Evaluated Characteritics of Chicken Bone Marrow-derived Dendritic Cells Following LPS Induced Maturation
2018-10-10HuangXueweiMaSuntingXuYigangJiangYanpingCuiWenWangLiTangLijieandLiYijing
Huang Xue-wei, Ma Sun-ting, Xu Yi-gang, Jiang Yan-ping, Cui Wen, Wang Li, Tang Li-jie, and Li Yi-jing
College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
Abstract: Dendritic cells (DCs) are bone marrow-derived professional antigen presenting cells (APCs), they are crucial for initiation of both innate and adaptive immune responses. In this study, chicken bone marrow (chBM) cells were cultured in medium with recombinant chicken granulocyte-macrophage colony stimulating factor (rGM-CSF) and recombinant chicken interleukin-4 (rIL-4)for 7 days, displayed the typical morphology of DCs. These immature chicken bone marrow-derived DCs (chBM-DCs) showed significant up-regulation of the putative CD11c and of major histocompatibility complex class II (MHC II), but CD40 and CD86 co-stimulatory molecules were almost no up-regulated. However, maturation with lipopolysaccharide (LPS), surface expression of CD40, CD86 was greatly increased. The phagocytosis of chBM-DCs was assessed by neutral red, and the phagocytosis decreased after stimulation. In mixed lymphocyte responses (MLR), stimulated chBM-DCs were more effective to T-cell stimulators than nonstimulated chBM-DCs. In addition, mRNA expression levels of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α, CXCLi1 and CXCLi2 were assessed by real-time qPCR (qRT-PCR), and the results showed cultured chBM-DCs could be matured to a T helper cell type 1(Th1)-promoting phenotype by LPS stimulation.
Key words: dendritic cell, bone marrow, chicken, lipopolysaccharide
Introduction
In common with the vast majority of avian species, and other non-mammalian vertebrates, the chicken lacks lymph nodes (Wu et al., 2010). During growth period poultry birds encounter a wide range of pathogens,making them susceptible to infection (Rajput et al.,2013). Due to lacking of lymph nodes, antigen presentation occurs either in the spleen or at local sites of diffused structures (Wu et al., 2010; Li et al., 2011).Dendritic cells are the most known powerful APCs and the only one that can directly activate the initial T cells in vivo, they play an important role in inducing T cell activation, stimulating initial T lymphocyte proliferation, mediating immune tolerance, etc.DC-induced immune defense or immune tolerance depends on the mature state of DCs. Immature DCs are specialized in capture antigen, processing but are poor T-cell stimulators and mainly induce immune tolerance. Upon activation by pathogen associated molecular patterns, they migrate out of non-lymphoid tissues into T-cell regions of secondary lymphoid tissues where they complete their maturation (Wu et al., 2010). Mature DCs can highly express MHCⅡmolecules, various cytokines, chemokines, adhesion factors and costimulatory molecules (Dauer et al.,2003; Granucci et al., 1999; Winzler et al., 1997) and are specialized in presenting collected antigens (Ags)to effectively activate T cells to initiate immune responses, and the capacity of uptake is decreased.
However, DCs are rare populations in different body tissues, they are very necessary for the functional studies to expand and purify sufficient numbers in vitro (Cau et al., 1992; Inaba et al., 1992; Vremec et al., 1992; Lutz et al., 1999). In 1868, DCs were first described as Langerhans cells in the skin. In 1973,Steinman and Cohn (1973) identified DCs from mouse spleen and named them for their typical morphology(Banchereau and Steinman, 1998). Since the expansion of DCs in vitro with GM-CSF in 1993, the research of DCs has been rapidly developed (Inaba et al., 1993),especially in vitro culture of human and mouse DCs,which has greatly promoted the study of DCs function and clinical application. Subsequently, research work was carried out on experimental animals, such as rabbits and pigs, and the in vitro culture of chBM-DCs was established and carried out until 2010 (Wu et al.,2010), therefore, chBM-DCs immunology research is lagging behind.
Recently, chBM-DCs were cultured from bone marrow cells using rGM-CSF and IL-4 and their functions and phenotypes were characterized (Wu et al., 2010; Rajput et al., 2013; Liang et al., 2015;Yasmin et al., 2015). On the basis of this, a series of optimization methods were carried out for culturing dendritic cells in vitro, and chBM-DCs were successfully cultured. This study aimed to investigate the activation and maturation of chBM-DCs by stimulation with LPS and attempted to understand what changes had occurred in morphological phenotype and function of chBM-DCs stimulated by LPS compared to nonstimulated.
Materials and Methods
Culture of chBM-DCs
ChBM-DCs were generated from 4 to 6-week-old inbred line Hailan birds as previously described (Wu et al., 2010; Liang et al., 2013; Liang et al., 2015).Optimized the previous protocol. Briefly, birds were sacrificed and immersed in 75% alcohol for 10 min,the femur and tibia were removed from the surrounding muscle tissue using sterile instruments under sterile conditions. The bones were washed three times with 0.01 mol · L-1phosphate buffered saline (PBS),and cut the ends of the bone with scissors, then the marrow was flushed with RPMI1640 (Gibco, USA)using a 10 mL syringe with a 0.45-mm-diameter needle. Clusters within the marrow suspension were disaggregated by syringe piston. After two wash in RPMI1640, 1 000 r · min-1for 10 min, the cells were suspended in RPMI1640 and loaded onto an equal volume of Histopaque-1119 (1.119 g · mL-1at 25℃; Sigma-Aldrich, UK) and centrifuged at 2 500 r · min-1for 30 min at room temperature. Cells at the interface were collected and washed twice with RPMI1640, 1 500 r · min-1for 10 min. Cells obtained from femurs and tibias were cultured at a final concentration of 2×106cells · mL-1in six-well plates in the culture medium containing RPMI-1640, 10%chicken serum (Gibco, USA), 1% non-essential amino acids (Sigma-Aldrich, UK), 1 U · mL-1penicillin and 1 ng · mL-1streptomycin, 25 ng · mL-1rGM-CSF(Abeam, USA) and IL-4 (Kingfisher, USA) for 7 days at 37℃5% CO2. Two-thirds of the medium was replaced with fresh, prewarmed complete medium at day 2 and 4 to remove non-adherent cells. On the 6th day, LPS (200 ng · mL-1, Sigma-Aldrich) was used to stimulate immature chBM-DCs for 24 h as previously described (Wu and Kaiser, 2011). On the 7th day of culture, all the cells were harvested by gentle pipetting and centrifugal separation. Observed cell morphology on days 2, 4 and 7.
Phenotypic analysis by flow cytometry
The harvested non-stimulated and stimulated chBMDCs on day 7 were gently pipetted for characterization and washed one time with 0.01 mol · L-1PBS, the supernatant was removed by suction and cells were resuspended in 100 μL of FACS buffer (PBS with 1%bovine serum albumin). The immature chBM-DCs and mature chBM-DCs (1×106cells · mL-1) were stained with PE-conjugated mouse anti-human CD11c antibody (eBioscience, USA) at the recommended concentration of 5 μg · 100 μL-1of FACS buffer, fluorescein isothiocyanate (FITC)-labeled mouse antichicken MHC II antibody (Abcam, USA) at the recommended concentration of 0.5 mg · mL-1of FACS buffer and incubated in the dark for 30 min at 4℃.In addition, chBM-DCs were stained with mouse anti-chicken CD40 or CD86 antibody (Abd, UK;at the recommended concentration of 25 g · mL-1of FACS buffer) and then incubated for 30 min at 4℃,following washed with FACS buffer. These cells were then resuspended in 200 μL of FACS buffer containing PE-conjugated goat anti-mouse IgG second antibody diluted 1 : 2 000 (MultiScience, China) and incubated for 15 min at 4℃. After the final wash with PBS, cells were analyzed on a FACSCalibur (BD Bioscience,Cowley, UK).
Chicken cytokine and chemokine mRNA expression by chBM-DCs assessed by qRTPCR
The total RNA was extracted using a commercial kit according to the manufacturer's protocol (Omega Bio-Tek). The total RNA was reverse transcribed into cDNA using SuperScript Ⅱ reverse transcriptase and oligo (dT) primers (life technologies) according to the manufacturer's recommendations. Cytokine and chemokine mRNA levels were quantified using qRT-PCR, the primer sequences with corresponding GenBank primer database are shown in Table 1. The fluorescence of SYBR green dye was measured for every cycle at the end of extension. The dissociation of the amplified product was analyzed at the end of PCR.Chicken β-actin gene as the reference gene, the relative expression of each target gene was calculated and normalized to that of β-actin using ABI 7500 system SDS software (Applied Biosystems). The following PCR conditions were utilized: 95℃ for 10 min,followed by 40 cycles of 95℃ for 15 s and 60℃ for 1 min.
ChBM-DCs phagocytosis assay
Non-stimulated or LPS stimulated DCs (2×106cells · mL-1) were seeded to 24-well plates (500 μL · well-1). Five hundred μL 0.1% neutral red saline solution was added to each well and the cells were cultured at 37℃ with 5% CO2. ChBM-DCs were washed twice with 0.01 mol · L-1PBS after 2 h,then 500 μL 0.1% SDS dissolved the cells. Using a microplate reader (OD570nm) (Bio 'Teke Corporation,Beijing, China) to detect the dissolution of cells in different wells.
T-cells proliferation assay
To determine T-cell stimulatory capacity of chBMDCs, allogeneic mixed lymphocyte reaction (MLR)of DCs was assayed as previously described (Wu and Kaiser et al., 2011; Liang et al., 2013). Mitomycin C was added into non-stimulated and stimulated chBMDCs at final concentration of 25 ng · mL-1for 1 h. DCs were washed twice in 0.01 mol · L-1PBS, then nonstimulated and LPS-stimulated chBM-DCs were used as stimulator cells. Allogeneic T lymphocytes were isolated from chicken spleen from 4-6 weeks old,cells were separated using 1.077 separations, after centrifugation at 2 000 r · min-1for 30 min, the cells at the interface were collected, washed, counted and used as reaction cells. T lymphocytes were added to 96-well round bottom cell culture plates and 1×105well · 100 μL-1, giving stimulator: responder ratios of 1 : 1, 1 : 10 and 1 : 100 in a culture volume of 200 μL.Negative control cultures contained responder or stimulator cells only, and blank control contained complete 1640 medium only. All the experiments were performed at least in triplicate. Three days later, cell counting kit-8 (CCK-8) (Sangon Biotech, Shanghai,China) was used to determine T cell proliferation.Added 10 μL CCK-8 solution, and incubated for 4 h at 37℃. The absorbance of each well was measured at 450 using a microplate reader (Bio-Tech Instruments, USA).The stimulation index was calculated as formula:


Table 1 Sequence of primers
Statistical analysis
Independent-samples t-test and one way ANOVA followed by Tukey's post hoc analysis were used for group comparison using SPSS 16.0 software. p<0.05 was considered statistically significant. p<0.01 was considered extremely significant. The test was repeated three times.
Results
Observation of morphology
ChBM-DCs were cultured for 2 days after by chicken rGM-CSF and IL-4 majority of cells were observed adherent growth (Fig. 1a). On the 4th day, many cell aggregates were seen under an inverted light microscope (Fig. 1b). On the 6th day, most of the cells were shown firm adhesion with longer dendrites which gave typical dendritic cell morphology (Fig. 1c).These aggregates grew and became floating or loosely adherent on the 7th day, when the cell aggregates were stimulated with LPS for 24 h, these cells aggregates grew and became bigger and floating or loosely adherent (Fig. 1d), many individual cells and the peripheral cells of the aggregates displayed a large veiled or dendritic appearance as a sign of maturation.
Non-stimulated cells having phenotype of immature chBM-DCs and LPS stimulated cells having phenotype of mature chBM-DCs
Flow cytometry was used to evaluate the cell surface expression of markers typically expressed on chBMDCs. The cells were collected on the 7th day, surface expression of cell surface molecules is shown in Fig. 2.The results showed the immature DCs high expression of CD11c and MHC Ⅱ, low expression of CD40 and CD86, suggesting that these cells were relatively immature. When using LPS stimulated DCs, surface expressions of CD40 and CD86 were significantly increased, compared with non-stimulated DCs.
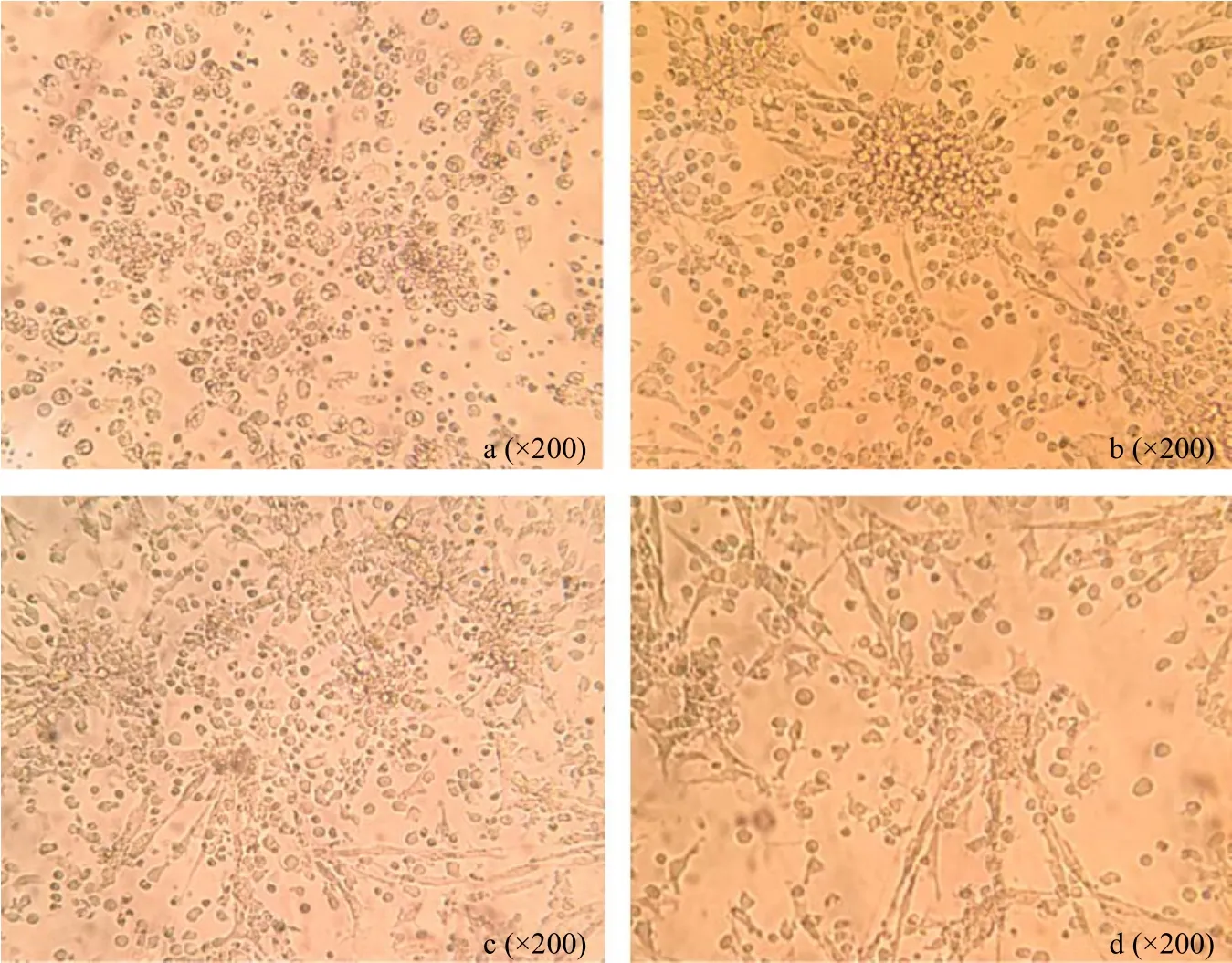
Fig. l Morphology of chicken bone marrow-derived dendritic cells (chBM-DCs) cultured in vitro

Fig. 2 Surface antigen expression on surface of chBM-DCs cultured for 7 days
Quantification of cytokines and chemokine gene expression
During the immune response, the cytokine environment was critical to the differentiation of initial T helper cells into subsets 1 and 2 (Th1 and Th2) (Wu et al., 2010). As a major antigen presenting cell, DCs had an unique function that elicited a T cell response and polarized Th1/Th2 differentiation by cytokine secretion (Rissoan et al., 1999). To evaluate the effect of LPS on the production of cytokines in chBM-DCs,DCs were stimulated by LPS for 6, 12 or 24 h.
Among Th1 cytokines (IL-12, IFN-γ and TNF-α) and Th2 cytokines (IL-4 and IL-10), and proinflammatory cytokines (IL-1β and IL-6) and chemokine (CXCLi1 and CXCLi2), relative expression of IL-12, IFN-γ and IL-1β and chemokine (CXCLi1 and CXCLi2) mRNA was significantly higher in LPS stimulated compared with non-stimulated (Fig. 3a, c, d), but TNF-α, IL-6 and Th2 cytokines (IL-4 and IL-10) expression levels were not significantly different between LPS treated and the control groups of both origins (Fig. 3a-d).
LPS stimulation results in decreased phagocytosis by chBM-DCs
To investigate whether these cells possess phagocytic ability, non-stimulated or LPS-stimulated chBM-DCs were co-cultured with neutral red. Research indicated that the phagocytic ability of the stimulated chBMDCs decreased compared with non-stimulated chBMDCs (Fig. 4).
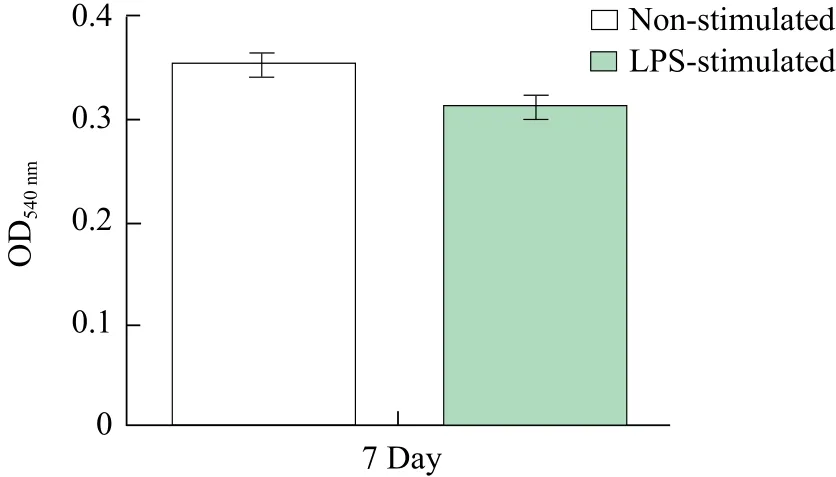
Fig. 4 Phagocytic ability of chBM-DCs treated with neutral red
ChBM-DCs stimulated proliferation of T cells in MLRs
After stimulation with LPS, chBM-DCs significantly evoked their allostimulatory capacities in MLR, compared to non-stimulated chBM-DCs (Fig. 5), especially when DC : T ratios of 1 : 1, 1 : 10, the capacity increased significantly.

Fig. 5 LPS-stimulated chBM-DCs stimulated proliferation of naive T cells in MLR
Discussion
DCs were key regulators of T and B-cell immunity,having superior ability to capture, process and present antigens compared to other antigen-presenting cells(APCs) (Kalaiyarasu et al., 2016). In avian immunology studies, the ability of chicken GM-CSF to differentiate, proliferate and survive in bone marrowderived dendritic cells has attracted more attention(Rajput et al., 2013). It was facilitating in development of both cellular and humoral immunity. Therefore,in recent years studies on the use of GM-CSF had attracted considerable attention (Warren and Weiner,2000; Parmiani et al., 2007).
Chicken bone marrow-derived dendritic cells were successfully cultured and characterized recently with the recombination of GM-CSF and IL-4 (Wu et al.,2010; Li et al., 2011). It was necessary to culture and expand DCs not only from bone marrow but also from peripheral blood to characterize and differentiate mature and immature cells to simulate in vivo immunological process. Kalaiyarasu et al. (2016)optimized the culture condition of chBM-DC with different concentrations of rGM-CSF and IL-4. This study described the effective generation of DCs from chicken bone marrow precursors, used a method of producing DCs from chicken bone marrow precursors and followed a large amount of DCs similar to that produced in chicken and mammals (Talmor et al.,1998; Bai et al., 2002). Chicken bone marrow cells were cultured for 7 days in the presence of rGMCSF and IL-4. The cultured cells had the morphology of typical mammalian DCs (Lutz et al., 1999; Inaba et al., 1992). The experiment optimized chBM-DCs culturing condition by refreshing two-thirds of the medium. For the functional verification, a neutral red method was adopted to verify the phagocytosis of DCs, neutral red was an effective method in verifying phagocytosis.
It is known that LPS was a strong inducer of DCs maturation in mammals and avian species (Wurtzen et al., 2001; Bai et al., 2002), LPS was used to induce the maturation of chBM-DCs. Flow cytometry showed that majority of the non-stimulated chBM-DCs showed high expression of CD11c and MHC Ⅱ, moderate or low levels of costimulatory molecules, LPS stimulated chBM-DCs had up-regulated surface expression levels of costimulatory molecules CD40 and CD86 compared with non-stimulated chBM-DCs.
chBM-DCs maturation with LPS was stimulated and mRNA expression levels of cytokines and chemokines were detected by qRT-PCR. Wu et al.(2010) research showed LPS-stimulated chBM-DCs could be matured to a Th1-promoting phenotype.Overall, the increased expression of Th1-type cytokines and proinflammatory cytokines compared with non-stimulated chBM-DCs; however, the expression level of Th2-type cytokines decreased or slightly increased. Among Th1-type cytokines IL-1β, TNF-α reached peak in initial 6 h of induction itself whereas IL-12, IFN-γ and IL-6 reached peak in 12 h, implying that LPS treated chBM-DCs could promote Th1 polarization and inflammatory response. These findings were well correlated with earlier studies that DC differentiation was associated with increased production of IL-6 and IFN-γ mRNA level. Reduction in TNF-α and IL-1β was observed during the differentiation progress suggested that these cytokines might have been utilized by DCs for their maturations(Kalaiyarasu et al., 2016). Compared with nonstimulated chBM-DCs, the expression of IL-4 was decreased by LPS-stimulated. Because Th2 cells differentiation was suppressed by Th1 cells derived IFN-γ. Chemokine (CXCli1 and CXCli2) also upregulated in LPS induced cells of both origin as demonstrated in chBM-DCs by Wu et al (2010).Chemokines were another important cytokine secreted by DCs, after gram-negative bacterium stimulation,raising Th1 and Th2 cells to reach the inflammatory site through the expression of the chemokines of DCs,balance Th1/Th2, play an inflammatory effect, and thus play a protective role in the body.
The results of the verification of the functionality showed that LPS-stimulated chBM-DCs decrease in their capability phagocytosis than non-stimulated chBM-DCs, whereas chBM-DCs significantly enhanced their allostimulatory capacities after LPS stimulation in MLR, chBM-DCs absorbed and processed antigens in vitro to deliver to T cells and eventually became the strongest condition for the production of immuno-stimulatory chBM-DC by this method. Thus, LPS showed chBM-DC functional mature inducers.
Conclusions
chBM-DCs were successfully cultured on the basis of optimization of the experimental method which had been characterized in terms of their morphology,phenotypic expression of DCs markers and function(phagocytosis and ability to present antigen as characterized by MLR). LPS was inducer of chBM-DC maturation, as evident by down regulation of phagocytic capacity, up-regulation of costimulatory molecules CD40 and CD86, and up-regulation of the capacity to stimulate proliferation of naive T cells in vitro.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Design and Experiment of four Lines Trailed Potato Fertilization Seeder
- Predictive Modeling for Growth and Enterotoxin Production of Staphylococcus aureus in Milk
- Evolution and Expression Patterns of Forkhead Box N1 in Pig
- Distribution of Selenium and Mercury in Heilongjiang Province and Its Effect on Body of Beef Cattle
- A Novel Bacillus thuringiensis Cry57 Protein Domain Swap In fluence on Insecticidal Activity
- Comparative Transcriptome Profiling of Glycine soja Roots Under Salinity and Alkalinity Stresses Using RNA-seq
