到明显的Tb3+的特征发射峰,其中,波长为485,545,580和620 nm的发射峰分别对应于5D4→7F6,5D4→7F5,5D4→7F4和5D4→7F3的电子跃迁[18]。结果表明,ACAC与Tb3+之间发生了有效的能量传递,杂化材料可以在紫外灯下发出明亮的绿光(图5)。对比Tb(ACAC)3的荧光发射光谱发现,Tb(POSS-ACAC)3的5D4→7F6,5D4→7F5的电子跃迁发射峰强度明显提高。这表明,POSS-ACAC与Tb3+之间发生了更有效的能量传递,改善了它的荧光性能。
圖4 稀土配合物和POSS基稀土杂化材料的荧光表征
Fig.4 Fluorescence characterization of rare earth
complexes and POSS based rare
earth hybrid materials
图5 紫外灯下的杂化材料Tb(POSS-ACAC)3照片
Fig.5 Digital photo of the hybrid material
Tb(POSS-ACAC)3 under UV-light
3 结 论
(1) 选用β-二酮类有机配体ACAC,通过化学键与含有活性羟基官能团的POSS进行键合,制备了ACAC功能化的杂化材料POSS-ACAC。通过红外光谱和紫外光谱分析确定了POSS骨架上存在共价键键合的有机官能团ACAC。
(2) 利用配位化学原理,将Tb3+引入到POSS-ACAC骨架中,合成了杂化材料Tb(POSS-ACAC)3。对材料的结构和性能进行了表征分析,得出POSS-ACAC可以很好地敏化中心Tb3+发光,并且有效提高荧光强度。
(3) 杂化材料Tb(POSS-ACAC)3显示了很强的Tb3+特征发射和较好的热稳定性。
参考文献:
[1] 刘政,孙丽宁,施利毅,等.近红外稀土荧光在功能材料领域的研究进展[J].化学进展,2011,23(1):153-164.
[2] 曹俊,陈连平,李翠云.稀土掺杂铝酸锶长余辉薄膜制备方法的研究现状[J].有色金属材料与工程,2016,37(4):171-175.
[3] SUN L N,ZHANG Y,YU J B,et al.Design and synthesis of near-IR luminescent mesoporous materials covalently linked with tris(8-hydroxyquinolinate) lanthanide(Ⅲ) complexes[J].Microporous and Mesoporous Materials,2008,115(3):535-540.
[4] FENG J,YU J B,SONG S Y,et al.Near-infrared luminescent xerogel materials covalently bonded with ternary lanthanide[Er(Ⅲ),Nd(Ⅲ),Yb(Ⅲ),Sm(Ⅲ)]complexes[J].Dalton Transactions,2009(13):2406-2414.
[5] BINNEMANS K,LENAERTS P,DRIESENA K,et al.A luminescent tris(2-thenoyltrifluoroacetonato) europium(Ⅲ) complex covalently linked to a 1,10-phenanthroline-functionalised sol-gel glass[J].Journal of Materials Chemistry,2004,14(2):191-195.
[6] MISHRA S,JEANNEAU E,LEDOUXC G,et al.Lanthanide complexes in hybrid halometallate materials:interconversion between a novel 2D microporous framework and a 1D zigzag chain structure of iodoargentates templated by octakis-solvated terbium(Ⅲ) cation[J].Dalton Transactions,2009(25):4954-4961.
[7] 刘舒曼,徐征,刘峰奇,等.稀土掺杂ZnS纳米晶中稀土离子与纳米基质之间的能量传递[J].中国稀土学报,2001,19(6):565-569.
[8] WANG J Y,GROENEVELD A,OIKONOMOU M,et al.Revealing and tuning the core,structure,properties and function of polymer micelles with lanthanide-coordination complexes[J].Soft Matter,2016,12(1):99-105.
[9] CHENG M L,TAO F,CHEN L T,et al.Lanthanide(Ⅲ)-based coordination monomers and polymers of 3,4-pyrazoledicarboxylate:Extended synergy within the ligand,structures and magnetic properties[J].Inorganica Chimica Acta,2015,429:22-29.
[10] ANSARI S A,LIU L S,RAO L S.Binary lanthanide(Ⅲ)/nitrate and ternary lanthanide(Ⅲ)/nitrate/chloride complexes in an ionic liquid containing water:optical absorption and luminescence studies[J].Dalton Transactions,2015,44(6):2907-2914.
[11] LI Z Q,WANG J,CHEN M,et al.Lanthanide luminescence improvement by using a functional poly(ionic liquid) as matrix and co-ligand[J].Chemistry-An Asian Journal,2016,11(5):745-749.
[12] HE Z C,ZHONG M Q,YANG Y,et al.Synthesis of POSS-based star-shaped poly(ionic liquid)s and its application in supercritical CO2 microcellular foaming of polystyrene[J].Journal of Polymer Research,2106,23:243.
[13] JEON J H,TANAKA K,CHUJO Y,et al.Synthesis of sulfonic acid-containing POSS and its filler effects for enhancing thermal stabilities and lowering melting temperatures of ionic liquids[J].Journal of Materials Chemistry A,2014,2(3):624-630.
[14] YANG J,LI Z Q,XU Y,et al.Zirconia-based luminescent organic-inorganic hybrid materials with ternary europium(Ⅲ) complexes bonded[J].Optical Materials,2016,55:78-82.
[15] JIMENEZ G L,REYES-RODRIGUEZ J L,PADILLA I,et al.Reducing the photo-bleaching effect of a new europium complex embedded in styrene butadiene copolymer[J].Optical Materials,2018,76:271-277.
[16] BEHZAD S K,AMINI M M,GHANBARI M,et al.Synthesis,structure,photoluminescence,and electroluminescence of four novel europium complexes:Fabrication of pure red organic light emitting diodes from europium complexes[J].European Journal of Inorganic Chemistry,2017,2017(30):3644-3654.
[17] XU H,YIN K,HUANG W.Novel light-emitting ternary Eu3+ complexes based on multifunctional bidentate aryl phosphine oxide derivatives:Tuning photophysical and electrochemical properties toward bright electroluminescence[J].The Journal of Physical Chemistry C,2010,114(3):1674-1683.
[18] HARRIS S M,SRIVASTAVA K,LEAGUE A B,et al.Achieving selectivity for copper over zinc with luminescent terbium probes bearing phenanthridine antennas[J].Dalton Transactions,2018,47(7):2202-2213.
