单层镍锰层状双金属氢氧化物用于锂-氧电池双功能催化剂
2018-10-12侯雪丹郭守武王晓飞
侯雪丹 郭守武*, 王 倩 王晓飞*,
(1陕西科技大学材料科学与工程学院,西安 710021)
(2西安工业大学材料与化工学院,西安 710021)
0 Introduction
Lithium-oxygen (Li-O2)batteries have captured significant scientific interest due to their ultrahigh theoretical energy density of ~3 505 Wh·kg-1[1-5].However,the current Li-O2batteries suffer from sluggish kinetics of oxygen reduction reaction(ORR)and oxygen evolution reaction (OER)during the discharge/charge processes.Developing low cost and high-efficient air cathode is one of the effective methods to improve the electrochemical performance of Li-O2batteries[6-9].
In themajority of cases,carbon material such as carbon nanotube (CNT),reduced graphene oxide and carbon aerogels have been widely employed as cathode for Li-O2battery due to their high conductivity,light weight,especially for CNT,which possess unique features such as high aspect ratio,low cost and abundant pore structure[10].Although CNT-based air cathode have been proved to be feasible cathode for Li-O2battery,bare CNT usually suffers from high overpotential and low capacity due to the low ability in promoting the formation/decomposition of ideal discharge product-Li2O2,as a remedy,combining or depositing high-efficient electrocatalystswith excellent ORR and OER activity on CNT is one of the efficiencyway to increase theoverall cathode activity[11].So far,various catalysts such as noble metals,transition metal oxides and perovskite oxides have been widely studied as ORR or OER catalysts[12-17].Although noble metals exhibit superior catalytic properties,the high cost and element scarcity limit their widespread application.Layered double hydroxides(LDHs),a class ofmulti-metal hydrotalcite-like compounds,have recently attracted extensive interest due to their considerable catalytic activities even superior to thatof noblemetals,the general formula of LDHs is[M2+1-xM3+x(OH)2]z+[An-]z/n·y H2O,where M2+,M3+and An-represent the divalent,trivalentmetal cations and charge compensating anions[18],because of the unique characteristics such as composition flexibility,ease of preparation,high redox activity and low cost,making LDHs as an ideal alternative to precious metals in Li-O2batteries[19-21].The unique properties mainly derives itsmetal-contained layered matrix,and especially transition metal cations contained LDH materials,such as Ni,Co,Fe and Mn-containing LDHs,have exhibited excellent electrocatalytic activity,especially for OER[22].Unfortunately,the poor electronic conductivity and large particle size of the bulk LDHs are themajor problems to be overcome.Liquid phase exfoliation is a facilemethod to enhance the catalytic activity of bulk LDHs.The exfoliated single-layer nanosheets have the same chemical compositions but significantly higher OER activity,more exposed active sites and faster charge transfer compared to the bulk LDHs,and the exfoliated thin single-layer nanosheets also facilitate the improvement of the electronic conductivity[23-25],but the catalytic performance of the exfoliated single-layer LDHs has not been revealed in Li-O2batteries up to now.
Herein,Ni3Mn-LDHswas prepared and exfoliated into single-layered LDHs by a facile liquid phase exfoliation,then incorporated with CNT via selfassemble method,the as-prepared Ni3Mn-LDHs/CNT composites demonstrated excellent electrochemical performance in Li-O2batteries.
1 Experimental
1.1 M aterials synthesis
Typically,NiCl2·6H2O (1.426 2 g),MnCl2·4H2O(0.395 8 g),and Hexamethylenetetramine(HMT,0.7 g)were dissolved in 60 mL deionized water and heated at 100℃for 12 h to form bulk Ni3Mn-LDHs.Then,0.1 g bulk Ni3Mn-LDHs were introduced into 50 mL formamide solution,magnetic stirring for 24 h and centrifuged under 1 500 r·min-1for 30 min to obtain single-layer Ni3Mn-LDHs colloidal suspension(positively charged LDHs).Negatively charged CNT was obtained by treatingCNT in H2SO4/HNO3solution(VH2SO4/VHNO3=3).The Ni3Mn-LDHs/CNT samplewas obtained through the following electrostatic self-assembly.50 mL single-layer Ni3Mn-LDHs colloidal suspension(1.2 mg·mL-1)was added dropwise into 50mL acidification CNT (1 mg·mL-1)under magnetic stirring over the course of 1 h.The the resulting suspension was then standing at room temperature for 24 h.The final product was isolated by centrifugation,washed with copious amounts of deionized water until the pH value is about 7,and were placed in a vacuum oven and dried at 80℃for 24 h.
1.2 Characterization and electrochem ical measurement
The samples were characterized by X-ray diffraction (XRD,Rigaku D/Max2200 V/PC),with Cu Kα radiation(λ=0.154 06 nm)at a scan speed of 8°·min-1.The operating voltage was 40 kV and the currentwas 40 mA.The operating voltage of scanning electron microscopy (SEM,JEOL S4800)was 5 kV and transmission electron microscopy (TEM,FEI TecnaiG2 F20 S-TWIN).Swagelok-type Li-O2batteries containing a Li foil anode,a nickel foam cathode,a celgard 2500 membrane and 100μL electrolyte(0.9 mol·L-1lithium bis(trifluoromethanesulphonyl)imide(LiTFSI) in tetraethylene glycol dimethyl ether(TEGDME))was assembled inside an Ar-filled glove box.The air cathodes were prepared by mixing 90%(w/w)CNT or Ni3Mn-LDHs/CNT and 10% (w/w)polyvinylidene fluoride (PVDF)in a mortar.A few drops of N-methyl pyrrolidone(NMP)was added and ultrasound to form an ink.The ink was pasted onto nickel foam current collector(1.5 cm-2).Comparable air cathode (Ni3Mn-LDHs+CNT cathode)was also fabricated by coating a mixture of Ni3Mn-LDHs,CNT and PVDF in a conventional weight ratio of 3 ∶6∶1 on the nickel foam current collector (1.5 cm-2).These cathodeswere dried at 110℃under vacuum for 12 h.After standing 2 h,The galvanostatic tests were carried out using a Neware battery testing system with a potential rang of 2.0~4.5 V.The capacities and current densitieswere calculated based on themass of CNT (for the CNT and Ni3Mn-LDHs+CNT cathodes)or Ni3Mn-LDHs/CNT (for the Ni3Mn-LDHs/CNT cathode).The electrochemical impedance spectroscopy(EIS)was recorded using CHI660E electrochemical workstation in the frequency range from 100 kHz to 10 mHz.Cyclic voltammetry (CV)was also recorded using CHI660E electrochemical workstation at a scan rate of 0.5mV·s-1.
2 Results and discussio n
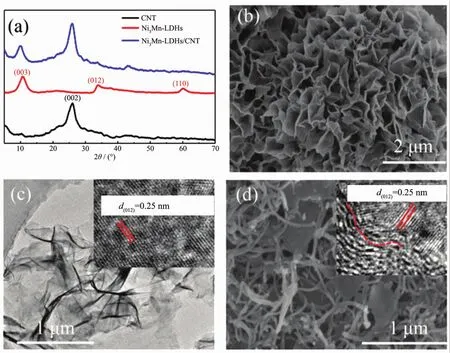
Fig.1 (a)XRD patterns of CNT,Ni3Mn-LDHs and Ni3Mn-LDHs/CNT;(b)SEM image of bulk Ni3Mn-LDHs;(c)TEM image of single-layer Ni3Mn-LDHs nanosheets;(d)SEM image of Ni3Mn-LDHs/CNT
Fig.1(a)shows the XRD patterns of the CNT,bulk Ni3Mn-LDHs and Ni3Mn-LDHs/CNT composites.The peaks at 2θvalues of 11.34°,34.41°and 59.98°corresponded to the (003),(012)and (110)crystal planes of Ni3Mn-LDHs,respectively[26].The peak at 26.5°and 43.5°indicated the present of CNT in the Ni3Mn-LDHs/CNT composites,however,only one significantdiffraction peak at11.34°was in accordance with the peaks of the bulk Ni3Mn-LDHs,maybe the exfoliated single-layer Ni3Mn-LDHs decreased the crystallinity of Ni3Mn-LDHs.From the SEM image in Fig.1(b)it could be found that the bulk Ni3Mn-LDHs nanosheets presented a flower-like structure,and the average diameter size was about 2.35 μm and thicknesswasapproximate 10 nm.However,only single nanosheets with an average lateral size of about 400 nm could be found after exfoliation,indicating that the exfoliation process destroyed its original structure,the HRTEM image insert in Fig.1(c)presents that the interplanar spacing is 0.25 nm,corresponding to the(012)plane of Ni3Mn-LDHs,as shown in Fig.1(c),indicating the delaminated small scale single-layer nanosheets maintained the unique structure of bulk Ni3Mn-LDHs.Fig.1(d)shows the SEM image of the obtained composites,it could be found that the singlelayer Ni3Mn-LDHsmixed uniformly with CNT,which ensure the maximum contact of Ni3Mn-LDHs with CNT and lead to a fast electron transport during the discharge-charge processes,from the HRTEM image insert in Fig.1(d)it could be found that there were slightly lattice distortions at the interfaces,facilitating to create more active sites and promote the catalytic performance[27-28].
Fig.2(a)shows the first discharge-charge curves of Li-O2batteries with CNT,Ni3Mn-LDHs+CNT and Ni3Mn-LDHs/CNT cathode cathodes at 100 mA·g-1,the discharge capacities of the CNT,Ni3Mn-LDHs+CNT and Ni3Mn-LDHs/CNT cathodewere 1 449,1 845 and 3 476mAh·g-1,respectively,the higher discharge capacity of the Ni3Mn-LDHs/CNT and Ni3Mn-LDHs+CNT electrodes than the bare CNT electrode indicated that the introduction of Ni3Mn-LDHs catalyst into air cathode could enhanced the discharge capacity,enhancing the ORR process.More importantly,compared with the Ni3Mn-LDHs+CNT electrode,it was interesting to find that the discharge-charge voltage gap of the Ni3Mn-LDHs/CNT electrode was significantly lower and delivered higher discharge capacity,this probably due to themaximum contact of Ni3Mn-LDHs with CNT.Meanwhile,the results also indicated that the exfoliated Ni3Mn-LDHs enhanced OER/ORR catalytic activity although they have the same chemical structure as the bulk LDHs,and the increased defects that derived from the lattice distortion provided more active sites and facilitating the deposition of the discharge products.
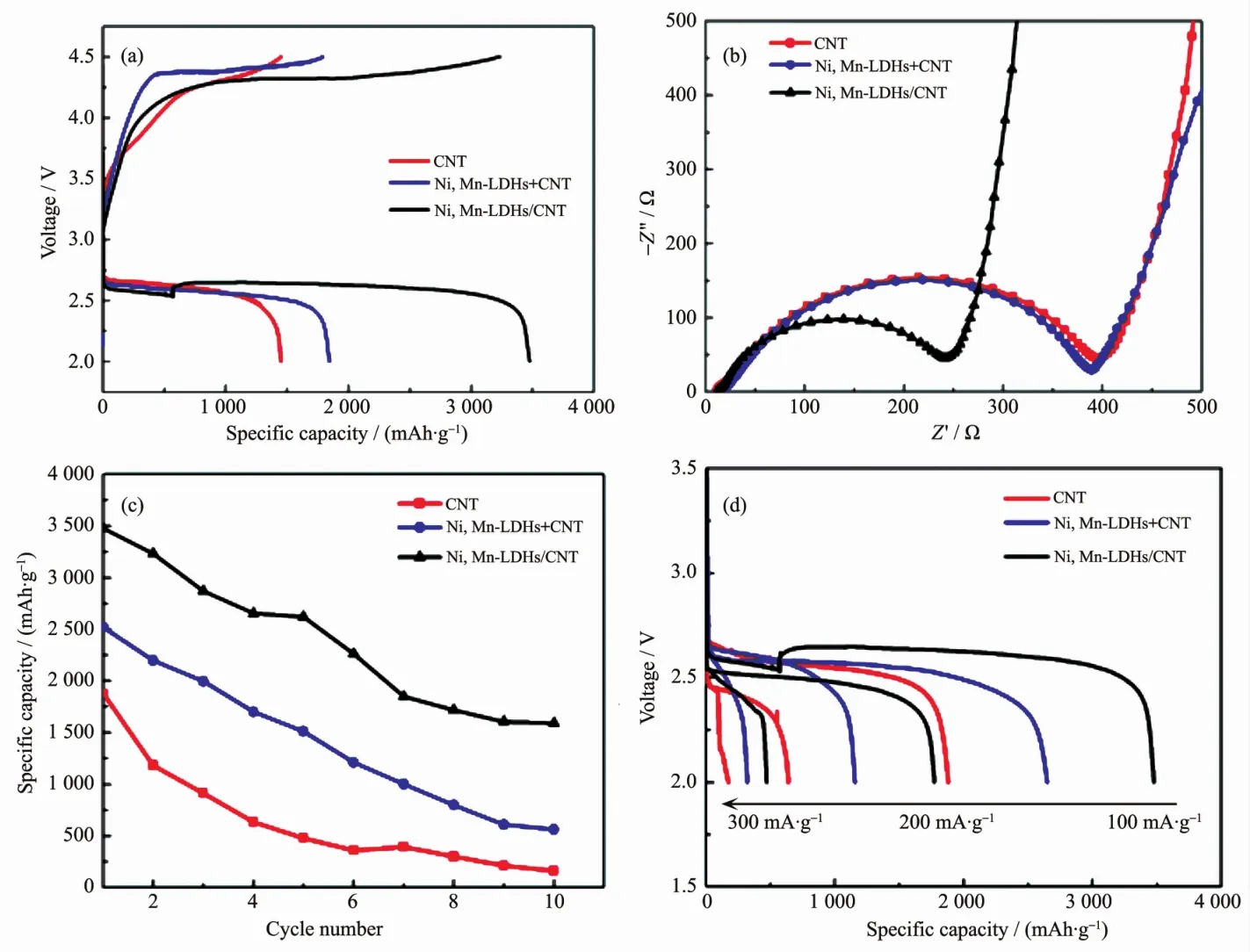
Fig.2 (a)First discharge-charge curves,(b)EIS spectra atOCV,(c)cycle ability and(d)rate performance of the CNT,Ni3Mn-LDHs+CNT and Ni3Mn-LDHs/CNT electrodes
Further information could be achieved from EIS tests at open circuit voltage(OCV),as shown in Fig.2(b),although the three cathodes have the same interfacial electron transfer in the highfrequency region,which is related to the contact between the air cathode and the electrolyte,the charge transfer resistance (Rct)which is related to the charge transfer reaction were obviously different,the Ni3Mn-LDHs/CNT cathode exhibited lowest Rctthan that of the Ni3Mn-LDHs+CNT and CNT electrodes,indicating the fastest kinetics of ORR[29].In other words,the high catalytic active of the Ni3Mn-LDHs/CNT cathode facilitated the nucleation of Li2O2[30-31].Based on above results,we further compared the rate and cycling performance with the bare CNT to reveal the excellent OER/ORR activities of Ni3Mn-LDHs/CNT cathode.Fig.2(c,d)shows the corresponding full dischargecharge cycling stability at100mA·g-1and rate performance,respectively,the 10th discharge capacity of the Ni3Mn-LDHs/CNT cathode decayed to 45.7%,the Ni3Mn-LDHs+CNT cathode decayed to 22.4%and the CNT cathode decay to 8.5%of the first discharge capacity.Although the specific capacity decreased upon increasing the current density for all cathodes,the capacity of the Ni3Mn-LDHs/CNT cathode was higher than that of the Ni3Mn-LDHs+CNT cathode and the CNT cathode,the capacity retentions of the Ni3Mn-LDHs/CNT cathode at 200 and 300 mA·g-1were 51.1% and 13.3%,respectively,while the corresponding Ni3Mn-LDHs+CNT cathode exhibited low capacity retentions of about 43.6%and 27.7%,respectively,and CNT cathode only exhibited an low capacity retentions of about 34.1% and 9.1%,respectively,further conforming the enhanced reaction kinetics of the Ni3Mn-LDHs/CNT cathode.
The enhanced ORR catalysis of the Ni3Mn-LDH could be further confirmed from the CV curves,as shown in Fig.3(a),for the ORR process,itwas clearly to find that the peak current of the Ni3Mn-LDHs/CNT cathode ismuch higher than that of the Ni3Mn-LDHs+CNT cathode and CNT cathode,meanwhile,only one cathodic peak is observed for all cathodes,suggesting the formation of one kind of discharge product(Li2O2)for all cathodes.And the Ni3Mn-LDHs/CNT cathode also exhibited slightly higher peak current during the following OER process,indicating the improved OER catalytic activity.From the SEM analysis in Fig.3(b~d),it could be found that all first discharged cathodes exhibited film-like Li2O2,however,the first discharged of CNT cathode contained many large and compact particles while the first discharged of Ni3Mn-LDHs/CNT and Ni3Mn-LDHs+CNT cathode cathode revealed a relative smaller and looser surface than that of the discharged CNT cathode.Based on the high discharge capacity,the improved ORR/OER processes and the different SEM images of the Ni3Mn-LDHs/CNT cathode,we have reason to believe that the introduced Ni3Mn-LDHs/CNT caused the improvement of the electrochemical performance of the Li-O2battery.
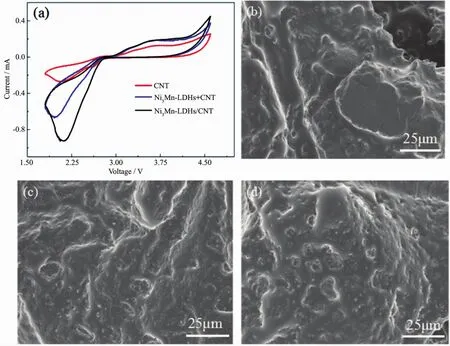
Fig.3 (a)CV curves of the CNT,Ni3Mn-LDHs+CNT and Ni3Mn-LDHs/CNT cathodes,SEM images of the first discharged of CNT(b),Ni3Mn-LDHs+CNT(c)and Ni3Mn-LDHs/CNT(d)electrodes
Commonly,capacity-limiting cycle is another widely used test to evaluate the cyclability ofa specific cathode material,and the cycling performances were conducted with a limited specific capacity of 500mAh·g-1at a current density of 100 mA·g-1.however,the CNT cathode and Ni3Mn-LDHs+CNT cathode rapidly failed within 20 cycles even using the capacitylimiting cycling protocol,as shown in Fig.4(a~d),and the charge potential almostmaintained the same with that of the full discharge/charge CNT cathode and Ni3Mn-LDHs+CNT cathode.When the Ni3Mn-LDHs/CNT cathode is cycled with a limited capacity output of 500 mAh·g-1,no capacity loss could be found over 30 cycles,and the 30th charge potential was even lower than thatof the first charge potential of the CNT cathode,as shown in Fig.4(e~f),meanwhile,it is clear to find that the first charge potential dropped to 3.5 V,which was about 750 mV lower than the charge potential under full discharge/charge conditions,this phenomenon was also observed in our previous research because a slight amount of the discharge product could be easily decomposed[32].Based on the above results,it is speculated that excellent performance of Ni3Mn-LDHs/CNT can be attributed to the following reasons:Firstly,the exfoliated single-layer Ni3Mn-LDHs nanosheets exposed more active sites and enhance the catalytic activity;Secondly,the incorporation of Ni3Mn-LDHs with CNT induced the generation of lattice distortions,the altered surface state of the composite facilitated the exposure of defects and enhanced the adsorption and dissociation of oxygen[33].In other words,the unique structure of Ni3Mn-LDHs/CNT composites promoted the deposition and decomposition of the discharge products.
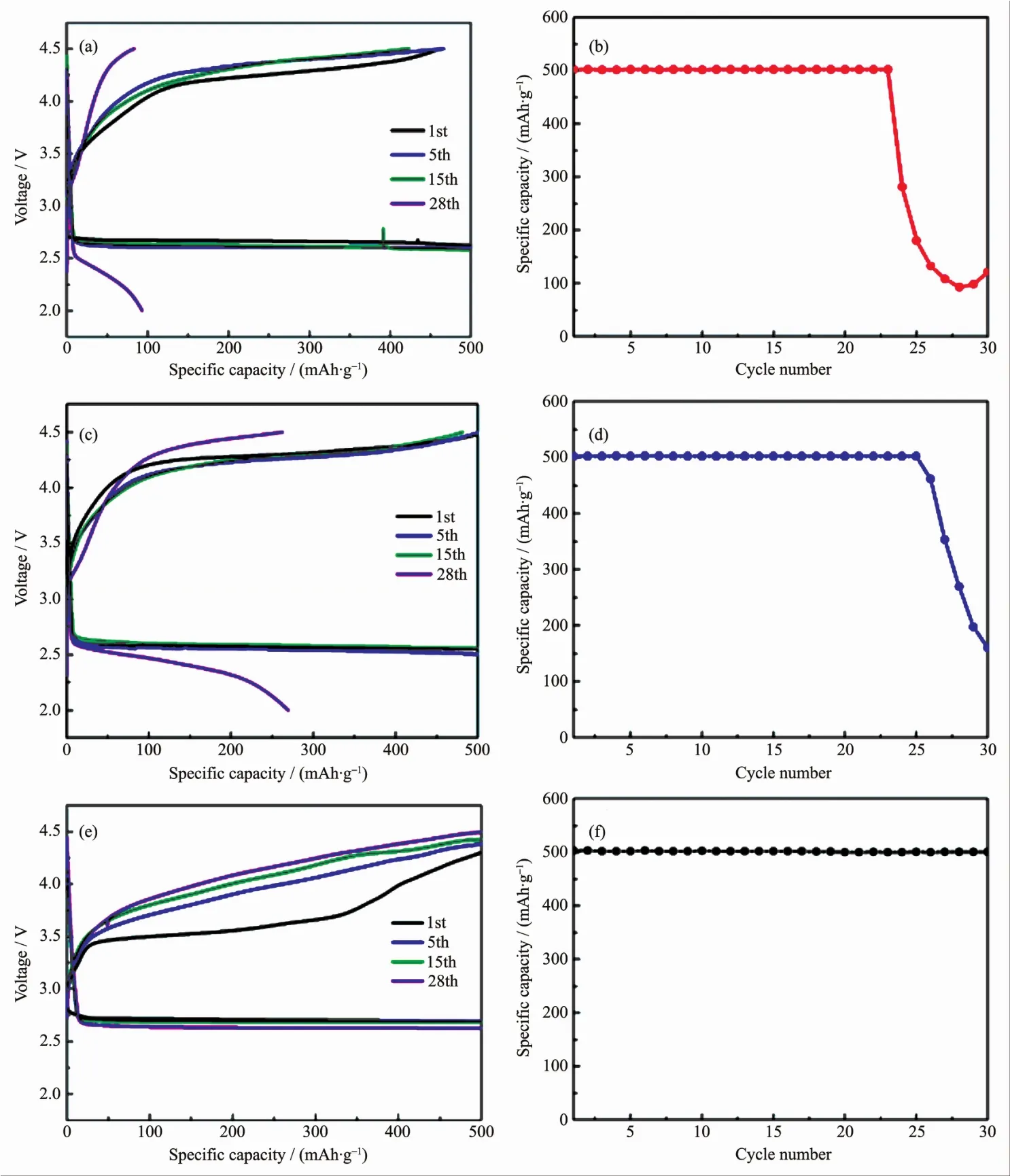
Fig.4 Discharge-charge curves and cycling performances of the(a,b)CNT,(c,d)Ni3Mn-LDHs+CNT and(e,f)Ni3Mn-LDHs/CNT electrodes
3 Conclusions
In this work,bulk Ni3Mn-LDHs was exfoliated into single-layer nanosheets and then incorporated with CNT via a self-assemble method.The formed composites facilitated the ORR/OER kinetics of the cathode and thus significantly improve the discharge capacity,rate and cycling performances of the Li-O2batteries.The facile and synthetic strategy can be easily extended to other LDHs-based composites.
