金属铜配合物的合成、晶体结构、抗癌活性及与牛血清白蛋白的相互作用
2018-10-12孟祥高程功臻贾士芳
张 燕 孟祥高 蔡 苹 程功臻 贾士芳
(1太原科技大学化学与生物工程学院,太原 030024)
(2华中师范大学化学学院,武汉 430079)
(3武汉大学化学与分子科学学院,武汉 430072)
0 Introduction
Cancer is one of themajor causes of death in the world.Current clinical treatment of cancer is limited to surgery,radiotherapy,and chemotherapy.Although surgical resection and/or radiotherapy could cure early-stage tumor,most patients are diagnosed with advanced disease and distant metastases.In fact,chemotherapy is the main treatment of hematological andmetastatic tumor[1].Developmentofnew compounds with specific anticancer activity is a burning issue[2].During recent decades,metal-based antitumor drugs have been playing a relevant role in antiblastic chemotherapy.Among the different metal complexes,copper complexes have shown great potential and remain the subject of extensive drug discovery efforts[3-4].In addition,Schiff bases linked by diazo have regularly been studied as ligands in coordination chemistry as a result of their good metal binding ability.The azo linkage is responsible for the biological activity and lone pair electrons in nitrogen atom play chemical and biological role.The bidentate and multidentate Schiff bases with delocalized-orbitals are suitable ligands for themetals ofbiological importance,and the study of the chemical properties of such metal-Schiff base complexes also represents a good strategy for the design and synthesis of models of biological systems[5-11]. Several Cuギ Schiff base complexes show notable biological activities and pharmaceutical properties,such as DNA binding and cleavage[12-14], antioxidant[15], antimicrobial[16], antifungal[17],antibacterial[18],antidiabetic[19],antitumor,and anticancer properties[20-21].CuギSchiff base complexes,which are known as favorable options to cisplatin as drugs in cancer chemotherapy,can inhibit in vitro tumor cell growth.CuギSchiff base complexes affect the function of proteins and target nucleic acids,and are able to bind and cleave DNA which leads to cell cycle arrest and apoptosis.It is generally accepted that only free drug molecules can pass through the cellmembranes to be effective against the tumor[22-23].Thus,the anticancer activitymay be strongly affected by drug-protein interactions in the blood stream.Serum albumin is the major transport protein and is capable of binding many endogenous and exogenous drugs reversibly and itmay aid in selective delivery of these studied drugs to tumor region and facilitate drug access into the cell.For effective therapeutic monitoring,it is necessary to know the parameters of binding to serum albumin during its destabilization.Most recently,many studies on the drug-serum albumin using techniques such as fluorescence[24-27],FT-IR[28-30],CD spectroscopy[31-32]and NMR[33-34]were reported.Fluorescence served as a sensitive and convenient means indicating the alteration of the fluorophore environment which can provide plenty of useful information.
In this work,one four-coordinated Cuギcomplex with Schiff base ligand was synthesized and characterized by the elemental analysis and infrared spectroscopy.The inhibitory activities of the complex on cancer cells in vitro was studied.In addition,Cuギcomplex with bovine serum albumin (BSA)was investigated by fluorescence spectroscopy.On the basis of the spectroscopic data,the quenching constants and thermodynamic parameters,binding constants,the number of binding sites and binding distance were calculated.The obtained results may have important applications in drug delivery and drug design procedures.
1 Experimental
1.1 M aterials
Hela (human cervical carcinoma),SGC-7901(human gastric carcinoma),MCF-7 (human breast cancer)and HepG2 (human liver cancer)cell lines were purchased from the American Type Culture Collection.Bovine serum albumin(BSA)was obtained from Sigma,the BSA binding studies were carried on in 5 mmol Tris buffer(pH 7.4),containing 50 mmol NaCl.The stock solution of the Cuギcomplex was prepared in DMSO.The BSA solutions were used freshly after preparation and its concentration was determined spectrophotometrically,using a molar excitation coefficient of 43 800 L·mol-1·cm-1at 280 nm.4-aminoazobenzene,salicylaldehyde and Cu(OAc)2·H2O were obtained from Wuhan Shenshihuagong.All other chemicals were of analytical grade and double distilled waterwas used in all the studies.
1.2 Apparatus
Microanalysis(C,H,and N)was conducted with a Perkin-Elmer 240Q elemental analyzer.1H NMR spectroscopicmeasurements(300MHz)were performed on a Bruker AM-300 NMR spectrometer,using CDCl3as solvent and TMS(SiMe4)as an internal reference at 25℃.Infrared spectra were recorded on a Perkin-Elmer Spectrum using KBr pellets.All fluorescence spectra were measured using a Shimadzu RF-5301PC fluorophotometer with a quartz cell of 1 cm path length,both excitation and emission slits were 5 nm,and the excitation wavelength of BSA was at 280 nm.UV spectra measurements were performed on a Shimadzu 3100 spectrophotometer using a 1 cm cell at 1.0 nm intervals.
1.3 Synthesis
1.3.1 Synthesis of the ligand L
4-aminoazobenzene (0.50 g,2.5 mmol)was dissolved in ethanol (40 mL),salicylaldehyde(240 μL)was added dropwise to the solution.The mixture was stirred at 90℃for 8 h.After being cooled to room temperature,the solution was concentrated,and the solid was collected by filtration,washed thoroughly with water and dried in vacuum over P2O5.Then the crude product thus obtained was recrystallized from ethanol.2-(4-phenylazo-phenylimino)-phenol(L)was obtained as yellow-orange powder (Yield:85.5%).Elemental analysis Calcd.for C19H15N3O(%):C 75.75,H 4.98,N 13.95.Found(%):C 75.78,H 4.97,N 13.94.1H NMR(300 MHz,CDCl3,298 K):δ6.98(m,1H,Ha),7.04(m,2H,Hh,Hj),7.41(t,4H,He,Hg,Hi),7.51(m,2H,Hb),7.93(d,2H,Hd),8.00(d,2H,Hc),8.70(s,1H,Hf).
1.3.2 Synthesis of Copperギcomplex
A solution of Cu(OAc)2·H2O(0.100 g,0.5 mmol)in ethanol(20 mL)was added to a solution of the ligand L (0.301 g,1 mmol)in absolute ethanol(40 mL).The mixture was stirred at 80℃.The precipitated complex was filtered,washed with cold ethanol,and dried in vacuo over P4O10.The product was dissolved by dichloromethane in the tube,which was put in the glass jar,with ethyl ether in it.Ethyl ether was diffused into the tube,and then a week later,yellow acicular crystalswere obtained.Elemental analysis Calcd.for C38H28N6O2Cu(%):C 68.67,H 4.22,N 12.65.Found(%):C 68.44,H 4.27,N 12.54.FT-IR(KBr,cm-1):1 601,1 527,1 437,1 315,1 279ν(C=N,N=N,C=C),1 184,1 143 ν(Ph-O),842,744 ν(=C-H),670,597,547ν(C-H).

Scheme 1 Synthetic routes of the ligand and complex
1.4 Crystal structure determ ination
X-ray diffraction data for the crystal was collected with graphite-monochromatic Mo Kαradiation(λ=0.071 073 nm)on a Bruker Smart Apex Ⅱ CCD diffractometer,by the φ-ω scan technique at ambient temperature.Multi-scan absorption correction was applied to the data.The crystal structure was solved by direct methods and refined by full-matrix leastsquareson F2.All the non-hydrogen atomswere located in successive difference Fourier syntheses and then refined anisotropically.Hydrogen atoms were placed in the calculated positions or located from Fourier maps,and refined isotropically with isotropic vibration parameters related to the non-hydrogen atoms to which they are bonded.All calculationswere performed with the SHELXL-97[35]programs.The crystallographic datawere listed in Table 1.
CCDC:1567198.
1.5 Anticancer activity studies
Standard MTT assay procedures were used[36].Cellswere placed in 96-wellmicroassay culture plates(8×103cells per well)and grown overnight at 37 ℃ in a 5% (V/V)CO2incubator.The tested complex were then added to thewells to achieve final concentrations ranging from 1.56 to 25 μmol·L-1.Controlwells were prepared by addition of culture medium (200μL).The plates were incubated at 37℃in a 5%(V/V)CO2incubator for 48 h.On completion of the incubation,stock MTT dye solution(20 μL,5 mg·mL-1)was added to each well.After 4 h,150 mL dimethyl sulfoxide (DMSO)was added to solubilize the MTT formazan.The optical density of each well was then measured with a microplate spectrophotometer at a wavelength of490 nm.The IC50valueswere determined by plotting the percentage viability versus the concentration and reading off the concentration at which 50%of the cells remained viable relative to the control.Each experiment was repeated at least three times to obtain themean values.Four different tumor cell lines were the subjects of this study:MCF-7(human breast carcinoma),SGC-7901 (human gastric carcinoma),Hela (human cervical carcinoma)and HepG2(human liver carcinoma).

Table 1 Crystallographic data of the Cuギcomplex
1.6 Interaction w ith BSA
All fluorescence measurements were performed on a Shimadzu RF-5301PC spectrofluorometer with water bath in a 1 cm quartz cuvette.The fluorescence spectra were measured from 290 to 450 nm with an excitation wavelength of 280 nm[37].The excitation and emission slits were both set to 5 nm.The scanning speed was 200 nm·min-1.To measure the polarity of Trp environment upon binding of the Cuギcomplex to BSA, intrinsic fluorescence experiments were performed.The fixed concentration of BSA(5.0μmol·L-1)was titrated with various concentrations of each Cuギ complex (0~24.0 μmol·L-1).The solutions incubated for 5min,before the spectra were recorded.The BSA solution were freshly prepared just before performing the measurements and the observed fluorescence intensities corrected for the dilutions.All experiments were performed at three different temperatures(287,303 and 313 K)and themaximum fluorescence intensity (about 340 nm)was used in order to calculate the thermodynamic parameters.
2 Results and discussion
2.1 1H NMR and IR Spectra
The1H NMR spectrum of L showed seven sets of signals in the aromatic region,as illustrated in Fig.1.The signal assignment was rather straightforward by the comparison of chemical shiftswith those of similar Schiff-base ligands,and the resonances atδ6.98,7.51 and 8.00 were assigned to the Ha,Hband Hcprotons,respectively,of the benzene ring.The resonances atδ7.93 and 7.41 were assigned to the Hdand Heprotons,respectively,of the phenyl spacer.The resonances atδ7.04 and 7.41 were assigned to the Hg,HhHiand Hjprotons,respectively,of the salicylaldehyde ring.It′sworth noting that there was a singlet atδ8.70,which indicated formation of-C=N in the Schiff base.

Fig.1 1H NMR of ligand L in CDCl3
Infrared spectra of the Cuギcomplex does not display any band near 3 300 cm-1,which suggested that the-OH of salicylaldehyde is deprotonated in the complex.IR spectra shows two bands at 1 601 and 1 527 cm-1,which are assigned to the absorption of ν(C=N)and ν(N=N),respectively[38].The absorption bands in the ranges of 1 437,1 315 and 1 279 cm-1correspond to theν(C=C)of benzene ring.The values ofν(C-O)being 1 184 and 1 143 cm-1,842 and 744 cm-1belong to theδ(=C-H)of benzene ring.
2.2 Electronic absorption spectra
The absorption spectra of the ligand and complex were recorded in ethanol(Fig.2).Ligand L displayed three absorptions peaks at 296,309 and 350 nm.These absorption peaks are attributed to intraligand n→π*andπ→π*transitions.The absorption band of Cuギcomplex at 340 nm corresponds to intraligand transition ofπ-π*orbitals of the ligand,and the band at 436 nm is assigned as the ligand tometal transition of Cuギcomplex.The low-energy band around 667 nm for Cuギcomplex is assigned as the metal d-d transition typical of copperギcomplexes[39].
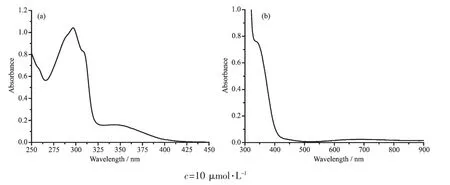
Fig.2 UV-Vis spectra of L(a)and Cuギcomplex(b)
2.3 Description of the structure
The selected bond lengths and bond angles are listed in Table 2.The molecular structure of Cuギcomplex is shown in Fig.3,and its packing in a unit cell in Fig.4.This complex ismononuclear molecule.The central metal atom is four-coordinated by two oxygen atoms(O(1)and O(1A))and two nitrogen atom(N(1)and N(1A))from the ligand.O(1),O(1A),N(1)and N(1A)are placed in equatorial sites.The bond anglesofO(1)-Cu(1)-O(1A),N(1)-Cu(1)-N(1A)and O(1)-Cu(1)-N(1)are 179.9°,180°and 89.5°,respectively,which obviously indicates that the Cuギcomplex is quadrilateral plane structure[40-41].

Fig.3 Molecular structure of the Cuギcomplex

Table 2 Selected bond lengths(nm)and bond angles(°)of Cuギ comp lex

Fig.4 Packing of Cuギcomplex in a unit cell
2.4 Anticancer activity
The cytotoxicity of Cuギcomplex towards the four cancer cell lines was evaluated using the MTT method.The cytotoxicity of the complexes was found to be concentration dependent.The cell viability decreasedwith increasing concentration of the complex.Table 3 lists the half inhibitory concentration(IC50)of the complex and carboplatin on cultured cancer cells in vitro.As shown in Fig.5 and Table 3,the Cuギcomplex have obvious inhibitory effect on four cancer cells,and the effect was better than carboplatin.The prominent cytotoxicity of the complex is probably related to the strong DNA binding involving hydrophobic interaction forces[42]or the dissociation of the complex in the cell,resulting in intracellular accumulation of high amounts of copper and the chelation with biological components such as proteins from the nucleus[43-44]. Recently,four novel Cuギcomplexes of bidentate Schiff base ligands were reported by Abbasi′s group.The in vitro anticancer activity of compounds was screened by MTT assays against gastric cancer cell line (MKN-45),the IC50values were 1.542~4.560 μg·mL-1[45].These results show that Cuギcomplex may be better potential candidates for further chemical optimization and cancer therapy.

Table 3 Inhibition action of com plexes to the cancer cell in vitro
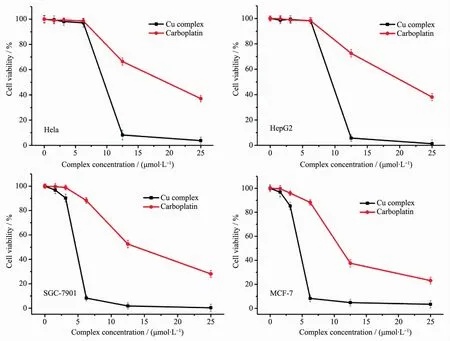
Fig.5 Representative graphs showing the survival of Hela,HepG2,SGC-7901 and MCF-7 cells grown for 48 h in the presence of Cuギcomplex and carboplatin
2.5 Fluorescence study of the interaction between Cuギcomplex and BSA
Among the several techniques,fluorescence spectroscopy is very useful to obtain quantity and quality information on the protein-drug interaction[46].Although BSA have two intrinsic fluorophores such as Tyr and Trp;most of fluorescence characteristic of this protein comes from the only Trp residue in subdomainⅡA,which is very sensitive to the environmental changes[47-48].In order to determine the quenchingmechanism of the interaction between BSA and Cuギcomplex,the fluorescence experiments were carried out at three different temperatures (287,303 and 313 K).The fluorescence emission spectra of BSA in the absence and presence of different concentrations of Cuギcomplex at 287 K are shown in Fig.6.As shown in Fig.6,the BSA fluorescence intensities were gradually decreased with increasing concentration of Cuギcomplex,indicating that the Trp residue was transferred into a more hydrophobic environment during the interaction.The similar emission profiles were observed as the experiments were repeated at 303 and 313 K.

Fig.6 Effectof Cuギcomplex on BSA fluorescence at different temperatures:(A)287 K;(B)303 K;(c)313 K
2.6 Determ ination of quenchingmechanism
There are two quenching types in characterizing the mechanism of the binding of quencher and macromolecules:static and dynamic (or collision)quenching.Static quenching refers to the formation of a non-fluorescence fluorophore-quencher complex.Dynamic quenching refers to that the quencher diffuses to the fluorophore during the lifetime of the exited state and upon contact,and the fluorophore returns to ground statewithout emission of a photon[49].Themechanism of the drug binding to serum albumin was probed using Stern-Volmer equation[50]:
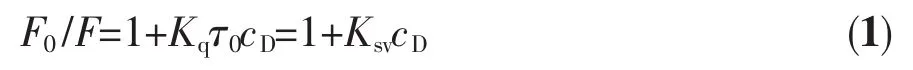
where F0and F are the fluorescence intensities in the absence and presence of drug,respectively,cDis the drug (complex)concentration,Kqis the biomolecular quenching rate constant,τ0is the average lifetime of molecule in the absence of drug and its value is 10-8s[51],Ksvis the Stern-Volmer quenching constant.For the drug-BSA system,the Stern-Volmer plots are presented in Fig.7 and the values of Ksvobtained from the plots are listed in Table 4.It is obvious that the Ksvdecreases with increasing temperature for Cuギcomplex.Furthermore,it was found all values of Kqwere larger than 2.0×1010L·mol-1·s-1,the maximum diffusion collision quenching rate constant of various quenchers with the biopolymer[52].So the quenching process between Cuギcomplex and BSA was static quenching and not dynamic quenching.

Fig.7 Stern-Volmer plots of quenching of BSA fluorescence by Cuギcomplex at different temperatures

Table 4 Quenching parameters of Cuギcom plex-BSA interactions
2.7 Binding constant and the number of binding sites
For the static quenching interaction,when drug molecules bind independently to a set of equivalent sites on a macromolecule,the binding constant(KA)and the number of binding sites(n)can be determined by the following equation[53]:
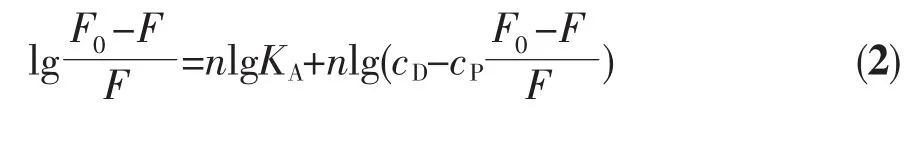
where F0and F are the fluorescence intensities in the absence and presence of the Cuギcomplex,cDand cPare the concentration of the Cuギcomplex and protein,respectively,KAis the binding constant and n is the number of binding sites.The values of n and KAat physiological pH 7.4 were obtained from the double logarithmic plots of lg[(F0-F)/F]versus lg cD. The binding constants and binding sites at three different temperatures are listed in Table 5.

Table 5 Binding constants(K A)and binding sites(n)at various temperatures
2.8 Thermodynam ic parameter and binding force
The thermodynamic studies reveal that free energy changes are negative for interaction between the Cuギ complex and protein which show that binding processes are spontaneous.The interaction forces between drug and biomolecules include hydrogen bonds and van der Waals forces as well as,electrostatic and hydrophobic attraction[54].The sign and magnitude ofΔS and ΔH for protein binding can account for the main force contributing to protein stability.When the temperature change is not very enormous,theΔH of a system can be regarded as a constant,and its value andΔS can be calculated from the van′t Hoff equation:

where KAis the binding constant,and R is the universal gas constant;ΔG,ΔH and ΔS are the standard free energy change,enthalpy change and entropy change for thebinding interaction,respectively.The values of thermodynamic parameters obtained through these equations are presented in Table 6.The values ofΔH for the binding reaction between Cuギcomplex and BSA are found to be negative,whileΔS are positive,which indicates that in this systemelectrostatic interaction plays a major role in the formation of the Cuギcomplex-protein adduct.

Table 6 Thermodynam ic parameters for the interaction of Cuギcomp lex and BSA
2.9 Calculation of binding distance
The binding distance concerning donor-acceptor pair can be obtained from the Frster theory of nonradiation energy transfer[55].It is well known that BSA contains two tryptophane (Trp 135,Trp 214),but the crystallography analysis reveals that many drugs are bound at subdomainsⅡA andⅢA,while Trp 214 is atⅡA.And in general,the fluorescence of BSA arises mainly from Trp 214.So the distance between the drug and BSA generally means the distance between Trp 214 and the drug.The rate of energy transfer depends on the extent of overlapping of the donor emission spectrum with the acceptor absorption spectrum,the relative orientation of the donor and acceptor transition dipoles,and the distance between the donor and the acceptor[56].
The energy transfer efficiency E is

Where F is the fluorescence intensity of BSA in the presence of the complex and the concentration ratio of BSA to complex is 1∶1.E is also expressed as:

where r0is the acting distance between the donor and acceptor and R0is a characteristic distance,called as the Förster distance or critical distance,at which the efficiency of transfer is 50%:

where K2is the spatial orientation factor describing the relative orientation in space of the transition dipoles of the donor and acceptor,K2=2/3,N is the refraction index for themedium,Φis the fluorescence quantum yield of the donor in the absence of the acceptor,N=1.33,Φ=0.13 and J is the overlap integral between the donor fluorescence emission spectrum and the acceptor absorption spectrum.J can be calculated by

In Eq.(8),F(λ)is the fluorescence intensity of the fluorescence donor atwavelength λ,ε(λ)is themolar absorption coefficient of the acceptor atwavelengthλ and its unit is L·mol-1·cm-1.
The over lapping of the absorption spectra of 5.00 μmol·L-1Cuギ complex with the fluorescence emission spectra of 50 μmol·L-1BSA were shown in Fig.8.The spectrum ranging from 290~450 nm was chosen to calculate J.By Eq.(7),the critical distance R0could be calculated.And the distance between the Cuギcomplex and tryptophan could be obtained from Eq.(6).The value of r0for the Cuギcomplex is 3.92 nm,less than the academic value (8 nm)[57],which indicates that the fluorescence quenching of BSA is also a non-radiation transfer process.

Fig.8 Overlapping of the fluorescence spectra of BSA with the absorption spectra of Cuギcomplex
3 Conclusions
One copperギcomplex has been synthesized by the reaction of 4-aminoazobenzene,salicylaldehyde and Cu(OAc)2·H2O.Structural analysis shows that the complex ismononuclearmolecule.The central copper atom is four-coordinated in a plane square structure.The inhibitory activity of complex on cultured cancer cells(Hela,HepG2,MCF-7,SGC-7901)in vitro was studied,and the results showed that it has obvious inhibitory effect on four kinds of cancer cells and may be better potential candidates for further chemical optimization and cancer therapy.The interaction between the complex and BSA was studied by the fluorescence quenching technique. The binding properties of Cuギcomplex and bovine serum albumin indicated that serum albumin can serve as a carrier of DNA binders.Under the conditions selected in this work,the binding constant,binding force and binding distance between Cuギcomplex and serum albumin were obtained.
These resultsmay be important,which may give us a better understanding of pharmacokinetics such as drug metabolism and distribution.And they may be useful for deciding the dosage in therapeutics as well as designing the new anti-tumor agents.Further studies on protein binding of drugswill be in progress in our laboratory.
Acknow ledgements:This work was supported by the National Natural Science Foundation of China (Grant No.21101121)and Doctor Fund of Taiyuan University of Science and Technology(Grant No.20172005).
