席夫碱类三明治型铽/镝金属配合物的合成、表征及磁性
2018-10-12杨立国郁有祝张永辉王大奇李大成
杨立国 王 芳 郁有祝 王 鑫 张永辉 杨 华 王大奇 李大成
(1安阳工学院化学与环境工程学院,安阳 455000)
(2聊城大学化学化工学院,聊城 252000)
0 Introduction
Single Molecule Magnets(SMMs),first emerging in a dodecametallic manganese-acetate(Mn12)with the slow relaxation ofmagnetization at low temperature[1-2],have attracted considerable attention due to the potential applications in high-density information storage[3],molecule-based spintronic devices[4],quantum computing devices[5]and refrigeration devices[6].Two important factors for the design of SMMs involve the large spin ground state(S)and uniaxial(negative)magnetic anisotropy (D),leading to an anisotropy energy barrier(Ueff)[7].With the large ground-state spin values,lots of polynunclear transition metal complexes have been synthesized as new SMMs to study the two ingredients(S and D)[8].But in recent years,the lanthanide ions such as Dyバ and Tbバ are widely studied due to their large intrinsic magnetic anisotropy which increase the D values for the complexes resulting in higher energy barriers compared to SMMs with 3d metals[9].So various lanthanide SMMs complexes have been synthesized,and one of the Dy SMMs has the largest effective energy barrier of 528 K in multinuclear SMMs[10].In addition,the ligand field as well as the coordination geometry strongly influences the local anisotropy of the lanthanide ion.That is to say,the interplay between the ligand field effect,the geometry,and the strength of themagnetic interaction between the lanthanide sites will govern the SMM behavior[11].Thus,it is necessary to study themagnetic interactions between the bridging ligand and the lanthanide ions.
Lots of organic ligands have been synthesized for discrete SMMs,for example,polyalcohols[10],carboxylic acid derivatives[12],oximate derivatives[13],and Schiffbase ligands[14].These polydentate ligands can mediate themagnetic interactions between themetallic centers.Recently,several sandwich-type complexeswith Schiffbase ligands have been reported[15],however,only one of them shows SMM behavior[16].Furthermore,to the best of our knowledge,the sandwich-type complex with Schiff base ligand N,N′-bis(4-methyloxysalicylidene)benzene-1,2-diamine(H2L)has not been reported.
In order to enlarge the structure database and further explore the magnetic interactions between the bridge group and lanthanide ions,two new tripledeckers sandwich complexes[M2L3(H2O)](M=Tb(1),Dy(2))with Schiff base ligand have been synthesized and characterized by X-ray single crystal diffraction.Complexes 1 and 2 show the antiferromagnetic couple.The slow relaxation and strong quantum tunneling of magnetization exist for 1 and 2.The deduced effective energy barrier(Ueff)and relaxation time(τ0)of 2 are 35.45 cm-1and 2.7×10-10s.
1 Experimental
1.1 Chem icals and instruments
The ligand H2L was prepared according to the published procedures[16].Allother reagents and solvents were used as received.
Elemental analyses were performed on an Elementar Vario MICRO CUBE elemental analyzer.Thermogravimetric analysis (TGA)experiments were carried out on a NETZSCH STA 449F3 thermal analyzer.IR spectrum was recorded as KBr discs on a Shimadzu IR-408 infrared spectrophotometer.Magnetic datawas collected onmagneticmeasurement system MPMS-XL 7.
1.2 Synthesis of[Tb2L3(H2O)](1)
Tb(OAc)3·6H2O(199.2 mg,0.2 mmol)and H2L(112.9mg,0.3mmol)weremixed inmethanol(10mL)in the presence of tetramethyl ammonium hydroxide.The solution was stirred for 5 h at room temperature and filtered.The filtrate was left undisturbed to allow slow evaporation of the solvent.Yellow single crystals suitable for X-ray diffraction were obtained after a week.Yield:36 mg,41.2% (based on Tb).IR(KBr cm-1):3 636(br),2 362(s),1 678(s),1 602(m),1 575(s),1 526(s),1 440(vs),1 381(s),1 357(w),1 310(m),1 234 (m),1 201 (m),1 116 (vw),1 034(vw),971(vw),831(w),786(vw),735(w),650(w),612(w).Anal.Calcd.for 1·DMF(C69H63Tb2O14N7,%):C,54.08;H,4.05;N,6.40.Found(%):C,54.11;H,4.09;N,6.35.
1.3 Synthesis of[Dy2L3(H2O)](2)
Complex 2 was obtained by following the procedure for 1 except that Dy(OAc)3·6H2O(199.9 mg,0.2mmol)was used instead of Tb(OAc)3·6H2O.Yellow single crystals suitable for X-ray diffraction were obtained after a week.Yield:30mg,40%(based on Dy).IR(KBr cm-1):3 652(br),2 989(s),1 602(s),1 578(s),1 524(vs),1 440(s),1 384(m),1 312(m),1 299(m),1 183(vw),1 116(vw),1 029(w),978(vw),831(w),755(w),739(w),659(w),605(w).Anal.Calcd.for[Dy2L3(H2O)]4·3DMF·7H2O(C273H259Dy8N27O62,%):C,52.75;H,4.17;N,6.09.Found(%):C,52.67;H,4.09;N,6.11.
1.4 X-ray crystallography
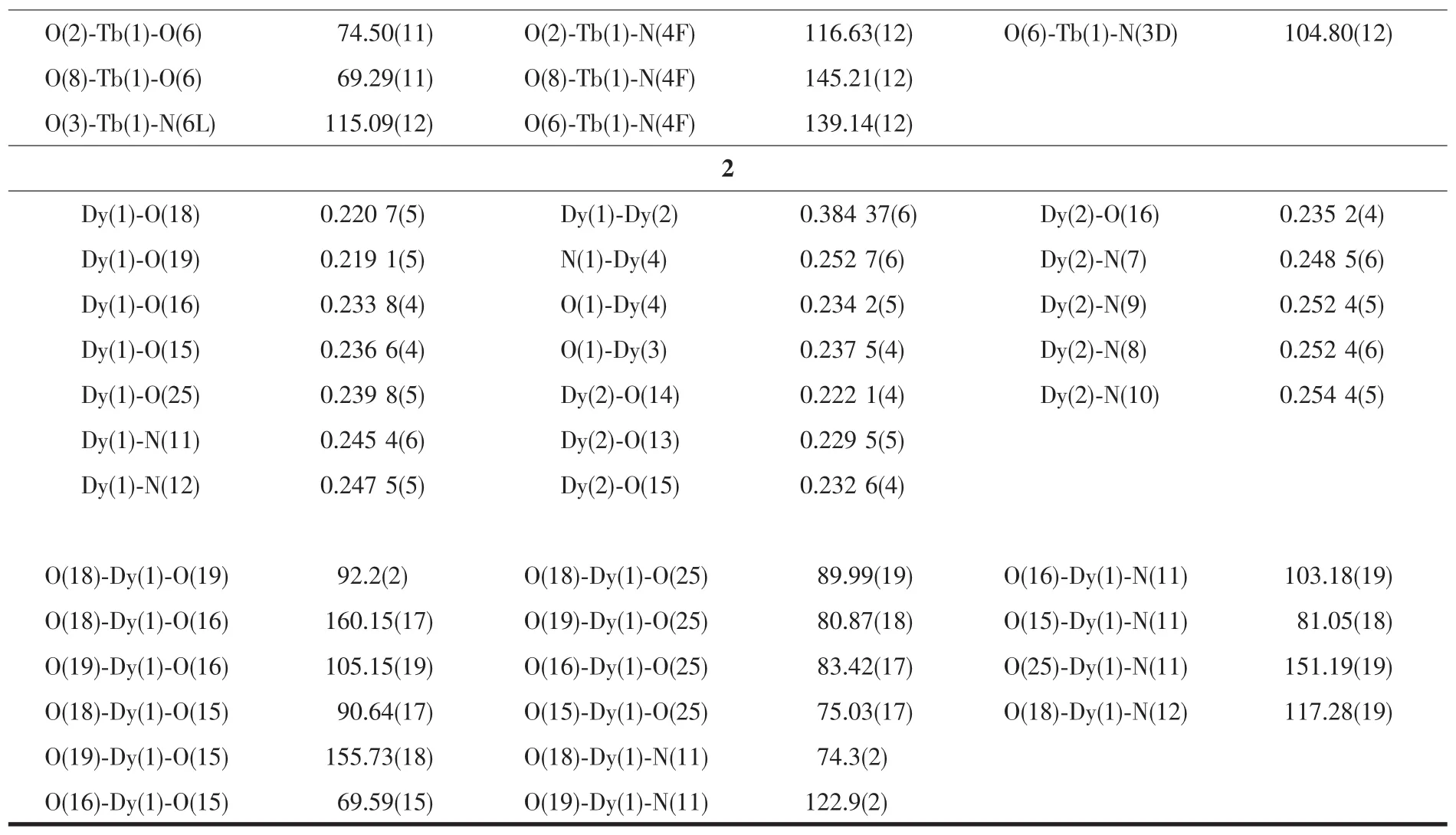
The crystal data were collected on an Oxford Diffraction Gemini E system with a Cu Kαsealed tube(λ=0.154 18 nm)at 296 K,using a ω scan mode with an increment of 0.3°.Preliminary unit cell parameters were obtained from 45 frames.Final unit cell parameters were derived by global refinements of reflections obtained from integration of all the frame data.The collected frames were integrated using the preliminary cell-orientation matrix.The SHELXL software was used for space group and structure determination,refinements,graphics,and structure reporting[17].There are some large residual peak in complex 2,and it is normal for the rare earth complexes.The crystallographic data and structural refinement parameters are provided in Table 1.Selected bond lengths and angles of complexes 1 and 2 are listed in Table 2.
CCDC:1818315 1;1818316,2.
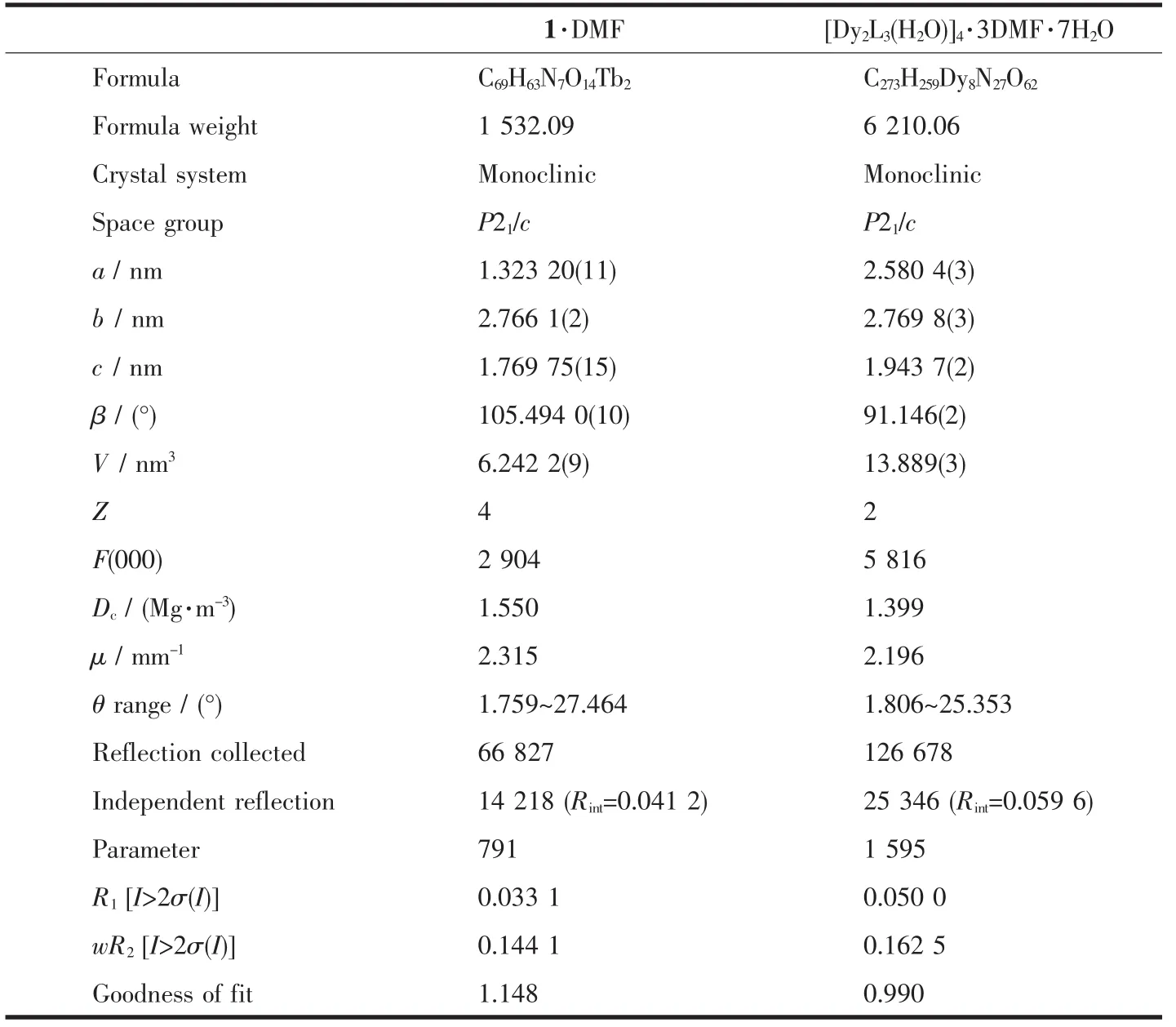
Table 1 Crystallographic data for com plexes 1 and 2
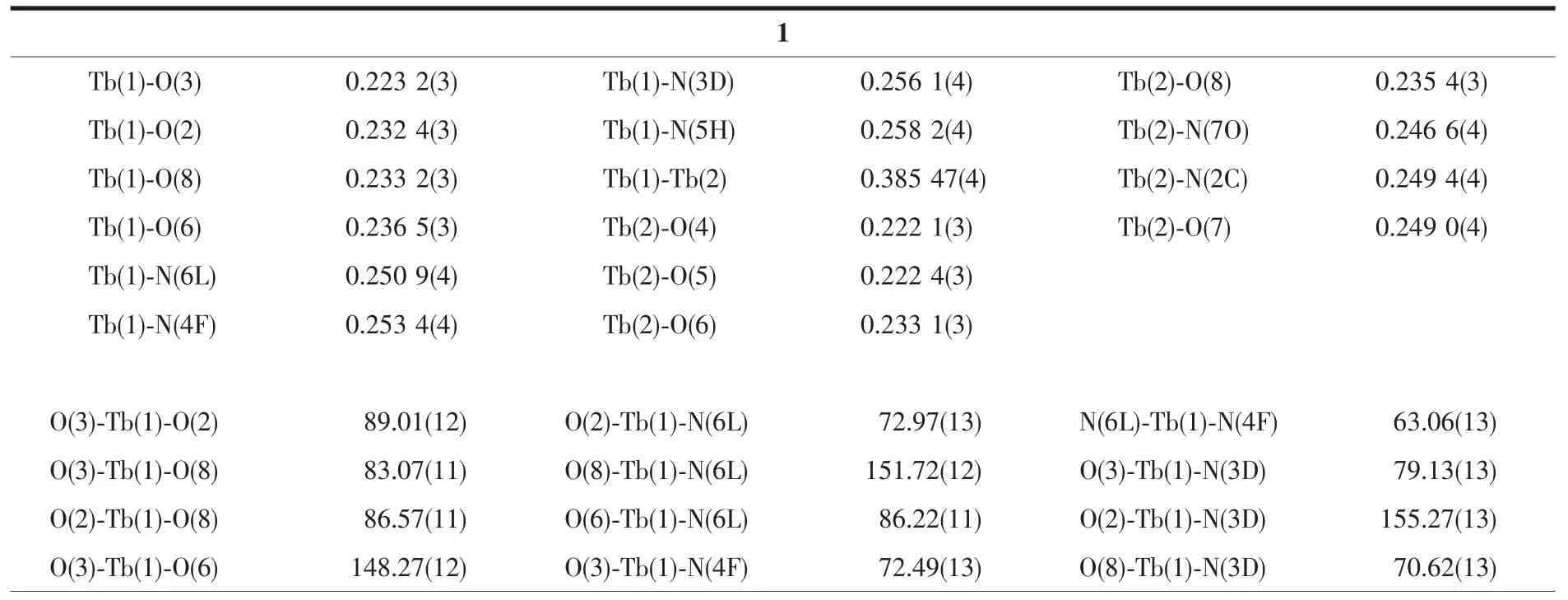
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
Continued Table 1
2 Results and discussion
2.1 Structure description
Complexes 1 and 2 crystallize in the monoclinic system with the similar structure. Herein, the molecular structure of complex 2 is described in detail as a representative example.As shown in Fig.1b,complex 2 is a triple-decker sandwich structure containing three Schiff base ligands,two Dyバions and one water molecule.One Dyバion is eightcoordinated and bridges two neighboring Schiff base ligands by four O atoms and four N atoms,while the other one is seven-coordinated by N2O2cavity of one outer Schiff base ligand,two oxygen atoms of the inner common Schiff base ligand,and one oxygen atom of additional water molecule.The average bond distancesofDy1-O and Dy1-N are 0.229 8 and 0.251 2 nm,respectively,which are similar to the corresponding distances of the reference reported[16].The average distances between Dy and the N2O2planes are 0.133 nm,which is almost consistwith the reference[16].The separation between the two intramolecular Dyバions amounts to 0.385 5 nm for 2,while the separation is 0.383 9 nm for 1,indicating the presence of intramolecular interionic magnetic interaction in terms of the metal-metal separation,which is almost consist with the similar complex reported[16].
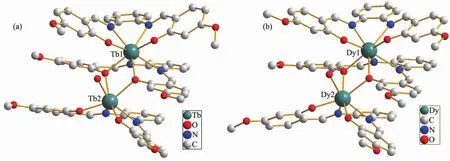
Fig.1 Molecular structures of 1(a)and 2(b)
2.3 Thermal stability
Thermal stability is an important aspect for the application of coordination complex.Thermogravimetric analysis(TGA)experiments were carried out to determine the thermal stabilities of 1 and 2(Fig.2).For complex 1,there are solventmolecules including DMF molecules and water molecules coordinated in the structure,and TG curve showing the first consecutive step of weight loss was observed in the range of 40~200 ℃,corresponding to the release of solventmolecules.Then,the continuously weight loss corresponds to the release of ligands (in the range of 180~800 ℃ ).For 2,TG curve showing the first consecutive step of weight loss was observed in the range of 40~200 ℃,corresponding to the release of solventmolecules.Then,the continuously weight loss corresponds to the release of ligands in the range of 300~600 ℃.

Fig.2 TG curves of complexes 1 and 2
2.4 M agnetic studies
The variable-temperature dc magnetic susceptibility measurement for complexes 1 and 2 were performed in the temperature range of 2~300 K under an applied magnetic field of 1 000 Oe.The collected data are plotted as χMT vs T in Fig.3.The χMT values of 1 and 2 are 24.15 and 28.06 cm3·K·mol-1at 300 K,respectively,which are consistentwith the expected values of 23.64 cm3·K·mol-1for two Tbバ ions(7F6,S=3,L=3,g=4/3)[18]and 28.34 cm3·K·mol-1for two Dyバ ions (6H15/2,S=5/2,L=5,g=4/3)[19].With the temperature decreasing,theχMT value of 1 almost keeps a constant until 50 K,and then drops rapidly to 8.62 cm3·K·mol-1at 2 K,and the χMT value of 2 decreases rapidly to 14.36 cm3·K·mol-1from 10 to 2 K,which ismostly due to the thermal depopulation of the Stark sublevels and/or significantmagnetic anisotropy in Tb3+/Dy3+ion systems.Compared to the complexes with similar structure without the radical contribution[20-21],it indicates the presence of antiferromagnetic coupling between the two Tb/Dy ions for 1 and 2,respectively.
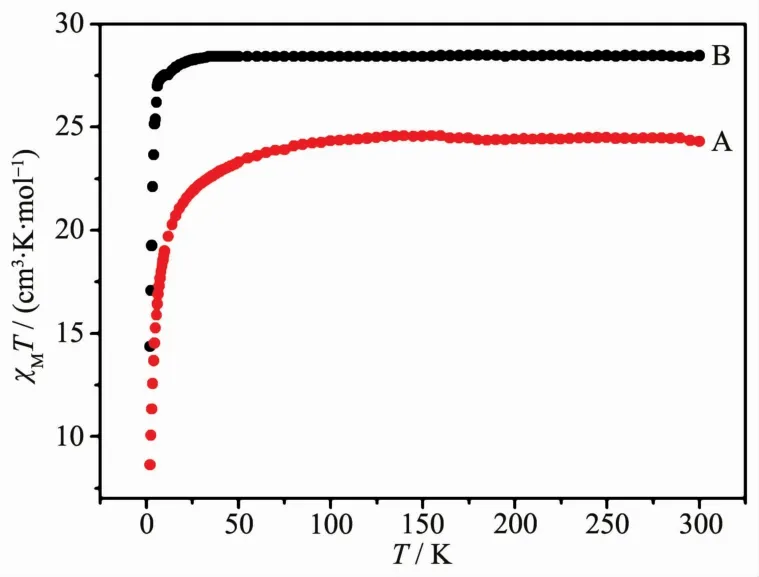
Fig.3 Temperature(T)dependence ofχM T for 1(A)and 2(B)at1 000 Oe
In addition, the magnetic-field-dependent magnetic properties of 1 and 2 have been studied.As shown in Fig.4,three observed non-superposition curves for 1 and 2 display a rapid increase at low field and eventually achieve the maximum value of 11.02μB~11.17μBfor 1 and 13.90μB~13.91μBfor 2 at 4 T,without reaching the theoretical magnetization saturation(18μBfor two Tbバ (7F6,S=3,L=3,g=3/2)ions and 20μBfor two Dyバ (6H15/2,S=5/2,L=5,g=4/3)ions,respectively),revealing the crystal-field effect on the Dyバ and Tbバ ions in the two triple-decker complexes.
To explore the dynamic magnetic property of the complexes,the temperature dependence of the alternating-current (ac)magnetic susceptibility of 1 and 2 under a zero direct-current(dc)magnetic field oscillating at 10~997 Hz is recorded in Fig.5~6.As can be seen,1 and 2 exhibit the frequency-dependent character in the out-of-phase (χ″)signals,indicating the slow relaxation ofmagnetization,which is generally attributed to the SMM nature of the two complexes.However,the frequency-dependent behavior was not observed in the in-phase(χ′)signals for 1 and 2.Furthermore,the peak was not observed in out-ofphase(χ″)signals for 1 and 2,which suggests the strong effect of quantum tunneling of magnetization(QTM).
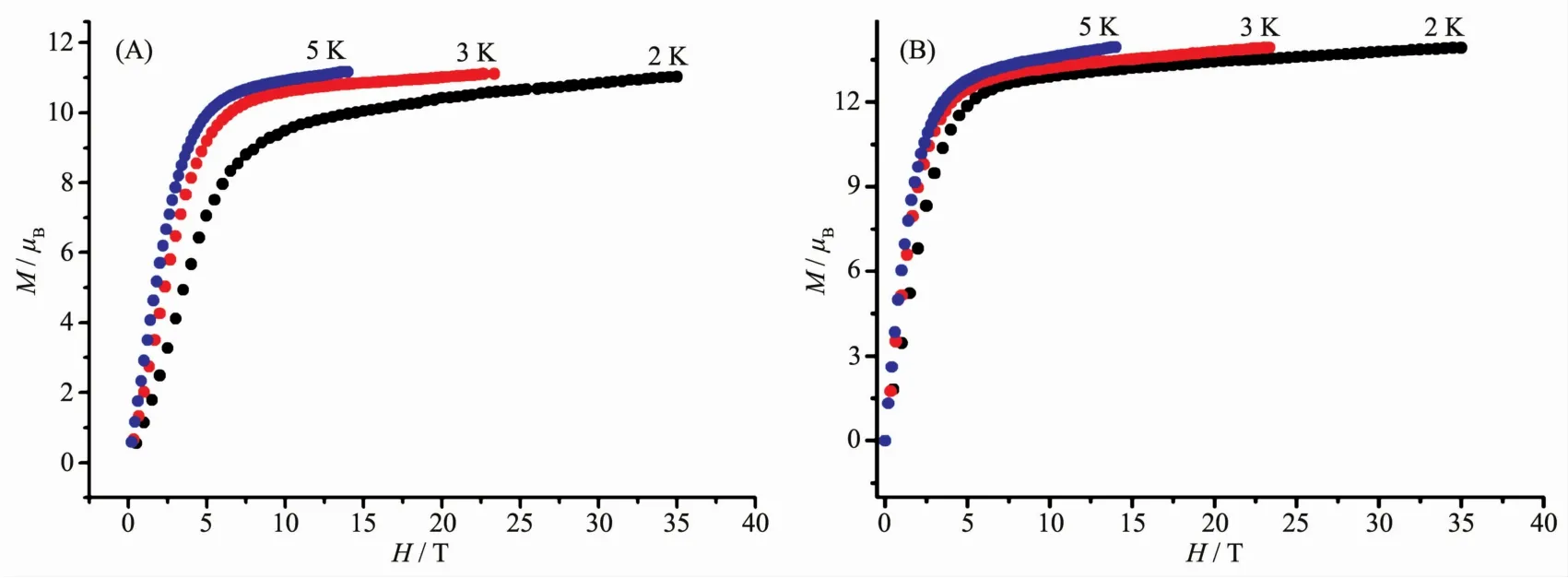
Fig.4 Plots of M vs H/T for 1(A)and 2(B)at different temperatures

Fig.5 Plots for temperature dependence of the in-phase(χ′)(A)and out-of-phase(χ″)(B)ac susceptibility of 1 under zero applied dcmagnetic field
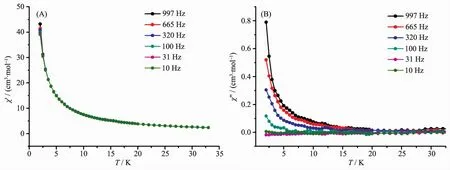
Fig.6 Plots for temperature dependence of the in-phase(χ′)(A)and out-of-phase(χ″)(B)ac susceptibility of 2 under zero applied dcmagnetic field
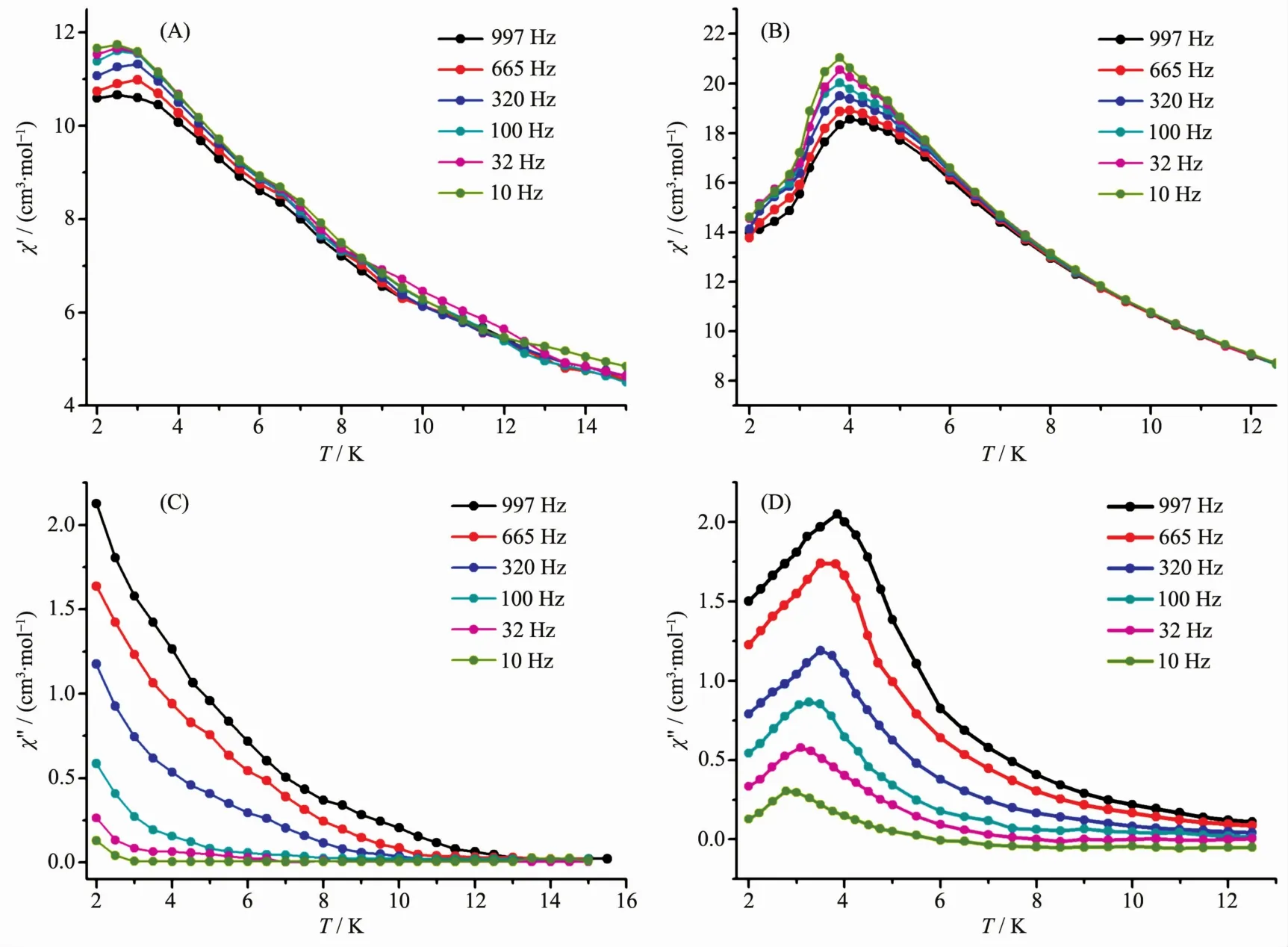
Fig.7 Plots for temperature dependence of the in-phase(χ′)and out-of-phase(χ″)ac susceptibility of 1(A,B)and 2(C,D)under 2 000 Oe applied dcmagnetic field
To reduce the effect of QTM,an optimal directcurrent field of 2 000 Oe was applied to the dynamic measurementover1 and 2.Asexpected,the frequencydependent character is still exist in both of the inphase( χ′)and out-of-phase( χ″)signals for 1 and 2,further confirming the SMM nature of the two complexes,as shown in Fig.7.In addition,the peak of the out-of-phase signal( χ″)for 2 could be observed until a frequency as low as 10 Hz,indicating the effective suppression of quantum tunneling of magnetization (QTM)under an applied 2 000 Oe magnetic field.On the basis of a thermally activated mechanism, τ=τ0exp[Ueff/(kT)] and τ=1/(2πν),the Arrhenius law fitting for the data under a 2 000 Oe magnetic field was carried out.As shown in Fig.8,a linear relationship exists between lnτand 1/T in the temperature range of 2.7~3.8 K for 2,which in turn result in a barrier Ueff=35.5 cm-1(51.0 K)and τ0=2.7×10-10s with R=0.98.However,the relaxation time and energy barrier of slow magnetic relaxation cannot be deduced for 1,most probably because of the stronger tunneling effect of complex 1 in comparison with 2.
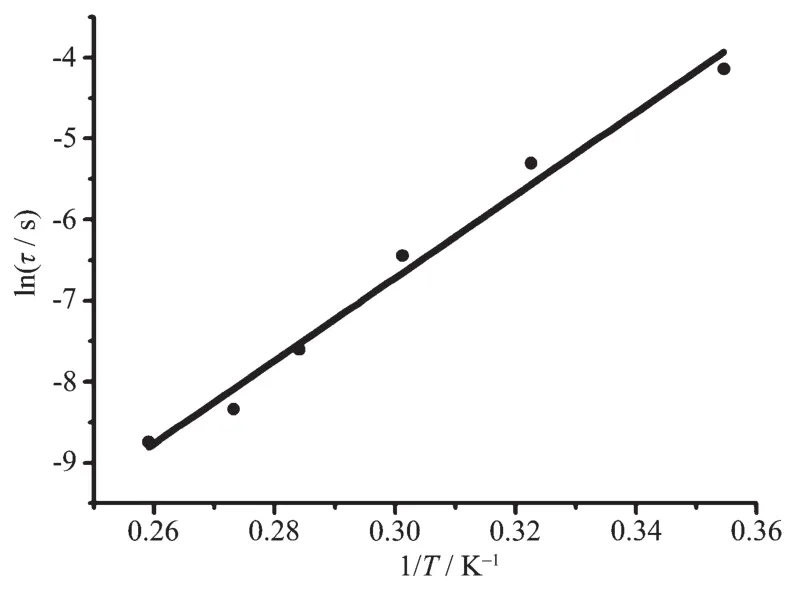
Fig.8 Plotof lnτvs 1/T for 2 under 2 000 Oe applied dc field
3 Conclusions
In summary,two new complexes[M2L3(H2O)](M=Tb(1),Dy(2))with Schiff-base ligands were synthesized and characterized by single crystal X-ray diffraction analysis.The structure analysis suggests the present of the magnetic interaction between lanthanide ions.The investigation ofmagnetic studies confirms the antiferromagnetic interactions between Ln ions.The frequency-dependent out-of-phase signals(χ″)demonstrated the SMM nature of1 and 2.We hope that the result is helpful for the study of themagnetic properties ofmultinuclear lanthanide based SMMs.
