基于β-二酮和四甲基咪唑鎓配体的Dyバ配合物的合成、结构和磁学性质
2018-09-03葛景园陈忠研马建平王海欧苏昆朋王海英
葛景园 陈忠研 马建平 黄 帅 杜 佳 王海欧 苏昆朋 王海英*,
(1杭州电子科技大学材料与环境工程学院,杭州 310018)
(2南京大学化学化工学院,配位化学国家重点实验室,南京 210023)
(3山东师范大学化学化工与材料科学学院,济南 250014)
0 Introduction
The coordination complexes consisting of anisotropic metal ions and organic ligands have received considerable research efforts overthe pastfew decades[1-3].There is a class of materials named as single-molecules magnets(SMMs)that features unique energy barrier and potential applications in quantum computing,high-density information storage,magnetic refrigeration and spintronics devices[4-5].Since the discovery of single-molecule magnetism in [TbPc2]-(Pc=phthalocyanine)[6],a growing number of lanthanide SMMs or lanthanide single-ion magnets (SIMs)have been reported.The advantage is that these types of complexes have large ground-state spin and large intrinsic magnetic anisotropy,especially dysprosiumバcomplexes[7-8].Recently,a breakthrough has been made in mononuclear Dy-SMM,[(Cpttt)2Dy]+(Cpttt=1,2,4-tri(tertbutyl)cyclopentadienide),which exhibits magnetic hysteresis at temperatures up to 60 K and a large energy barrier of 1 837 K[9-10].This result indicates that,with judicious molecular design,magnetic data storage in single molecules at temperatures above the symbolic temperature of 77 K should be possible.However,predicting the propertiesoflanthanide compounds is still a challenging task.Many researches reveal that the magnetic relaxation behaviors of 4f-SMMs/SIMs are particularly sensitive to several factors such as the symmetry-related magnetic anisotropy of individualmetalions,ligand field and intra-/intermolecular magnetic interactio[11-12].In this regard,continuous efforts should be devoted to synthesize and design new 4f-SMMs with diverse structural topologies and to deeply understand the mechanism of relaxation behaviors in SMMs/SIMs.
In addition to improving coordination symmetry to design SMMs with high performance,one of the most compelling trends is that improved properties through targeting the synthesis of structurally simple monometallicSMMs/SIMsratherthan polynuclear complexes,and/or diluting diamagnetic species to reduce the molecular interactions.In this paper,we synthesized a new dysprosiumバcomplex,(Tmim)[Dy(thd)4](1),which contains one mononuclear[Dy(thd)4]-anion as a salt of the non-coordinating Tmim+cation.Complex 1 crystallizes in an orthorhombic Fdd2 space group,and an approximately square antiprismatic configuration exists around the Dyバ centre.As expected,every[Dy(thd)4]-unit is separated regularly by Tmim+cation with the shortest Dy…Dy distance of 1.229 8 nm.Both static and dynamic magnetic properties of 1 were also investigated.
1 Experimental
1.1 Chemicals and synthesis
All chemicals and solvents were obtained from the commercial sources and used without further purification.Starting materials,Dy(thd)3·2H2O(Hthd=2,2,6,6-tetramethylheptanedione)and TmimI(1,3,4,5-tetramethylimidazolium iodide)were prepared according to literature methods[13-14].Complex 1 was synthesized according to the following method:to the ethanol solution of Dy(thd)3·2H2O(500 mg,0.7 mmol)added 1,3,4,5-tetramethylimidazolium chloride (89 mg,0.7 mmol)in solid form.The suspension became clear immediately.After stirring for 45 min,the solvent was removed in vacuo to obtain a white solid of complex 1.Suitable single crystals were obtained by the slow evaporation of the resulting solid over the period of three days in their pentane solution.This complex is quite stable in the air,and more importantly,it is very soluble in common organic solvents,such as methanol,ethanol and dichloromethane.Elemental analysis Calcd.for C51H89DyN2O8(%):C,60.01;H,8.79;N,2.74.Found(%):C,60.65;H,8.71;N,2.85.
1.2 Crystal structure determination
The crystal structures were determined on a Bruker Smart ApexⅡ CCD diffractometer(Mo Kα radiation,λ=0.071 073 nm).The cell parameters were retrieved and refined by using computer software(SMART and SAINT,respectively)[15].Corrections for incident and diffracted beam absorption effects were applied using SADABS supplied by Bruker[16].Both structures were solved and refined against F2by the full-matrix least-squares using the SHELXL-97 program[17].All non-hydrogen atoms were refined with anisotropic thermal parameters,and hydrogen atoms of the organic ligands and the hydroxide anion were calculated theoretically onto the specific atoms and refined isotropically with fixed thermal factors.A number of restraints were applied owing to disorder in the p-tert-butyl groups of thd-ligand.Crystal data,data collection parameters,and refinement statistics for complex 1 are given in Table 1.Selected bond distances and bond angles are listed in Table 2.
CCDC:1837597.

Table 1 Crystal data and structure refinements for 1

Table 2 Selected bond lengths(nm)and angles(°)for 1
1.3 Physical measurements
Elemental analysis was performed on an Elementar Vario MICRO analyzer.The static magnetic susceptibilities for 1 were collected on a Quantum DesignMPMS-XL7SQUID magnetometerinthe temperature range of 2~300 K for direct-current(dc)applied fieldsrangingfrom 0 to 70 kOe.The alternating current(ac)susceptibilities were obtained using an oscillating field of 5 Oe with the frequency ranging from 1 to 1 488 Hz.All magnetic data were corrected forthe sample holderby a previous calibration and for the diamagnetism estimated from Pascals tables[18].
2 Results and discussion
2.1 Crystal structure
The X-ray crystal structure analysis reveals that 1 crystallizes in the orthorhombic space group Fdd2.As depicted in Fig.1a,the asymmetric unit contains one mononuclear[Dy(thd)4]-anion and one Tmim+cation.In each[Dy(thd)4]-anion,the central Dyバion is coordinated by eight O atoms from four thd-ligands to form an octahedral[DyO8]coordination sphere.The Dy-O bonds range from 0.231 3 to 0.237 3 nm within the normal range according to the previous reports[19].The coordination environment of Dyバcenter was also confirmed by the continuous shape measures(CShM)values calculated by SHAPE 2.1 software.As shown in Table 3,Dy1 ion lies in a square antiprismatic(D4d)symmetry with a small SAPR-8 value of 0.316.Four O atoms from two thd-ligands compose an upper(O5,O6,O7 and O8)and a lower(O1,O2,O3 and O4)square planes.The twist angle (φ)between these two planes is 44.12°(Fig.1b),which is very close to that expected for an ideal D4dsymmetry (φ=45°).The two planes are in a nearly parallel arrangement with a slight dihedral angle of 0.334°,and the Dyバ ion is unevenly spaced between them with distances of 0.132 8 nm from the upper plane and 0.126 8 nm from the lower plane,respectively.
In the packing motif,[Dy(thd)4]-units stack together in an ABAB fashion in the bc plane(Fig.2a),and each[Dy(thd)4]-anions is surrounded regularly by the charge balanced Tmim+cations with the shortest DyN distance of 0.570 1 nm(Fig.2b).The existence of Tmim+cation makes a larger Dy…Dy separated distance(dmin=1.229 8 nm).No significant interlayer interactions have been found.

Fig.1 (a)Coordination environment around Dyバion in complex 1;(b)Perspective showing the φ structural features

Fig.2 Crystal-packing diagram of 1 in the bc plane(a)and ac plane(b)

Table 3 Continuous shape measures calculations(CShM)for 1*
2.2 Magnetic properties
Direct current(dc)magnetic susceptibility data for complex 1 were collected under an applied field of 100 Oe from 2 to 300 K on freshly prepared samples(Fig.3).The room-temperature χMT value of 14.23 cm3·K·mol-1is in good agreement with the value of one mononuclear Dyバ ion(14.17 cm3·K·mol-1,J=15/2,g=4/3)[11].As the temperature decreases,the χMT product decreases slowly and more rapidly below 50 K,which is likely due to the progressive depopulation of the Dyバexcited Stark sublevels and/or the possible antiferromagneticdipole-dipoleinteraction between the molecules[21].At low temperature,the χMT value remains almost unchanged at 11.23 cm3·K·mol-1,suggesting negligible magnetic interactions[21].The field-dependent magnetization of 1 from zero to 70 kOe dc field at 2,3,5 and 8 K is shown in Fig.3 inset in the form of M versus H.The curve shows a rapid increase before 10 kOe and then a more gradual increase to 5.59Nβ at 2 K and 70 kOe without saturation,which can be attributed to the presence of crystal-field effects and the low-lying excited states[11].This is also supported by the non-superposition of isotemperature lines in the M versus H/T curves(Fig.4).

Fig.3 Temperature dependence of χMT for complex 1 at 100 Oe field
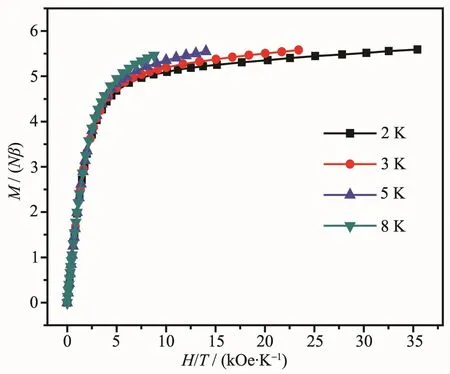
Fig.4 Experimental M versus H/T plots at different temperatures for complex 1
The alternating current(ac)magnetic susceptibilities were measured to detect the magnetic dynamic behavior of complex 1.The temperature-dependent ac susceptibility measurements under zero dc-applied field in the frequency range of 1~1 488 Hz are depicted in Fig.5,showing obvious in-phase signals(χ′)and out-of-phase signals(χ″).The obvious “tails”of the χ″signal at temperatures down to 10 K reveal the slow relaxation ofmagnetization,butno peaks were observed even at a low temperature of 1.8 K and high frequency of 1 488 Hz.The absence of peak maxima in χ″siganls is mostly resulted from the quantum tunneling of magnetization (QTM)process,which is often revealed in 4f-based SMMs[11].The energy barrier or characteristic relation time of 1 cannot be obtained according to the Arrhenius law: τ=τ0exp[(ΔE/(kBT)].However, fitting to ln(χ″/χ′)=ln(ωτ0)+Ea/(kBT)(ω:oscillating frequency of the ac experiment,τ0:preexponential factor of Arrhenius law,Ea:energy barrier,kB:Boltzmann constant)[22]allows us to roughly evaluate the energy barrier Ea/kBof 0.51 K(Fig.6).
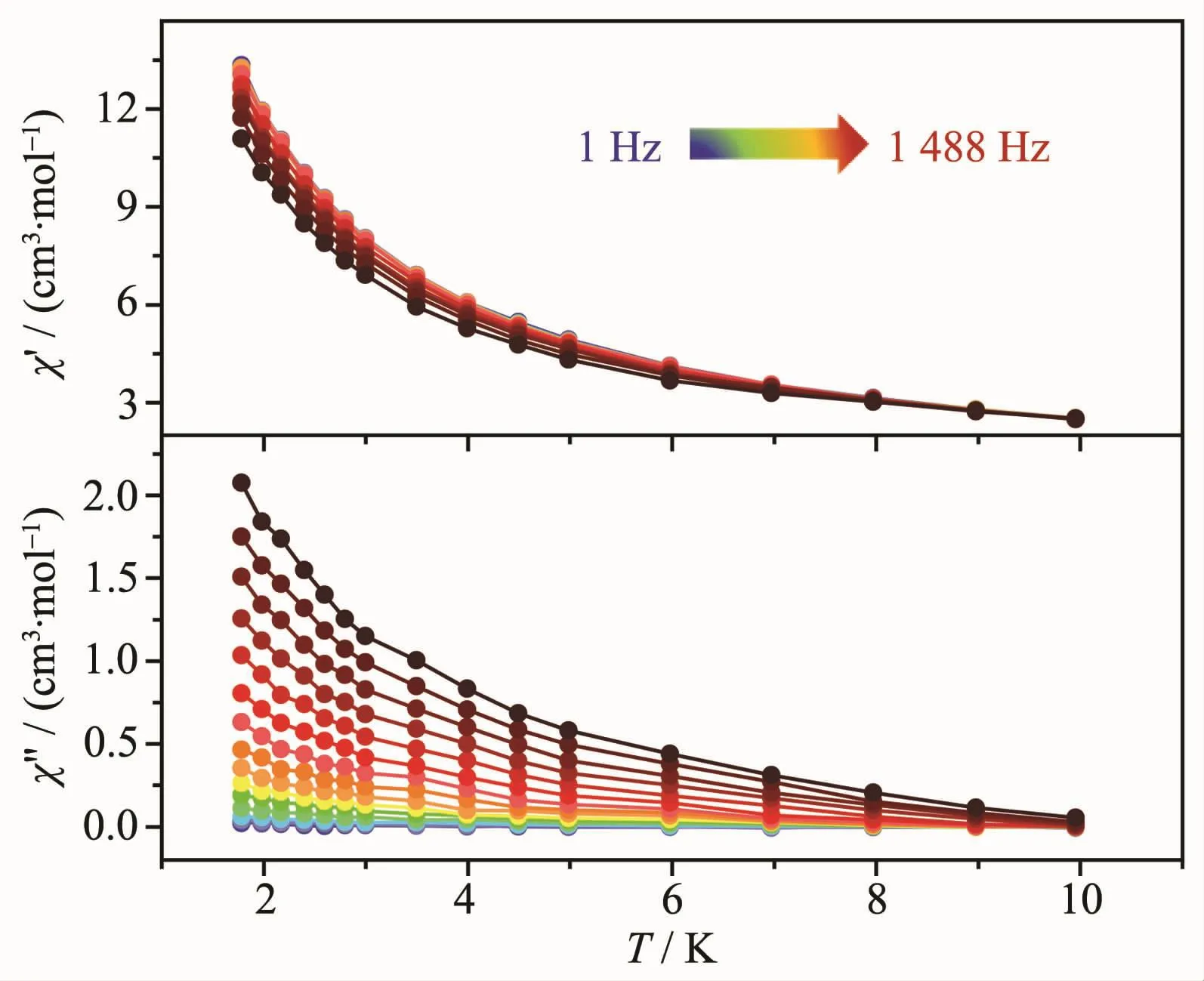
Fig.5 Temperature-dependent in-phase(χ′)and out-ofphase signals(χ″)ac susceptibility signals at different frequencies in a 5 Oe ac field oscillating at 1~1 488 Hz under zero dc field for 1
When a 500 Oe external field was introduced,the well-shaped peaks of χ″are fully observed(Fig.7).It is worth noting that the χ″signal at low temperature shows an increase trend with the cooling of temperature,indicating the existence of the other relaxation pathways.This type of multiple relaxation processes is not unusual in mononuclear SMMs/SIMs examples,which may be attributed to the intermolecularinteractionsorthe differentconformersof molecule resulting from the ligand disorder[21,23].The above result confirms the field-induced single-ion magnetic behavior of 1.Arrhenius law was allowed to afford the energy barrier Ea/kBof 30.9 K and the preexponential factor τ0=6.47×10-7s(R=0.995 7)(Fig.8).
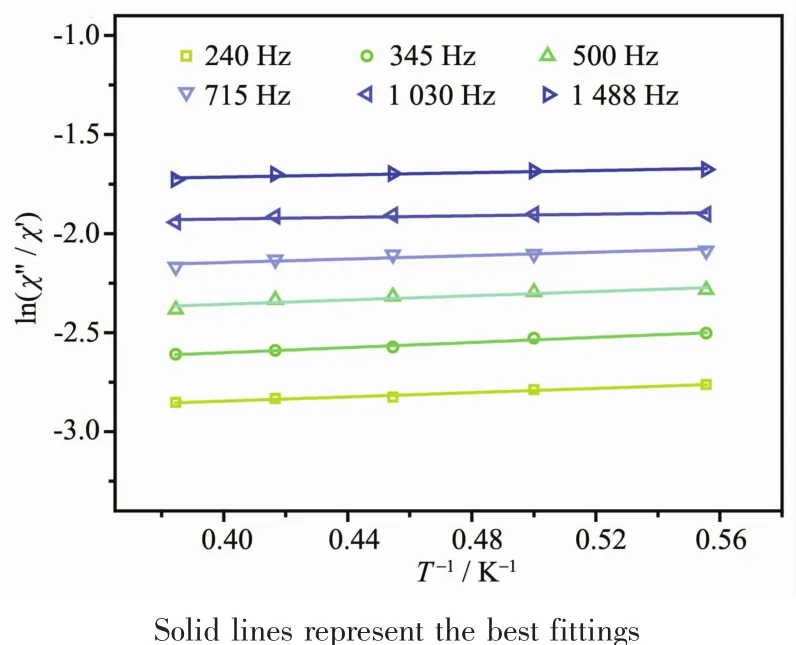
Fig.6 Plots of ln(χ″/χ′)versus T-1for 1 under zero dc field

Fig.7 Temperature-dependent in-phase(χ′)and out-ofphase signals(χ″)ac susceptibility signals at different frequencies in a 5 Oe ac field oscillating at 1~1 488 Hz under 500 Oe dc field for 1

Fig.8 Arrhenius fitting for lnτ vs T-1plot of 1 under 500 Oe dc field
The Dyバion in complex 1 locates in a high D4dsymmetry with the small SAPR-8 value of 0.316.Herein,the larger SAPR-8 parameters,the greater the deviation from an ideal D4dsymmetry.One[DyO8]family member[Hex4N][Dy(DBM)4](DBM=dibenzoylmethane;Hex4N+=tetrahexylammonium)with the more perfect square antiprismatic geometry of Dyバcenter has been reported,in which the χ″peaks under zero dc field can only be observed at frequencies higher than 3 160 Hz[21].Comparing 1 with[Hex4N][Dy(DBM)4],the dynamic magnetic behaviors for 4f-based SMMs/SIMsareaffected notonlyby the coordination geometry of central metal core,but also by several other factors such as the ligand field,magnetic anisotropy, magnetic interactions and multiple relaxation pathways[20].Yet further studies are required to determine exactly the relaxation mechanisms in this type of systems.
3 Conclusions
Insummary,anewdysprosiumバ complex(Tmim)[Dy(thd)4](1)was synthesized and structurally characterized.Dyバcentre locates in an approximately ideal square antiprismatic geometry.The[Dy(thd)4]-anions are separated regularly by Tmim+cations with the shortest Dy…Dy distance of 1.229 8 nm,suggesting that the incorporation of diamagnetic species into molecule magnets can be useful for minimizing intermolecular interactions between spin centers.Moreover,complex 1 exhibits a field-induced single-ion magnetic behavior with a high effective energy of 30.9 K.Taking the advantages of good solubility and the magnetic behavior,1 will have possible application in the preparation of organic films with slow magnetic relaxation.Further investigations to obtain new molecular architectures by choosing appropriate organic ligands to link paramagnetic metal ions together are underway in our laboratory.
