Incorporation of silica into the goethite structure:a microscopic and spectroscopic study
2018-10-25AbdullahMusaAliEswaranPadmanabhanHassanBaioumy
Abdullah Musa Ali·Eswaran Padmanabhan·Hassan Baioumy
Abstract Quartz and iron(hydr)oxide are reactive surface phases that are often associated with one another in soils and sediments.Despite the several studies on the coating of quartz with iron oxides,the reactivity of dissolved species(Si)leached from quartz with iron(hydr)oxides has received limited attention.In this study,goethite synthesized on quartz substrates were characterized using fi eld emission scanning electron microscopy,X-ray diffraction(XRD),transmission electron microscopy,and Fouriertransform infrared(FT-IR)spectroscopy.The SEM characterization revealed that bundles of thin parallel aligned goethite rods were formed at pH>10,while large pseudohexagonal crystals of twinned goethite needles were synthesized at pH≤10 after dehydration and hydration in the alkaline media.TEM analysis showed expanded and distorted lattice spacing of the crystal structure of iron(hydr)oxide due to silica incorporation.The characterization showed that silica increased the crystallite size of the goethite and transformed its acicular texture to a larger,twinned needlestructure.FT-IR and XRD analyses revealed band shifts in crystal bonds as well as new bond formations,which indicate the presence of changes in the chemical environment of Fe–O and Si–O bonds.Thus,the presence of sorbed silicates modi fi es the crystal and lattice structure of goethite.
Keywords Quartz·Goethite·Twinned goethite·Microscopic characterization(FESEM and TEM)·FT-IR spectroscopy
1 Introduction
Quartz is the most ubiquitous mineral in the Earth’s crust,and it is a fundamental material in reactions that affect physiochemical properties of siliciclastics(White et al.2001;Lasaga and Luttge 2001;Lüttge 2005;Brantley et al.2008;Ali and Padmanabhan 2014;Ali et al.2017).Meanwhile,the abundant nature and thermodynamic stability of iron minerals have motivated the study of quartz’s phase composition,crystallinity and polymorphic variations(White 1990;Rodgers et al.1991;Pigna et al.2006;Gotićand Music 2007).Quartz and iron(hydr)oxide are reactive surface phases often associated with one another in soils and sediments(Davis et al.1998).Experimental studies(Schwertmann and Murad 1977;Hu etal.2012,2013;Dai and Hu 2015)have shown the nucleation and growth of iron hydroxide on quartz.Related studies report that iron oxides bind strongly with quartz surfaces(Hendershot and Lavkulich 1983;Scheidegger et al.1993;Rusch et al.2010;Xu and Axe 2005).However,as Rusch et al.(2010)pointed out,little is known about iron-phase reactivity with dissolved silica leached from quartz.In addition,there remains the unresolved issue of whether the interaction is a chemisorption process or simply surface adsorption(Padmanabhan and Mermut 1996).Moreover,the impact of pH on the formation of goethite and hematite is not entirely known(Van Ranst et al.2016).
The characterization of the interactions between iron minerals and quartz will be valuable in determining how quartz or dissolved silica may affect the formation of iron(hydr)oxides in low-temperature and strongly oxidizing sedimentary environments.It will also ensure reliable prediction of quartz reactivity using laboratory experimental data as well as improve the understanding of the actual transformation of iron oxides into more crystalline products.Furthermore,the capacity of iron oxide phases to adsorb cationic Si species from aqueous solutions plays a central role in the mobility and transfer of inorganic species and ionizable organic compounds in environmental contaminants(Hanna 2007;Rusch et al.2010).The objectives of this paper are to morphologically characterize the outcome of silicate sorption on quartz surfaces and to describe the surface texture and lattice variations of iron oxide coatings.In essence,this study reports the morphology of the silica-induced transformation of iron oxides.At pH 4–7 and 20°C,the levels of silicate in solution prevent the transformation of iron(hydr)oxides over a particular length of time(Schwertmann and Murad 1977).Therefore,the experiments in this study were carried out at pH≥10 and at 60°C to re fl ect natural environments such as oxidized vapor dominated geothermal systems,crater lakes,and mine drainage settings as well as geothermal energy and oil residual phase extractions(White and Brantley 2003).
2 Materials and methods
To achieve the objectives of this study,two sets of experimentswere conducted:the synthesisofiron(hydr)oxides on quartz substrates and quartz dissolution in the presence of iron oxides.The quartz crystals that were used were fi rst crushed in a mortar grinder to increase the exposed surface area and then sieved to obtain>50 μm mesh fraction.After that,about 20 g of the crushed quartz crystals were ultrasoni fi ed in cold 0.1 mol L-1HCl solution to remove the carbonate coatings and adhering iron coating.The washed samples were then soaked in 35%hydrogen peroxide(H2O2)to remove organic debris.The quartz grains were washed several times in distilled water and subsequently oven-dried at 60°C for 24 h.Individual quartz grains ranged from 200 to 400 μm in size.This pretreatment procedure also dissolved sharp edges,adhering fi ne particles,heavy minerals,organics,surface artefacts and other impurities without introducing metals or corrosive agents to the system that might affect the subsequent dissolution rate measurements.The fi nal quartz samples were comprised of subhedral to subround grains with surface defects that included conchoidal fractures and cleavage planes(Fig.1).The procedure for synthesizing iron(hydr)oxides on quartz substrates was modi fi ed from earlier studies by Arias et al.(1997)and Rusch et al.(2010).In the second experiment,bulk quartz grains were dissolved in NaOH solutions that contained varying amounts of iron.

Fig.1 FESEM image of a clean subhedral quartz grain with conchoidal fractures and smoothed edges
2.1 Synthesis of iron(hydr)oxides on quartz
The iron(hydr)oxides were synthesized on quartz substrates by precipitating 0.4 mol L-1FeCl3with the addition of0.5 mol L-1NaOH.A seriesofsolutions comprising 15,20,and 25 mL of 0.4 mol L-1of FeCl3were added to 5 g of quartz substrates(qFe15,qFe20,and qFe25),and then mLdistilled water was added until samples were 50 mL.The proportions used were roughly equivalent to the addition of 0.75,1.0,and 1.25 g of Fe per 5 g of quartz substrate.With the exception of qFe20,the initial pH of approximately 2 was then increased to 12 to ensure iron(hydr)oxide precipitation by adding 100 mL of 0.5 mol L-1NaOH solution.Each suspension was agitated intermittently for 30 min to improve contact between the quartz and the initial products of the Fe hydrolysis.The samples were aged at 60°C.The sample aged in 20 mL of FeCl3solution(qFe20)was dehydrated after 5 days of ageing through oven drying at 60°C for 2 days.Afterwards,fresh NaOH solution was added to the sample,which was then left to age for 20 days at a pH of approximately 8.5.Silicate was strongly adsorbed on the surfaces of various iron(hydr)oxides,with a maximum in surface coverage near pH 9(Taylor 1995).This approach was also utilized to limit the silica dissolved in the system as well as to synthesize hematite.The experimental conditions of the synthesis are outlined in Table 1.The amount of silica in the system was measured using the Silica Molybdate spectrophotometry method.The pH of solutionwas monitored in all titration and ageing experiments using a pH meter(EUTECH,Model:Cyberscan).
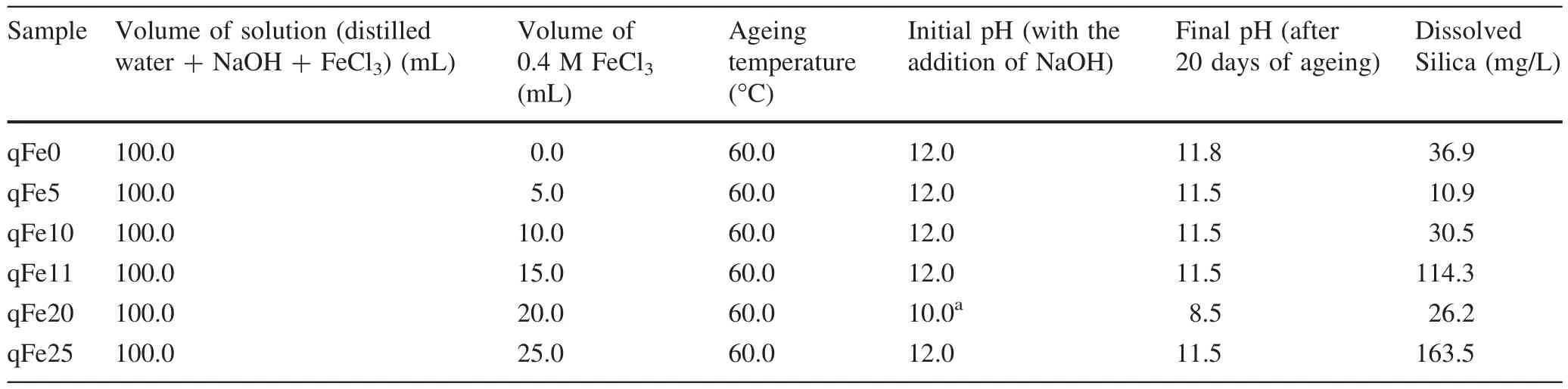
Table 1 Experimental condition for the synthesis
2.2 Quartz dissolution experiment
In a separate experiment,bulk samples of quartz grains were placed in the presence of iron oxides using the batch bottle system.The batch bottle system comprises sealed polyethylene bottles(50 mL) fl oating in a water bath shaker and heater.5 g of pure quartz grains were dissolved at 60°C in the separate bottles containing NaOH,and 5(qFe5),10(qFe10),15(qFe15),20(qFe20)and 25 mL(qFe25)of 0.4 mol L-1solutions of FeCl3fi xed at a pH of 12.Dilute sodium hydroxide(NaOH)and hydrochloric acid were used as neutralizing agents in the titration process to adjust the pH.1 mL of NaOH and HCl was added to the initial solvents to increase and reduce the pH by 0.1,respectively.The bottles were opened periodically to remove solution samples.The solvent was changed every 10 h.The amount of dissolved silica was measured using the silica molybdate spectrophotometry method(HACH D2800).Pure quartz substrate was included in the experiment as a control sample(qFe0).The bottles were mildly agitated from water circulation in the water bath to ensure the solution was always in contact with the quartz surface.Mass transfer limitations were not expected due to the slow dissolution rate conditions.
2.3 Sample characterization
Stereomicroscopic study of the iron(hydr)oxides synthesized on quartz was carried out to identify different mineral phases.The morphological characterization and surface composition analysis of the samples was performed using a high-resolution fi eld emission scanning electron microscope(FESEM:Carl Zeiss Supra 55VP;operated at 5–20 kV).A transmission electron microscope(TEM:Zeiss Libra 200FE)was used to provide high-resolution mineralogical and textural analysis to differentiate the quartz from the as-synthesized phases.The mineral composition of the iron(hydr)oxides precipitated on quartz was identi fi ed using an X-ray diffractometer(brand:Bruker-AXS,model:D8 Advance),with Ni fi ltered CuKα radiation(wavelength of 1.54056)generated at 40 kV and 40 mA.The operating parameters for XRD analysis also included step size and step time of 0.0001°and 1 s,respectively,with a 1 mm divergence slit,a 0.1 mm detector slit and a 1 mm antiscatter slit.Infrared spectra of the samples were obtained using Fourier-transform infrared(Shimadzu FT-IR 8400S)to identify the chemical bonds in the synthesized material.
3 Results and interpretation
3.1 Morphological characterization
The iron(hydr)oxides synthesized on quartz substrates were morphologically described.The stereomicroscopic characterization of the samples(Fig.2)indicated the presence of goethite and hematite as the iron bearing phases.The stereomicroscopic photomicrographs showed the coating of quartz grains with the synthesized hematite and goethite.It was observed that the iron bearing phases adhere onto the quartz surface,which gives the initial quartz grains a red tint(Fig.2a).The goethite particles(G)were characterized by star-shaped particles dispersed on the quartz surface(Fig.2b),while the hematite was enmeshed with the quartz grains(CQ)to form a distinct phase,possibly ferrisilicates.
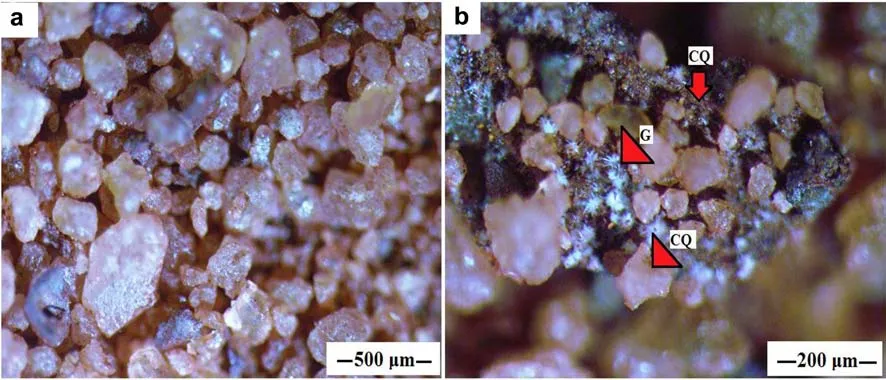
Fig.2 Stereomicroscope photomicrographs showing a iron oxide coated quartz grains and b closer view with arrows denoting star shaped goethite crystals(G),and hematite meshed with fi ner quartz grains to from a coating phase(CQ)

Fig.3 FESEM micrographs showing a distinctive needle shaped acicular goethite synthesized on quartz indicated by the inset arrow,b in fi lling of gouges and notches on the quartz surface with goethite laths
Goethite crystals were synthesized at pH 12,in solutions containing 15 mL(qFe15)and 25 mL(qFe25)of FeCl3.The FESEM micrographs showed needle/lath shaped goethite of aciculartexture(Fig.3).They occurred as(a)bundles of thin parallel aligned rods ranging from>1 to 3 μm in size for both samples.The goethite laths existed as a distinct phase,which was dispersed and admixed over the quartz surface(Fig.3a).The goethite laths were also observed to be non-uniform and discretely located in the stepped and concave areas of the quartz surface,indicating that etched and pitted areas of quartz ensured higher loadings of goethite material.A closer examination also showed that the goethite laths aggregated preferentially along the edges of the quartz crystal(Fig.3b),thus the goethite appeared to have been deposited abundantly at speci fi c sites.The very thin and elongated shape indicated an increasingly strong retardation of crystal growth in aand b-axes and a rapid growth/elongation in the crystallographic c-axis following rapid crystallization at high pH(Gotićand Music 2007).This phenomenon was attributed to the interference of OH-species in the growth of goethite(Cornell and Giovanoli 1985).
Large pseudohexagonal crystals of twinned goethite needles(TG)and Hematite(H)(Fig.4)were synthesized at pH 10 via the dehydration of initially hydrolyzed iron oxides and the subsequent rehydration with the base solution(NaOH).The twinned goethite was characterized by a larger crystalline structure compared to the goethite crystals synthesized at pH 12(Fig.4a,b).The twinned goethite crystals occurred as perfect goethite crystal,about 1 mm in length.Cleavage/twinning planes were evident.The synthesized twinned goethite and hematite adhered to planar and pitted surface,in fi ll crevices,notches,and fractures of the quartz grains(Fig.4a,b).The Fe-(hydr)oxides aggregated into isolated patches that partially covered the quartz grains(Fig.4a).EDX analysis of the twinned goethite needles showed 80.5 wt%for Fe and 4.0 wt%for Si(Fig.5),thus con fi rming the incorporation of Si into the goethite crystal.
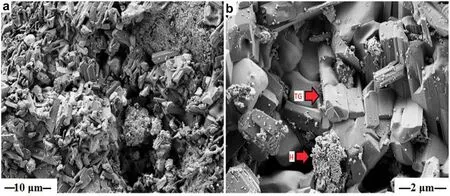
Fig.4 FESEM micrographs showing a well crystallized goethite needles in fi lling notches and completely covering the quartz surface,b synthesized twinned goethite needles[TG]and hematite[H]
3.2 Lattice structure of the twinned goethite
High-resolution TEM images were obtained to examine the lattice structure of the goethite laths dispersed on synthesized quartz substrates.The parallel goethite needles occurred as agglomerations of crystal lattices with different orientations.The goethite particles observed in the TEM images for the sample have dimensions in the order of 20×200 nm.The image depicted in Fig.6 shows goethite laths leaching into the silica lattice structure.The goethite crystals were characterized by acicularmorphology(Fig.7a),while the twinned goethite minerals(qFe20)were de fi ned by cross hatch twinning planes(Fig.7b).The length of the crystal lattice ranged from 100 nm up to 200 nm,with a diameter of approximately 5 nm.
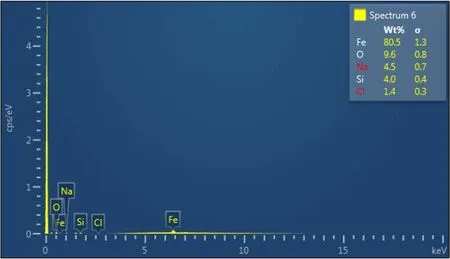
Fig.5 EDX spectrum showing the elemental concentrations of the twinned goethite
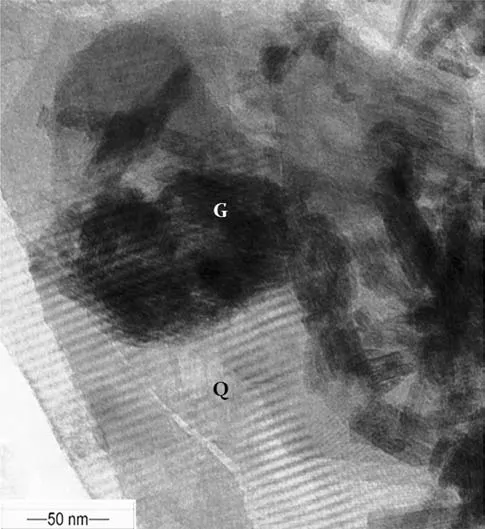
Fig.6 TEM micrograph of acicular goethite crystals(G)complexed with quartz(Q)showing goethite crystals intruding into the lattice structure of quartz
The well faceted crystals of the twinned goethite(Fig.8a)showed different lengths and curvatures,with some of the angles between the lattice fringes deviating signi fi cantly from 120°,although some of the hexagonal arrangement showed almost perfect projections(Fig.8b).The length of the crystal lath ranged from 1000up to 2000,with varying widths of 45–55(Fig.8b).In some places,the parallel goethite needles overlapped into bundles with a single orientation.The overlapping bundles appeared as rafts of crystals,with each single crystal projected at the edges of the rafts.However,two or more crystals were superimposed to form moiréfringes,as shown in Fig.7a.The depicted imperfections showed discontinuous lattice rows in the crystal(Fig.8a).The laths showed a prominent 40lattice repeat,separated by four 23.5fringes.The crystals also showed 25lath fringes perpendicular to a 30structure(Fig.8a).In some areas,the 3regularity was interrupted by shorter(25)or longer(50)repeats(Fig.7b).The lattice spacings[d()]expanded signi fi cantly from the characteristic d-spacing values of goethite(4.18,2.69,2.452,2.490,2.192)to 4.839 and 3.225.The contrasting lattice arrangement/alignment and changes in the stacking sequence of the goethite crystals arose from positional,or electron density variation.
3.3 X-ray diffraction(XRD)spectra analysis
XRD spectra were obtained to determine the mineralogy of iron(hydr)oxide synthesized on quartz substrates.Some of the samples(qFe15,qFe20 and qFe25)were selected for the analysis.Goethite and hematite were found to be the dominant phases of the precipitates,as shown in Fig.9.Ferrihydrite was the precursor mineral from which goethite and hematite were synthesized,the former from dissolution and re-precipitation,while the latter from thermal dehydration and aggregation(Cornell and Schwertmann 2003).The peaks at d-spacing=4.26,2.46,2.28,1.817 and 1.541were assigned to quartz.The peak at d-spacing=2.83 was typical for trioctahedral chlorites with Fedominant octahedral cations(Bailey 1988).The chlorite was also denoted by weak re fl ectiońs at 3.53.Goethite was identi fi ed with d-spacing of 2.69 A˚in substrates synthesized with 20 and 25 mL ofsolution[qFe15 and qFe25].A d-spacing value of 2.52indicated the presence of hematite in qFe20.Similar to the TEM results,shifts in basal d-spacings for goethite were identi fi ed.The characteristic peaks for goethite shifted from 4.18,2.69 and 2.49 to 4.238/4.20, 2.70/2.71/2.73 and 2.50/2.52/2.53,respectively.
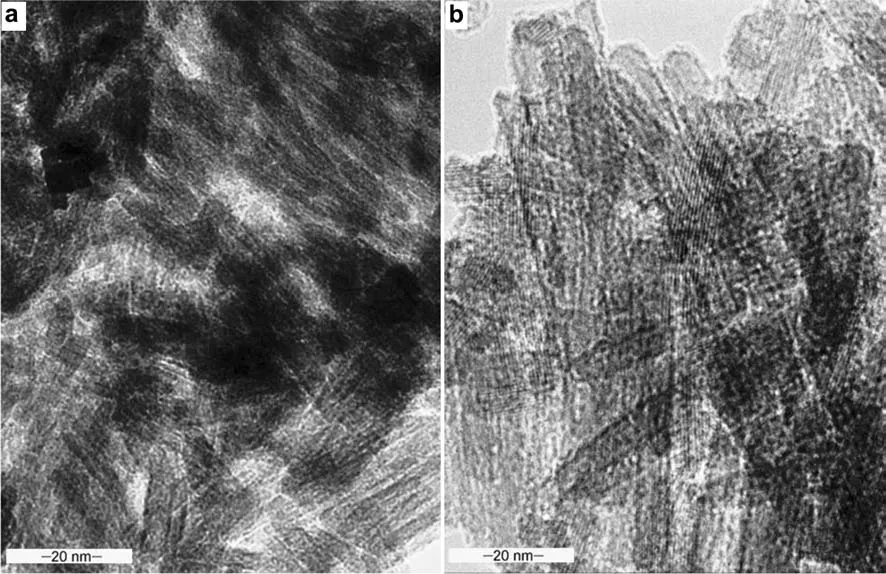
Fig.7 TEM micrographs showing a laths of goethite crystals,b twinned goethite
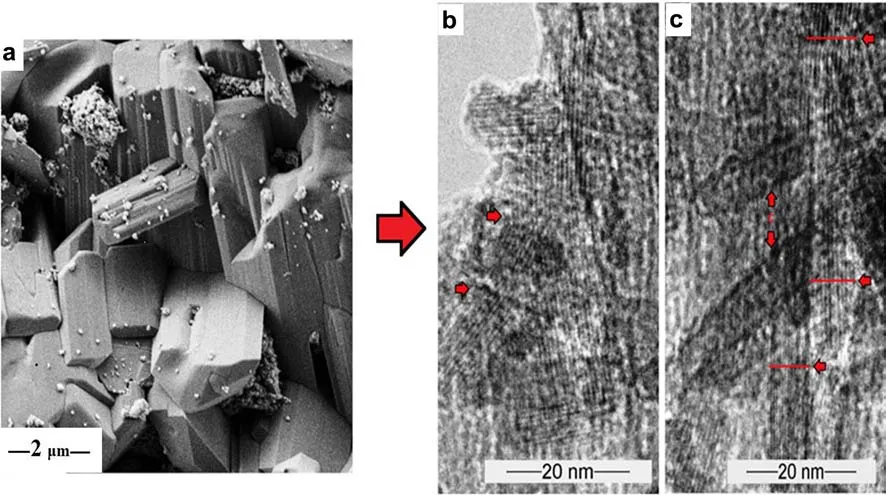
Fig.8 a FESEM image of the twinned goethite needles,b TEM micrograph of goethite lath showing lattice distortion and disparity in crystallite width(as indicated by the inset arrows)due to silica absorption
3.4 FT-IR spectra for the identi fi cation of chemical bonds
The FT-IR spectra of Fe(hydr)oxides synthesized on quartz grains is shown in Fig.10.A spectrum of pure quartz substrate was included as a means to identify any variance resulting from the coating by Fe(hydr)oxide.The IR bands at 789 and 780 cm-1present in both the pure quartz and Fe-oxide coated quartz grains denoted Si–O–Si inter tetrahedral bridging bonds in SiO2.The IR band at 889 cm-1,which is conspicuously absent in the pure quartz can be assigned to OH-deformation linked to Fe3+,suggesting unambiguously that goethite was coated on the quartz surface.This also suggested the formation of Si–O–Fe bond,which is characteristic of Fe–Si hydrous oxides and silicates(Gallup and Reiff 1991).Si–O-Fe bonds in Fe–Si hydrous oxides and silicates indicated the spatial absorption and bonding of the Fe cations to the quartz siloxanes to form ferrisilcates.The broadening peak at 1347 cm-1for the Fe-oxide coated quartz grains was assigned to Fe–O as Si cage.In addition,the IR band at 1084 cm-1in the spectra of pure quartz(qFe0)suggested Si–O stretching.
The appearance of the peak at 1080 cm-1may also indicate enhanced asymmetric stretching of SiO4(Xu and Axe 2005).The symmetrical peak at 1635 cm-1for the Feoxide coated quartz grain was assigned to H–O–H bending of water(OH deformation of water).The considerable shift from 1080 cm-1to asymmetric Si–O–Si stretching band at 1100 cm-1and symmetric peak at Si–O–Si band at 1635 cm-1indicated increasing lattice substitution of trivalent Fe3+.This is consistent with similar research that reported that the substitution of Fe3+for Si4+caused a substantial shift of the Si–O–Si stretching band(1080 cm-1)to lower frequencies(Nowok et al.2001;Schwertmann and Thalmann 1976).H–O–H stretching bonds and H–O–H bending bonds of absorbed water presented at approximately 3377 cm-1of the synthesized Feoxides,which was absent in the pure quartz sample.The peak at 1437 cm-1for qFe20 sample(Fig.10),which denoted Fe–O as Sicages,furthersupported the chemisorption of iron oxides on quartz.This peak was absent in the other series.
3.5 Quartz dissolution in the presence of iron oxides
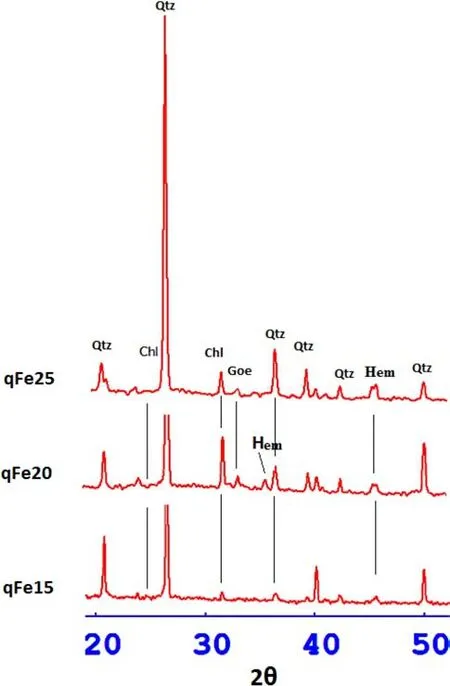
Fig.9 X-ray diffraction spectra of Fe(hydr)oxides synthesized on quartz substrates at 60°C,indicating the presence of goethite and hematite(Chl chloride,Qtz quartz,Hem hematite,Goe goethite)
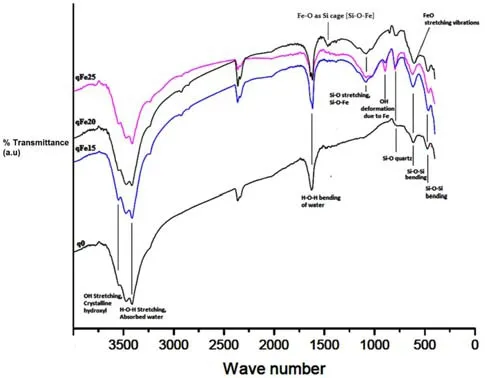
Fig.10 Stacked FT-IR spectra of pure quartz(qFe0)and Fe-oxide coated quartz grains(qfe)showing disparity in characteristic peaks
The change in pH with time as Fe-(hydr)oxides were synthesized on quartz is observed in Table 1.The pH was monitored daily for 60 days to detect any deviations.After 20 days of ageing,the silica concentration of the solvent was measured.The pH set at 12 remained relatively steady for the ageing duration,whereas the solution with pH set at 10 declined to 8.5.The parameters of the ageing experiment is outlined in Table 1.The silica concentrations for qFe0,qFe5,qFe10,qFe15,qFe20 and qFe25 were 36.9,10.9,30.5,114.3,26.2 and 163.5 mg/L,respectively.The amounts of dissolved silica was observed to increase with increasing iron content.
The dissolution or chemical disintegration of quartz is mostly pH dependent.To fully elucidate quartz—Fe-(hydr)oxide interactions,quartz dissolution experiments were conducted in the presence of iron.Silica concentration was measured after the agitation of quartz grains for 60 h in NaOH solutions containing Fe ions.The silica dissolution rates in the presence variable iron concentrations were directly calculated from the experimental data and plotted against time,as shown in Fig.11.The silica concentration for qFe5–qFe25 varied from 6.7 to 4.9 mg/L in the fi rst 10 h and steadily increased for the next 20 h,and then gradually declined for the last 20 h(Fig.11).Equilibrium(steady state)was attained after 60 h of dissolution.In contrast,high silica value(36.5 mg/L)was recorded for NaOH solution containing no Fe ions for the initial 20 h,but gradually decreased to 4.7 mg/L after 30 h of dissolution.
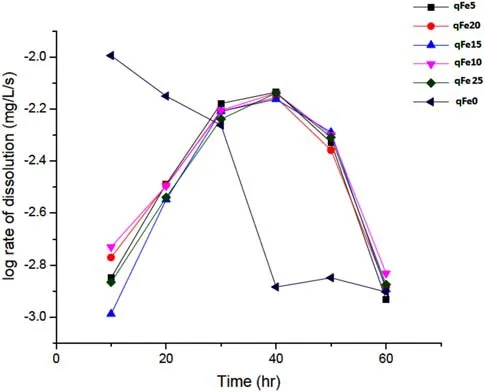
Fig.11 Plot of log rate of quartz dissolution(mg/L/s)with time
Table 1 and Fig.11 indicates quartz dissolution varies in tandem with pH and Fe content,although in a nonsequential pattern.The variation in pH with time can be explained by the following mechanism.The trivalent compounds(ferric chloride)basically hydrolyze in water solution and ionize to iron(ferric)and chloride ions;the water ionizes to hydrogen and hydroxide ions,making the solution strongly acidic.Afterwards,the ferric ions partially combine with the hydroxide ions released from the dissociation of water to form ferric hydroxide,a compound that is only slightly soluble and precipitates from the solution as a brown solid.This process is catalyzed by heating up the solution and adding a base.As a base was added to the FeCl3solution,the OH-ions depleted and neutralized the available H+ions to form water,causing the pH value to rise until the pH of the base being added was attained.The further addition of a base had little effect,with the pH remaining relatively constant based on the excess amount of OH-in the system.Within this range,OH-became constantly absorbed by Fe cations overtime to form oxyhydroxide species.Thus,the OH-ions were neutralized by the trivalent and H+ions,leading to the decline in pH.However,the pH of the null sample of only pure quartz remained constant at 12.5,possibly due to the absence of neutralizing agents.
4 Discussion
The formation of Fe-(hydr)oxides is generally dependent on precursor materials and conditions of synthesis(Schwertmann and Taylor 1977;Lewis and Schwertmann 1979).In this study,goethite crystals were formed in solution from dissolved Fe3+ions,while hematite was synthesized by means of internal dehydration and reordering within the goethite aggregates.Thermodynamics may indicate that goethite and hematite are the most stable Fe oxides,but chemicalequilibriumisfrequentlyimpededbyslowkinetics(Van Ranst et al.2016).The synthesis of hematite via dehydration of goethite can be attributed to its relatively higher magnetic ordering temperature and magnetic hyperf i ne fi eld(Murad2010).Althoughbothgoethiteandsilicaare negatively charged,suggesting electrostatic repulsion,the plasteringofgoethiteonquartzsurfaceindicateelectrostatic,van der Waals,and/or chemical bonding.At low silica concentrations and pH 10,the initial goethite is transformed by dissolution and re-precipitation to hematite and large pseudohexagonal crystals of twinned goethite by internal rearrangement and crystal lattice displacement,Thus,the morphology of hematite synthesized from ferrihydrite is in fl uenced by the presence o.In contrast,the higher silica concentrations at pH 12 and 60°C suppressed the transformation of ferrihydrite to hematite.
Therefore,the pH and presence of silica in solution in fl uence the synthesis of goethite since silicate anions typically coexist with Fe-(hydr)oxides and retard or inhibit the transformation of these minerals to more crystalline products(Schwertmann 1985;Schwertmann and Taylor 1989;Vempati et al.1990).The silicate adsorption appears to have a distinct effect on the adsorption of other anions,or,the displacement of the limiting pH for anion adsorption to lower values,but it has little effect on cation adsorption(Taylor 1995;Pham et al.2012).At pH above 10,the adsorption of silica is slow in comparison with many ionic adsorption processes,and it may take several weeks to approach equilibrium at ambient temperature.At pH below 10,there is a relatively lower level of silica in the system,which can strongly in fl uence the course of iron oxide interconversion reactions,by inhibiting the nucleation of product phases.
The role of silica in limiting the growth of Fe-(hydr)oxides extends beyond a surface phenomenon as indicated by Eggleton and Fitzpatrick(1988).The substitution of Fe3+with tetrahedral Si introduces lattice distortions and crystal expansion due to variable Fe:O:H ratio[considering the composition of goethite crystals having an internal stoichiometry FeO(OH),with an OH surface].The interaction between the dissolved silica and goethite nanocrystallites alters the intrinsic crystal structure of goethite,thus inducing morphological changes.The leached silica does not bridge related silanol groups in the presence ofFe-(hydr)oxide butinstead differentially absorbs into the lattice structure of the Fe(hydr)oxides,thus spatially modifying the goethite minerals(Fig.8).Therefore,in a way,the Fe-(hydr)oxides do affect quartz by creating a micro environment for the incorporation of silica.The mobilization of Fe generally occurs via reductive reactions.As the reduction condition transforms to oxidation with a drop in pH,the Fe may become immobilized(Krauskopf and Bird 1995).The few nuclei of goethite that form grow gradually to large crystalline structures.The silicate ions are taken up by the iron oxides via a ligand-exchange mechanism between silicate coordinates and iron oxide precursor-ferrihydrite(Cornell and Schwertmann 2003),while Dzombak and Morel(1990)earlier asserted that the mechanism most likely involves ligand exchange in which hydroxyl radical surface groups are replaced by silicates as illustrated in Eqs.(1)(2)and(3)shown below:
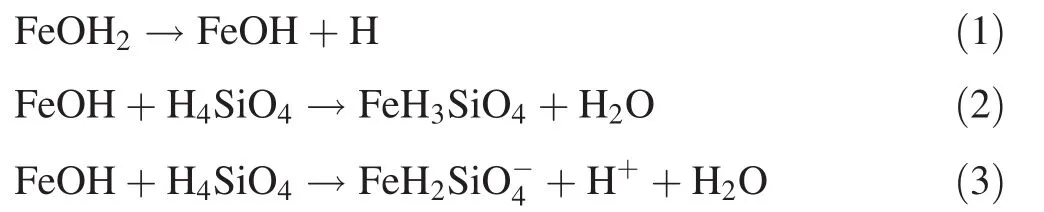
On the other hand,for steric reasons,Si4+should not replace structural Fe3+in the Fe-(hydr)oxides.However,in a reverse mechanism,tetravalent Si4+can be replaced by the trivalent Fe3+at speci fi c locations,leaving uncompensated charges in the structure(Kronenberg 1994).To maintain electrical balance,monovalent cations such as H+,Na+,and/or K+enter the quartz structure in interstitial spaces,not at original Si-locations.The differential lattice spacing suggests expansion of the lattice structure due to the incorporation of silica.The different crystallite spacing values indicate the preferential sorption of silica,which in turn spatially modi fi es the goethite minerals.Silica are adsorbed by ligand exchange onto the goethite surfaces,as evidenced by the infrared spectroscopy for the formation of unidentate surface complexes of the=Fe-OSi(OH)3type at 889 cm-1.
5 Conclusion
The pH parameter was shown to be the key component in the formation of Fe-(hydr)oxides.Quartz also acted as a media for the precipitation of Fe-(hydr)oxides.Goethite was the only Fe-(hydr)oxide formed at pH 12,while hematite and twinned goethite were synthesized at pH 10.The dissolved silica favored the formation of hematite and twinned goethite.The Fe-(hydr)oxides formed discrete mineral coatings on the quartz surface and aggregated in surface defects such as edges,pits,and fracture sites.Vibrationalcharacteristicsindicated bonding between synthesized Fe-(hydr)oxides and dissolved silica as well as expansion in the lattice spaces of goethite due to incorporation of silica.Based on the TEM images and FT-IR analysis,alkaline media and relatively low dissolved silica in the system can promote crystallization of goethite and in fl uence the direction of its aggregation as well as induce transformation to twinned goethite.
AcknowledgementsThis research was supported by the graduate assistantship scheme(GA)from Universiti Teknologi Petronas and partly from the research fund awarded to Associate Professor Eswaran Padmanabhan.
杂志排行
Acta Geochimica的其它文章
- The effects of clay minerals and organic matter on nanoscale pores in Lower Paleozoic shale gas reservoirs,Guizhou,China
- Age and geochemistry of Early Ordovician A-type granites in the Northeastern Songnen Block,NE China
- Origin and genetic family of Huhehu oil in the Hailar Basin,northeast China
- Genesis of fahlore in the Tianbaoshan lead–zinc deposit,Sichuan Province,China:a scanning electron microscopy–energy dispersive spectroscopy study
- The morphological characteristics of gully systems and watersheds in Dry-Hot Valley,SW China
- State of rare earth elements in the rare earth deposits of Northwest Guizhou,China
