一种简单的提高无定形TiO2光催化活性的方法及其在增透膜中的应用
2018-09-03李远洋晏良宏
李远洋 晏良宏 江 波*,
(1四川大学化学学院绿色化学与技术教育部重点实验室,成都 610064)
(2中国工程物理研究院激光聚变研究中心,绵阳 621900)
0 Introduction
TiO2-based coatings can be applied easily on transparent substrates such as glass and plastics to provide self-cleaning function.However,the TiO2coatingsdeveloped thusfaralwaysenhance the surface reflection of transparent substrates due to the high refractive index (n>2)[1].To meet the conflicting requirements of antireflection (AR)and self-cleaning function,an effective way is to apply a low-refractiveindex material such as SiO2into TiO2coatings.Common practices include:(i)the preparations of the TiO2and SiO2composite film[2-3],(ii)the design of a double layer structure composed of a SiO2bottom layer and a porous/density ultrathin TiO2top layer[4-5],(iii)the synthesis of solid/hollow SiO2&TiO2core-shell particles[6-7]and(iv)loading TiO2-nanoparticles into a highly porous SiO2network[8].
However,all these methods realized self-cleaning AR coatings using anatase TiO2,which will usually need high-temperature treatments to crystallize TiO2.Or for the studies employed preformed anatase TiO2powder,although high temperature treatments can be avoid,the redispersibility of the TiO2nanoparticles is also a great challenge.Amorphous TiO2,which can be prepared at room-temperature,then becomes a better choice to provide AR coatings with self-cleaning function.
It is widely believed that the crystal structure of TiO2is the most primitive and essential property to predict photocatalytic activity.And it is commonly considered that amorphous TiO2contains high concentrations of defects,which will function as electron-hole recombination centres,and render the amorphous TiO2inactive[9-10].But other physical properties such as surface area[11],particle size[9]and surface hydroxyls[12]also have a great impact on photocatalytic activity of TiO2.In order to enhance the photoactivity of amorphous titania,extensive efforts have been conducted including constructing special microstructures[11],fabricating hydro-oxygenated amorphous[13-14]and the additives of metal oxides[15].
In our research,a facile,fast and simple method was developed to fabricate self-cleaning AR coatings with amorphousTiO2and mesoporousSiO2.The SiO2&amorphous-TiO2films were prepared by sol-gel dip-coatings method at room temperature.The amorphous TiO2nanoparticles with small-size and limited amount were deposited on mesoporous SiO2layer.The large surface areas of the mesoporous SiO2layer favour dispersion of TiO2nanoparticles.This structure greatly increased the surface areas of the amorphous TiO2particles,which facilitates the enhancement of photocatalytic activity and preserved the optical performance of the films in the same time.
These SiO2&amorphous-TiO2films exhibita similar AR function with the ideal single-layer AR coatings.Meanwhile,these films show a great enhancement of photo-catalytic activity compared with the single amorphous TiO2layer.Surprisingly,the SiO2&amorphous-TiO2even exhibit a faster photocatalytic degradation rate than the counterparts with anatase phase,i.e.SiO2&anatase-TiO2.To explain this abnormal finding,the chemical structures of the amorphous and anatase-TiO2films were studies. Notably, the mesoporous structure of the SiO2coating was spontaneously formed during the sol-gel dip-coating process without the need of post heat treatment.So the entire coating process can be processed at room temperature and the species of the feasible substrate materials can be remarkably expanded.
1 Expermimental
1.1 Materials
Methyltriethoxysilane(MTES)and Tetraethoxysilane (TEOS)were purchased from Alfa Aesar(China)Chemical Co.,Ltd.Titanium n-butoxide(n-BuTi,99%)was purchased from Acros.Ethanol(EtOH),NH3·H2O(13.4 mol·L-1),concentrated hydrochloric acid(HCl,37%(w/w))and stearic acid were purchased from Kelong Chemical Reagents Factory.The water was deionized.All chemicals were used without further purification.
1.2 Preparation of SiO2sols
The SiO2sols were prepared by a modified classic Stber method using MTES and TEOS as co-precursors and ammonia as catalyst.These reagents were successively added into a glass bottle.nSi(total amount of MTES and TEOS)∶nH2O∶nEtOH∶nNH3was 1∶3.25∶37.6∶0.17 finally.And the molar ratio of MTES/TEOS was 0.3 for the coating with a refractive index of 1.15.The concentration of equivalent SiO2was 3%(w/w)(assuming that 1 mol of TEOS (or MTES)gives 1 mol of SiO2).The solution was stirred at 30℃for 2 h,and then aged at room temperature for 10 days.Before deposition,the sols were diluted with equal amount of anhydrous ethanol.
1.3 Preparation of TiO2sols
TiO2sol was prepared by mixing EtOH,H2O and HCl,followed by dropwise adding n-BuTiunder stirring.The molar ratio of nn-BuTi∶nH2O∶nC2H5OH∶nHClwas 1∶3.55∶49.75∶0.22.The solution was stirred in a closed glass container at 30℃for 2 h,and then aged at room temperature for 7 days.Before deposition,the sols were diluted with anhydrous ethanol,and the final concentration of TiO2was 0.75%(w/w)(assuming that 1 mol of n-BuTi gives 1 mol of TiO2).
1.4 Films preparation
BK-7 substrates(Φ=35 mm,d=3 mm)were cleaned by ultrasonication in acetone for 10 min and wiped carefully before dip-coating.The SiO2and TiO2sols were deposited on the well-cleaned substrates successively under ambient condition of 30%RH(relative humidity)and 25℃.Approximately an interval of 2 minutes was needed to ensure complete evaporation of solvent.The thicknesses of the films were tuned by changing the withdrawal rates.The obtained SiO2&amorphous-TiO2films were labelled as Sx-ATy,where Sx represents the withdrawal rate applied on SiO2layer was x and Ty represents the withdrawal rate applied on TiO2layer was y.A denotes the amorphous state of TiO2nanoparticles.Sx-CTy represents the SiO2&TiO2films with crystallized anatase TiO2and the Sx-CTy films were prepared by heat-treated the Sx-ATy films at 100℃for 1 h and then 400℃for 2 h.
1.5 Characterizations
The surface topography of the coatings was studied with atomic force microscopy (AFM,SEIKO SPA-400,Japan).The surface root-mean-square(RMS)roughness values were obtained from the analysis of atomic force microscopy (AFM)images.Images of sol particles were obtained by the transmission electron microscope(TEM,JEM-100CX,JEOL)with an acceleration voltage of 200 kV.Cross-sections and top-view morphologies of the coatings were investigated by scanning electrons microscope(SEM,Hitachi S-4800,Japan)with an accelerating voltage of 3 kV.The transmittance spectra were measured with an UV-Vis spectrophotometer(Mapada,UV-3100PC,China)over the range of 400~800 nm.The refractive indices and thicknesses of the coatings were determined by an ellipsometer(SENTECH SE850,Germany).The crystallinity of the TiO2powders was estimated using an X-ray diffractometer(XRD,Philips X′PertMRD,Netherlands)that was operated at 40 kV and 35 mA with Cu Kα radiation (λ=0.154 2 nm)in the 2θ range between 20°and 80°.Raman spectra of the TiO2films coated on glasses were recorded on a Raman spectrometer(Horiba LabRAM HR,Japan)with an excitation wavelength of 532 nm.The nitrogen adsorption-desorption isotherms of the SiO2xerogels were detected on a BET equipment(Autosorb SI,Quanachrome,USA)and the multi-point BET method was applied to determine the pore size distribution and surface area.To determine particle size and distribution,the SiO2and TiO2sols were analyzed by dynamic light scattering(DLS,Malvern Nano-ZS,England,wavelength of 632.8 nm)at 25℃.The chemical binding states and compositions of titania films were measured by XPS(kratos,AXIS Ultra DLD,England)using a monochromatized Al Kα(1 486.6 eV)X-ray source under ultra-high vacuum (UHV)of 1.33×10-6~1.33×10-8Pa.For determination of the photocatalytic activity,stearic acid was deposited on the samples by dip-coating of a 50 mmol·L-1solution in ethanol at 180 mm·min-1withdrawal rate.After drying at room temperature,the stearic acid coated samples were then directly irradiated with UV light at ambient atmosphere.UV irradiation was provided by a UV reactor with high pressure mercury lamp (250 W)as light source and circulating-water to remove the effect of heat.The photocatalytic decomposition ofstearic acid was monitored by periodic FTIR (Bruker,Tensor 27,Germany)in transmission mode.
2 Results and discussion
2.1 Surface morphology of the SiO2 amorphous-TiO2films
The SiO2sols were prepared by a modified classic Stöber method[16]using MTES and TEOS as coprecursors.As our group previously reported[17],these base-catalysed silica particles randomly stack on the substrates;the inter-particleand particleinterior mesopores result in the coating with a low refractive index.The incorporation of methyl groups in the MTES enlarges the pore size in the silica particles.The refractive index of the SiO2coatings ranged from 1.22 to 1.10 by varying the molar ratio of MTES to TEOS(Fig.S1).The SiO2film with the refractive index of 1.15 was selected as the bottom layer.
The top-view SEM image of the bottom SiO2coating is shown in Fig.1a.The SiO2coating exhibits a porous structure just like a honeycomb.The N2sorption-desorption isotherms of SiO2xerogels are a typeⅣisotherm,typical of mesoporous solids.The pore size of the SiO2xerogels is determined to be~3 nm.And the SiO2xerogels has a large specific surface area of 747 m2·g-1(Fig.S2).The large surface areas of the mesoporous SiO2layer will favour the dispersion of TiO2nanoparticles.
The SiO2particles were monodisperse with size of ca.17.11 nm,verified by the TEM image and the size analyses(Fig.1(b,f)).Under the acidic conditions,TiO2tends to form linear chain network composed mostly of small primary particles[18].The TiO2particles have a much smaller size of ca.2.99 nm (Fig.1f).The TEM image of the TiO2nanoparticles is not shown as single TiO2particle cannot be seen due to conglomeration.From the XRD pattern (Fig.1c)and Raman spectrum(Fig.S3)of the as-prepared TiO2sample without heattreatment,no peak was observed,which proves that it is amorphous.After depositing the limited small-sized amorphous TiO2particles,the loose porous structure disappeared,as shown in SEM image of S140-AT100 in Fig.1d.Meanwhile,from the cross-section SEM images (Fig.1e),it could be seen that the thickness value of the S140 and S140-AT100 films are very close,estimated to be ca.103 and 109 nm,respectively,and no distinct boundary between mesoporous SiO2layer and TiO2nanoparticles can be seen in the S140-AT100 films.These phenomena indicate that the TiO2nanoparticles were dispersed well on the SiO2support and not enough to form a dense layer with a sufficient thickness.
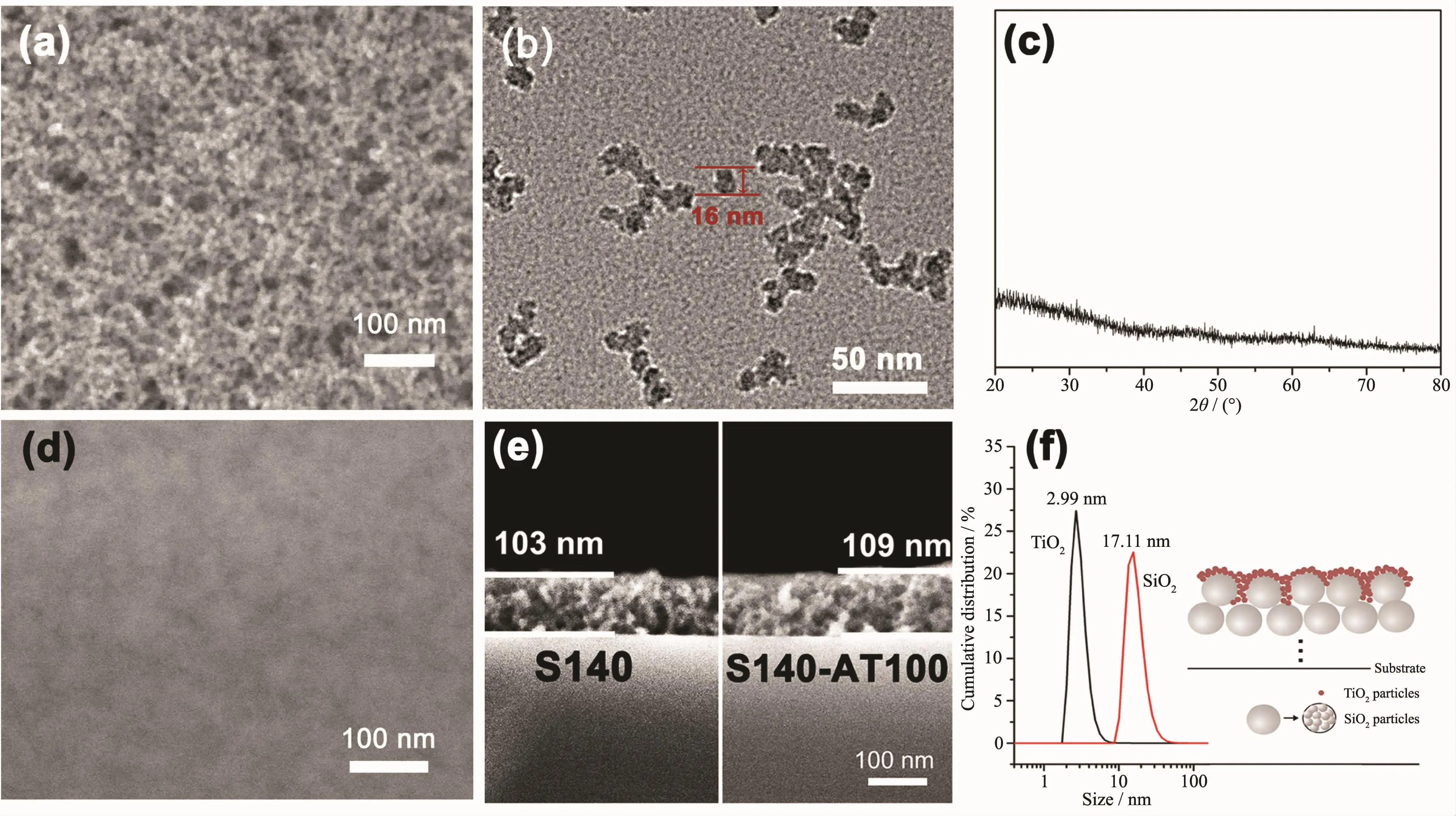
Fig.1 (a)Top-view SEM image of S140;(b)TEM image of the SiO2sol;(c)XRD patterns of TiO2powders obtained by natural evaporation at room temperature;(d)Top-view SEM image of S140-AT100;(e)Cross-section SEM image of S140 and S140-AT100;(f)Size distribution of SiO2and TiO2nanoparticles,and schematic illustration of the SiO2&amorphous-TiO2films
Fig.2 shows the 3D-AFM images for S140(Fig.2a),S140-AT60 (Fig.2b),S140-AT100 (Fig.2c)and AT100 (Fig.2d)films.Fig.2a shows that the SiO2film appears as a coarse surface with numerous surface protuberances,several nanometers high and bamboo shoot-shaped.The root-mean-square(RMS)roughness(Rq)value of the S140 film was 2.37 nm.After depositing TiO2particles on the porous SiO2film,the SiO2&amorphous-TiO2composite films became flat(Fig.2(b,c))and the Rqvalue of the S140-AT60 and S140-AT100 coatings decreased to 1.31 and 1.29,respectively.It could be seen as the small TiO2nanoparticles filled into the porous SiO2matrix and flatted the coarse SiO2surface.But due to the limited quantity and the much smaller size of TiO2particles compared to SiO2particles,the S140-AT60 and S140-AT100 films still presented a coarse surface and the bamboo shoot-shaped protuberances were still visible in the 3D-AFM images.In contrast,the AT100 film(Fig.2d)was very smooth with an Rqvalue of 0.75 nm,and this smooth and flat surface resulted from the tightly packed TiO2nanoparticles.From the AFM and SEM analyses,it can be concluded that the large surface areas of the bottom SiO2layer favor the dispersion of TiO2nanoparticles,enabling the limited and small-sized TiO2nanoparticles to be dispersed effectively instead of forming a dense layer.
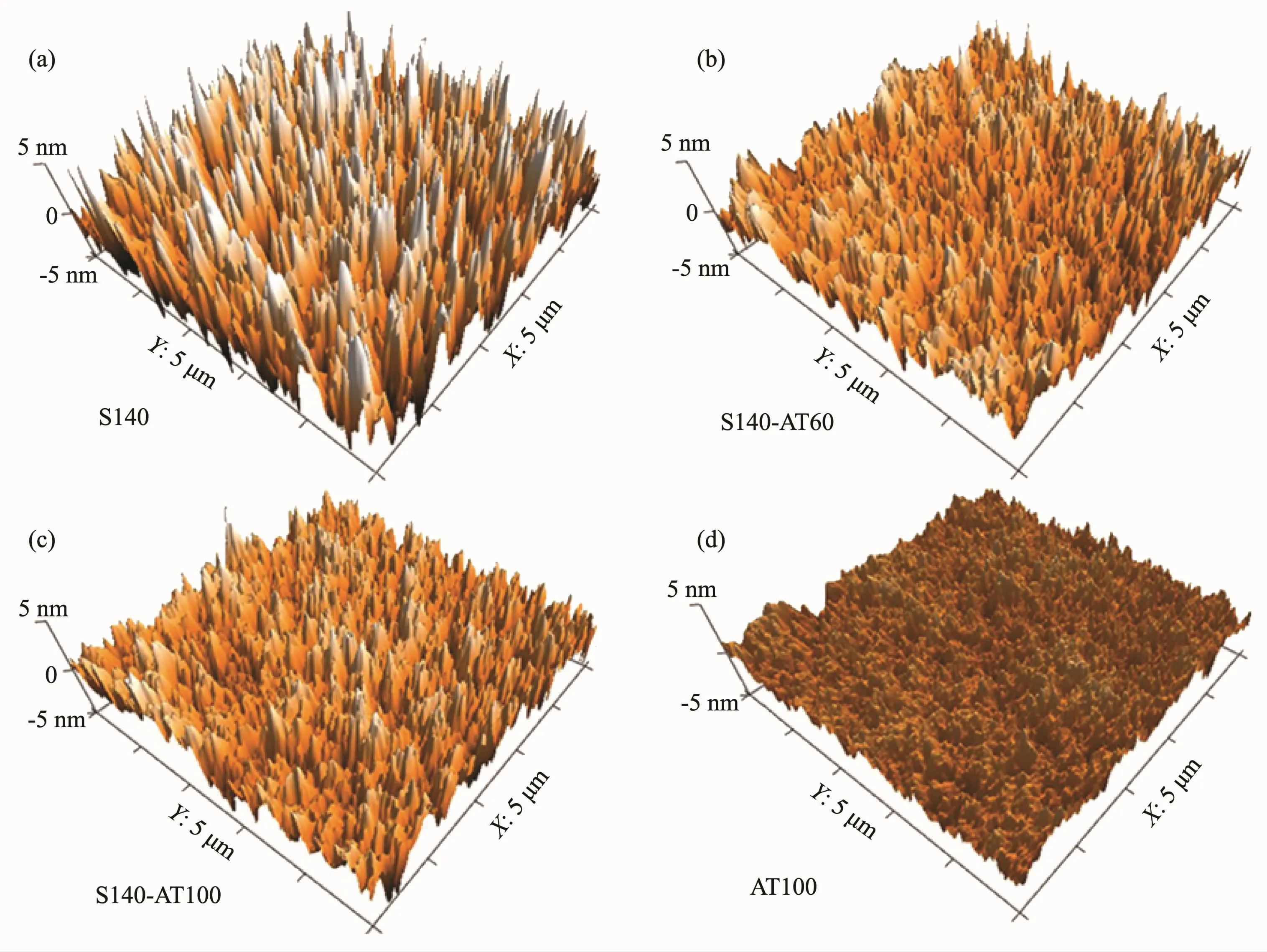
Fig.2 3D-AFM images of S140(a),S140-AT60(b),S140-AT100(c)and AT100(d)films
2.2 Optical performance of the SiO2&amorphous-TiO2films
The SiO2&amorphous-TiO2films were optimized by adjusting the refractive index and thickness of the SiO2layer,or altering the thickness of the TiO2layer.The SiO2film with the refractive index of 1.15 was selected as bottom layer,as in the preliminary experiments,it matchswell with the TiO2nanoparticles and exhibit a better optical performance.The thicknesses of the films were optimized by changing the withdrawal rates.The relative content of the SiO2and TiO2can be assessed by the thickness of the monolayer SiO2and TiO2coatings.The thicknesses of the monolayer of S140,AT60,AT100 and AT140 were measured to be 101,13,16 and 21 nm,respectively.The transmission spectra of the resultant SiO2&amorphous-TiO2films coated BK-7 glass are shown in Fig.3.These SiO2&amorphous-TiO2films exhibit a similar AR function with the ideal single-layer AR coatings.The maximum transmittances of S140-AT100 and S140-AT140 coated BK-7 glasses both reached as high as 99.97%,and the S140-AT100 coated glasses had the highest average transmittance of 99.91%in the range of 400~800 nm,enhanced greatly compared to that of blank BK-7 glasses(91.8%).
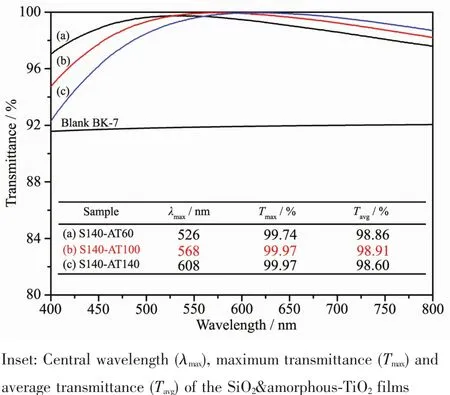
Fig.3 Transmission spectra of the SiO2&amorphous-TiO2 films coated BK-7 glasses compared with the blank BK-7 glass in the range of 400~800 nm
2.3 Photocatalytic activity
The photocatalytic activities of the SiO2&TiO2coatings were evaluated by the decomposition of stearic acid under UV illumination.Stearic acid can readily form a homogeneous layer onto the surface of the as-prepared coatings.By monitoring the FTIR spectra of stearic acid during UV illumination,the decomposition of stearic acid can be measured.
Fig.4a shows the evolution of the FTIR spectra of the CH2groups of stearic acid coated S140-AT100 under UV-illumination,the bands at 2 916 and 2 848 cm-1stemming from the symmetric and asymmetric CH stretching of CH2,respectively.The absorbances of the CH2bands greatly decreased at the first 10 min.Then the absorption peaks gradually weaken and nearly completely disappeared after 40 min UV-irradiation.It can be concluded that the SiO2&TiO2coating exhibits efficient degradation for stearic acid under UV illumination,suggesting that this nanocomposite film is an effective photocatalyst.
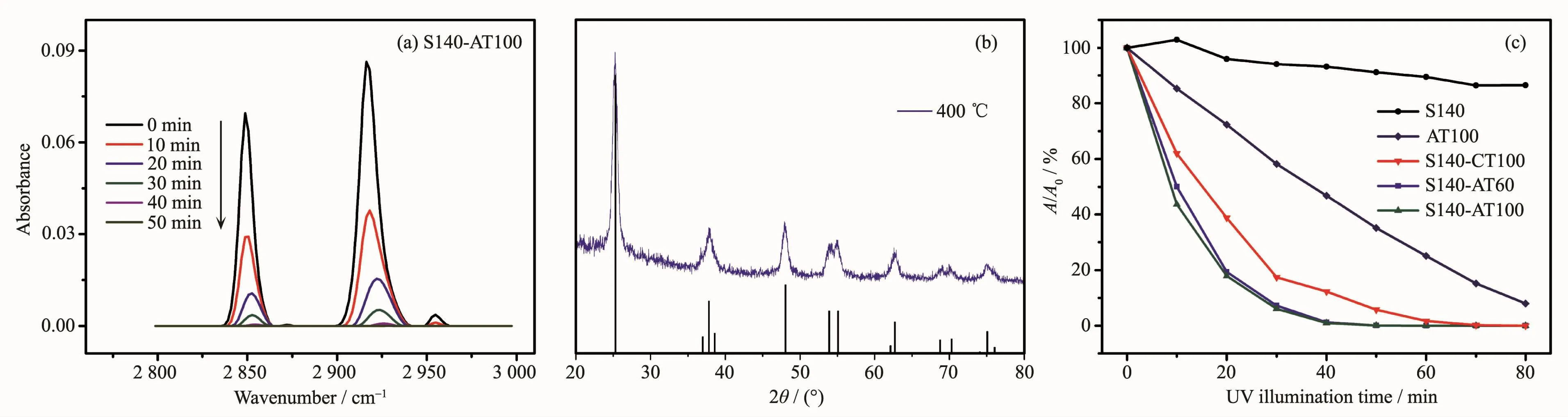
Fig.4 (a)Evolution of FTIR spectra of stearic acid coated S140-AT100 film with UV illumination;(b)XRD patterns of TiO2 powders heat-treated at 400℃compared with standard anatase TiO2in PDF No.21-1272;(c)Absorbance ratio(A/A0)as a function of UV irradiation time for stearic acid coated samples
The photodegradation experimentswere also carried out on S140-AT60,S140-CT100,AT100 and SiO2coatings.The anatase-type TiO2were obtained by annealing amorphous TiO2at 400℃.The crystal forms of the anatase TiO2were verified by XRD analyses(Fig.4b)and Raman spectra(Fig.S3).The XRD peaks agreed well with the standard anatase TiO2(PDF No.21-1272).The decreases in absorbances at 2 916 cm-1versus irradiation time are shown in Fig.4c for all samples (The evolutions of IR spectra for all the samples are shown in Fig.S4).A and A0are the absorbance at 2 916 cm-1after the UV irradiation and that from the initial surface,respectively.In the case of SiO2coating,no significant change in FTIR absorbance was observed after 80 min of UV-irradiation,indicating thatstearic acid decomposition arose exclusively from the photocatalytic activity of the TiO2particles.Compared the films with mesoporous SiO2as matrix (i.e.S140-ATx coatings),the monolayer TiO2film (AT100)shows a much weaker photocatalytic activity.This superior photocatalytic activity of the S140-ATx films can be ascribed to the efficient dispersion of the TiO2particles in a very accessible mesoporous matrix,which can greatly enhance the surface areas of TiO2particles.It is known that an increase in surface area can facilitate the formation of more effective adsorption sites,which might promote the photocatalytic activity by increasing the concentration of contaminants and reaction intermediates near the TiO2surface[19].More inspiringly,the S140-AT100 films show a higher photocatalytical activity compared to its counterpart with anatase phase,i.e.S140-CT100.
2.4 Structure-photocatalytic activity correlations
Inspired by the outstanding activity of S140-AT100,chemical properties of these films were investigated to disclose the correlations between structure and performance.The XPS analyses were conducted on amorphous and anatase titania,respectively.The XPS survey spectra confirms that the as-deposited films are composed of Ti,O,and residual C(Fig.S5).The high resolution XPS spectra of Ti2p and O1s are shown in Fig.5.As can be seen in Fig.5a,the binding energy difference between the two peaks Ti2p3/2and Ti2p1/2(at 458.7 and 464.4 eV for amorphous titania,and at 458.4 and 464.1 eV for anatase)is always 5.7 eV,corresponding to the spin-orbit coupling of Tiビchemical state[19].
Fig.5b shows the high resolution XPS spectra of the O1s core level.The O1s spectra were resolved into two peaks.The peaks at 529.4 eV for amorphous TiO2and 529.9 eV for anatase were assigned to surface Ti-Os[20-21].The other O1s peak respectively located at 530.7 and 529.9 eV for amorphous and anatases titania corresponded to surface hydroxyl groups(-OHs)[22-23].The peaks of amorphous titania slightly red-shifted relative to those of anatase titania,which may be ascribed to the difference of binding energy surrounding by the different chemical states of amorphous and anatase titania samples.
The peaks fitting parameters,such as binding energies,peak areas and the calculated atom ratios,are listed in Table 1.The molar ratio of Ti-Osto Ti are both~2 (listed in Table 1),calculated from the integrated areas of Ti2p and O1s at 529.4 eV or 529.9 eV(i.e.Ti-Os)XPS peaks divided by their corresponding sensitivity factors (Ti2p=2.001,O1s=0.780).However,the amorphous titania show a much higher contentofsurface hydroxylgroupsthan anatase sample,and the molar ratios of-OHsto Ti are 1.12 and 0.53 for amorphous and anatase titania,respectively.The abundant surface hydroxyl groups have a great effect on photocatalytic activity.
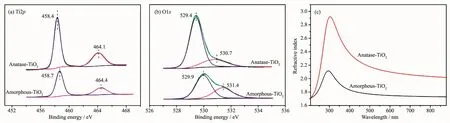
Fig.5 XPS spectra of Ti2p(a)and O1s(b)of amorphous and anatase TiO2samples;(c)dispersion curve of amorphous and anatase TiO2films
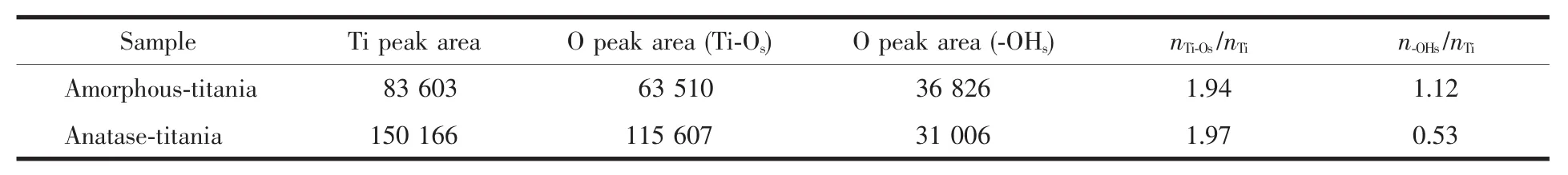
Table 1 Peaks fitting parameters for the O1s and Ti2p regions of amorphous and anatase titania
The widely accepted photoactivation mechanism is as follows:When a TiO2photocatalyst absorbs a photon with energy equal to or greater than its bandgap,electrons are excited from the valence band to the conduction band;subsequently,the photogenerated electrons(e)and holes(h)migrate to the surface of the photocatalyst and are trapped there.The trapped electron and hole react with acceptor(e.g.,O2)or donor molecules (e.g.,RH),respectively,or recombine at surface trapping sites[24].The photocatalytic reactions kinetics of TiO2depend on both the amount of substrates adsorbed on the surface to be reduced or oxidized by photoexcited electron (e-)or positive hole(h+),respectively,and rate of the recombination of eand h+[25].
Surface Ti-OH groups play an important role in trapping charge carriers,which is needed in the photocatalysis process to suppress recombination and increase the probability of interfacial charge transfer.The Ti-OH groups trap electron and hole forming Ti3+-OH groups and Ti4+-·OH radicals,respectively.The role of the Ti-OH groups trapping hole was disputed by some researcher[12],but the contribution of surface Ti-OH groups to electron trapping has been verified experimentally and theoretically[26].Meanwhile,the surface OH groups can protect the recombination of the excited carriers from the defective states by compensate the dangling bonds at defects and thus prolong the lifetime of the photoexcited carrier[13,27].
In addition,the refractive index of the amorphous and anatase TiO2are 1.74 and 2.10 at 632.8 nm,respectively (Fig.5c),indicating the prepared amorphous TiO2films possessing a relative loose structure compared with the anatase films.The loose structure can lead to a faster migration of the photoexcited electrons and holes from the internal to surface and better photocatalytic activity[28].
These differences between amorphous and anatase TiO2mainly result from the further condensation between the surface-OH groups[29],and the films shrinkage and crystallization during the heat treatment process.In a word,the amorphous TiO2nanoparticles featured abundant hydroxyl groups and loose structure.These characters may be preferable to crystal structure for enhancing photocatalytic activity.Thus the Sx-ATy films exhibit a slight higher photocatalytic decomposition rate compared to Sx-CTy films.
3 Conclusions
In summary,this article presents a simple and facile method to enhance the photocatalytic activity of amorphous TiO2by dispersion of amorphous TiO2nanoparticles onto a mesoporous SiO2coating.This structure greatly increased the surface areas of the TiO2particles and the SiO2&amorphous-TiO2films exhibit an enhanced photocatalytic activity than the monolayer amorphous TiO2film.The SiO2&amorphous-TiO2even exhibit a higher photocatalytic activity than the counterparts with anatase phase.This higher photocatalytic activity is attributed to abundant surface hydroxyl groups and relative loose structure of the amorphous TiO2nanoparticles,which can help enhance photocatalytic activity through reducing recombination of electron-hole pairs and increasing the migration rates of the photoexcited electrons and holes,respectively.The SiO2&amorphous-TiO2filmsalso exhibit excellent AR properties.These AR films with efficient photocatalytic activitycan find potential applications in energy-related instruments and optical devices.
Supporting information is available at http://www.wjhxxb.cn
