两个基于氮、膦混合配体的银ガ配合物的合成、表征和荧光性质
2018-09-03匡晓楠杨玉平李中峰韩洪亮金琼花
匡晓楠 王 宇 朱 宁 刘 敏 杨玉平 李中峰 韩洪亮 金琼花*,
(1首都师范大学化学系,北京 100048)
(2北京工业大学材料科学与工程学院,北京 100124)
(3中央民族大学理学院,北京 100081)
So far,the structural diversity and potential applications of complexes had been studied in great detail[1-5].Complexes with coinage metals are applied to many areas and are widely served as functional materials,such as nanomaterials,magnetic materials,catalysts and antitumor drugs[6-10].
Until now,a number of mixed-ligand Agガdiimine phosphine complexes such as[Ag(N-N)(P-P)]+system(P-P=various bis(phosphine)ligands)have been reported and their fluorescence properties have been investigated in various solvents and different temperatures[11].As we all known,silverガsalts are classified as a kind of soft acid.According to the Hard-Soft-Acid-Base(HSAB)theory,as P-donor ligands,the rigid bis(diphenylphosphino)methane(dppm)and flexible bis(diphenylphosphino)propane (dppp)can easily coordinate with Agガsalts to help improve crystal quality.For the N-donor ligands,the chelating 1,10-phenanthroline ligand and one of its derivatives (dpq)were often used to synthesize complexes.In our previous reports,we have obtained some complexes with the novel structure and good fluorescence properties by the reaction of similar ligands and closed-shell d10metal[12-16].
In this paper,two novel silverガcomplexes,namely[Ag2(dppp)2(phen)2](CF3SO3)2(1)and[Ag2(dppm)2(dpq)2](CF3SO3)2·3CH3OH (2),where dppp=bis(diphenylphosphino)propane,dppm=bis(diphenylphosphino)methane,phen=1,10-phenanthroline,dpq=pyrazino[2,3-f][1,10]phenanthroline,have been synthesized and characterized by X-ray diffraction,IR,1H NMR,fluorescence spectra and THz time domain spectroscopy(THz-TDS).The luminescent properties of these complexes are discussed.
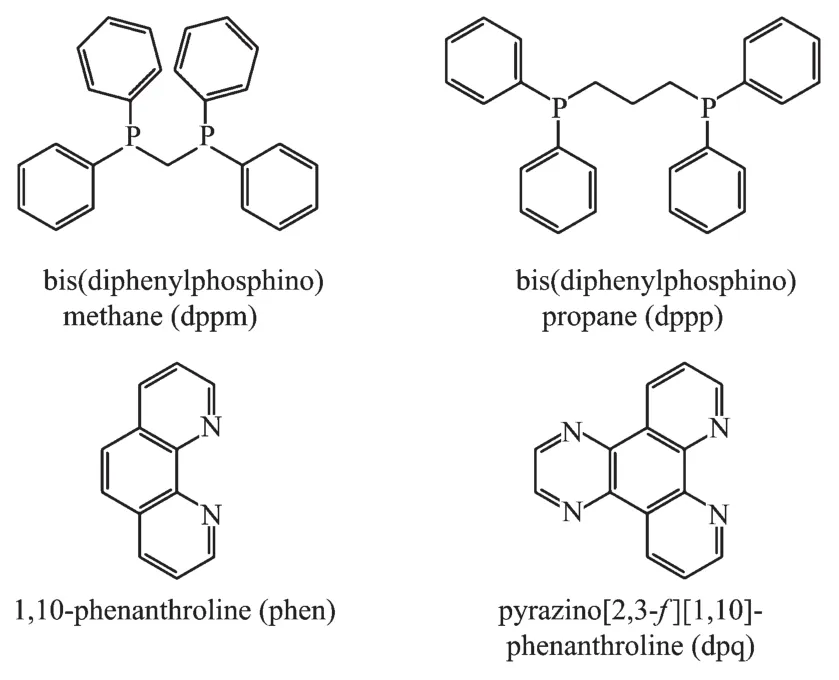
Scheme 1 Structures of the ligands
1 Experimental
1.1 Materials and measurement
All chemical reagents are commercially available and used withoutfurthermore treatment.FT-IR spectra (KBr pellets)were measured on a Perkin-Elmer Infrared spectrometer.C,H and N elemental analysis were carried out on an Elementar Vario MICRO CUBE (Germany)elemental analyzer.Roomtemperature fluorescence spectra were measured on F-4500 FL Spectrophotometer.1H NMR was recorded at room temperature with a Bruker DPX 600 spectrometer.The THz absorption spectra were recorded on a THz time domain device of the Capital Normal University of China,carried out in a N2atmosphere to avoid the influence of water vapor,based on photoconductive switches for the generation and electro-optical crystal detection of the far-infrared light,effective frequency in the range of 0.2~2.8 THz[17-18].
1.2 Synthesis of[Ag2(dppp)2(phen)2](CF3SO3)2(1)
Complex 1 was prepared by the reaction of Ag(CF3SO3)(0.051 4 g,0.2 mmol),dppp(0.082 5 g,0.2 mmol)and phen(0.036 0 g,0.2 mmol)in the mixed solvents of 5 mL CH2Cl2and 5 mL CH3OH.The mixture was stirred for 6 hours and filtered.Colorless crystals were obtained from the filtrate after standing at room temperature for several days.Yield:71%.Element analysis Calcd.for C80H68Ag2F6N4O6P4S2(%):C,56.55;H,4.03;N,3.30;Found(%):C,56.27;H,4.13;N,3.28.IR data (cm-1,KBr pellets):3 439m,3 056w,1 621w,1 588w,1 570w,1 510m,1 485w,1 436m,1 422m,1 264s,1 224m,1 151s,1 100m,1 032s,999w,841m,758m,745m,730m,695m,664w,638s,572w,525m,482w,439w.1H NMR(600 MHz,DMSO-d6,298 K):δ 7.1~8.6(m,dppp-aromatic rings,phen-Heteroaromatic rings),2.47(t,dppp-CH2)
1.3 Synthesis of[Ag2(dppm)2(dpq)2](CF3SO3)2·3CH3OH(2)
Complex 2 was prepared in a manner similar to the described for 1.A mixture of AgCF3SO3(0.051 4 g,0.2 mmol),dppm (0.076 9 g,0.2 mmol)and dpq(0.046 4 g,0.2 mmol)was dissolved in a mixture of 5 mL CH2Cl2and 5 mL CH3OH,stirred for 6 h and filtered.Yellow crystal was obtained from the filtrate after standing at the room temperature for several days.Yield:57%.Element analysis Calcd.for C80H60Ag2F6N8O6P4S2(%):C,55.00;H,3.46;N,6.41.Found(%):C,54.66;H,3.53;N,6.30.IR data (cm-1,KBr pellets):3 447m,3 050m,2 945w,1 581m,1 525w,1 472m,1 435m,1 391m,1 257s,1 223m,1 156s,1 121m,1 099m,1 081m,1 053w,1 030s,999m,815m,775m,741s,717m,693s,637s,572m,516m,479m,439m,413w.1H NMR(600 MHz,DMSO-d6,298 K):δ 7.91~9.42(m,overlap with the solvent peak signal,dpq-heteroaromatic rings,dppm-aromatic rings),2.48(s,dppm-CH2)
1.4 Structure determination
Single crystalsofthe title complexeswere mounted on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated Mo Kα (λ=0.071 073 nm)radiation at 298 K.Semi-empirical absorption corrections were applied using SADABS program[19].All the structures were solved by direct methods using SHELXS program of the SHELXTL-97 package and refined with SHELXL-97[20].Metal atom centers were located from the E-maps and other nonhydrogen atoms were located in successive difference Fourier syntheses.The final refinements were performed by full matrix least-squares methods with anisotropic thermal parameters for non-hydrogen atoms on F2.The hydrogen atoms were generated geometrically and refined with displacement parameters riding on the concerned atoms.
Crystallographic data and experimental details for structural analysis are summarized in Table 1,and selected bond lengths and angles of complexes 1~2 are summarized in Table 2.The hydrogen bonds of complex 2 are listed in Table 3.
CCDC:890658,1;1571774,2.

Table 1 Crystallographic data for complexes 1~2

Table 2 Selected bond distances(nm)and bond angles(°)for complexes 1~2

Table 3 Hydrogen bonds of complex 2
2 Results and discussion
2.1 Syntheses of the complexes
Functional Agガcomplex 1 has been synthesized by one-pot reaction of silverガ salts with dppp and phen ligands(Scheme 2).Complex 2 was prepared in a manner similar to the described for 1,except for using dppm and dpq ligands.As is known to all,ligand is one of the factors that can influence the structures of the compounds.The dppp ligand has a long carbon chain and dppm ligand has an extremely strong rigid structure,so it′s easy for the above two ligands to form a binuclear structure[21-25]like complexes 1~2.In complex 2,there are hydrogen bonds and CH…π intramolecular forces in molecular structure;however,there are no such forces in complex 1.
Scheme 2 Synthetic routine for complexes 1 and 2
2.2 Description of crystal structures
By Single-crystalX-ray diffraction analysis,complex 1 crystallizes in the triclinic crystal system with space group P1.The asymmetric unit(Fig.1)is comprised of two Agガions,two dppp ligands and two 1,10-phenanthroline (phen)ligands,forming a binuclear heteroleptic complex with a 0D coordination polymer.The Agガion adopts two types of fourcoordinated modes,which are combined with two N atoms from phen ligand and two P atoms from dppp ligand establishing a distorted tetrahedral geometry for the metal.Ag-N(0.242 1(5),0.244 5(4)nm)and Ag-P bond lengths(0.241 6(11),0.245 3(11)nm)are typical Agガ-N and Agガ-P distances in coordination compounds[26],respectively.Compared with the complex[Ag(dppp)]2(NO3)2[27],there are almost no so obvious differencesin Ag-P bond distances (0.241 9(3),0.242 4(3)nm)and bond angle(139.8(1)°)in complex 1,implied that substituting anions have no effect on complex structure.
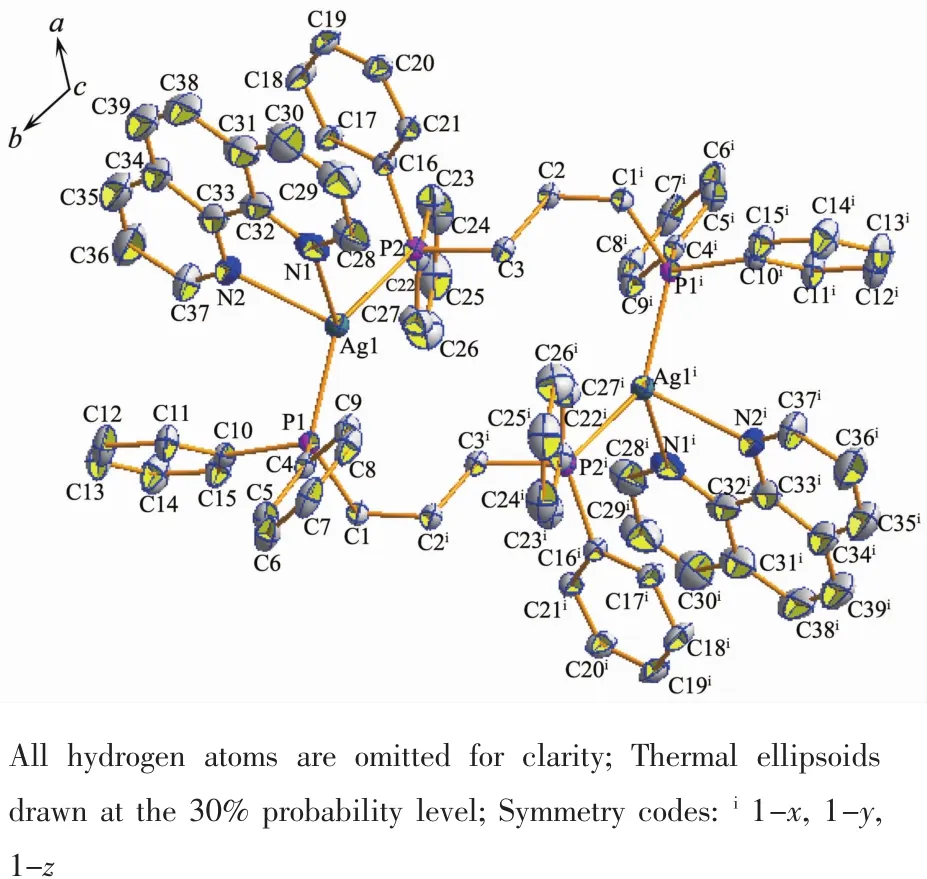
Fig.1 Molecular structure of complex 1
Unlike complex 1,2 crystallizes in the monoclinic crystal system with space group C2/c.Complex 2 is a binuclear heteroleptic complex formed with the same Agガ,dppm and dpq ligands in 1 ∶1∶1 molar ratio.Each Ag atom is four-coordinated surrounded by two P atoms from dppm ligand and two N atoms from dpq ligand (Fig.2).The angles between Ag,N and P atoms are ranging from 67.3(2)°to 141.84(6)°,indicating that the geometry around Ag atom is distortedly tetrahedral.Compared with complex[Ag2(μ-dppm)2(phen)2](NO3)2[28],there are also no so obvious difference in bond distances(0.238 9(3),0.240 6(9),0.245 6(9),0.246 1(3)nm)and bond angles(68.9(9)°~44.5(3)°),since dpq ligand is the derivative of phen ligand.The C-H…N intramolecular hydrogen bonds are observed (H…N 0.251 nm,C-H…N 100°).Moreover,the intramolecular C-H…π forces are also observed(H…Cg 0.287 nm)in complex 2,but in complex 1,these two forces are not exist.
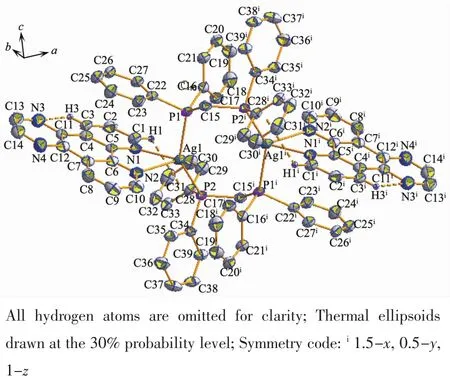
Fig.2 Molecular structure of complex 2
2.3 Infrared spectroscopy and fluorescence spectra
The infrared spectra of 1 and 2 show C-C stretch vibration of the phenyl rings whose absorptions are found in 1 436 and 1 435 cm-1.The middle absorptions around 3 056 and 3 050 cm-1are caused by C-H vibration of the phenyl rings.The absorptions at 1 032 and 1 030 cm-1are derived from trifluoromethanesulfonate(CF3SO3-)in complexes 1 and 2.
At room temperature,the solid-state excitation and emission spectra of complexes 1~2 and all the ligands were measured.When being excited at 322 nm,the dppm ligand displays a fluorescence emission peak at 431 nm,and the emission peak of the dppp ligand is found at 424 nm with excitation at 356 nm.It was found that the emission peak is centered at 525 nm with λex=386 nm for complex 1,and it is centered at 434 nm with λex=395 nm for complex 2(Fig.3).Why is there such a big difference in the emission of the two complexes?Firstly,there is no obvious difference on the part of diphosphine ligands.On the other hand,change of nitrogen ligand may be the majorcontributors to the distrinctemission in complexes 1 and 2.Compared with the dppp and dppm,complexes 1 and 2 all had different degrees of red-shift (101 and 10 nm,respectively).Moreover,complex 2 is red-shifted by about 17 nm compared with the dpq ligand which is excited at 381nm and shows a fluorescence emission peak at 417 nm.These shifts of emission peak are derived from ligandcentered π-π*transition.
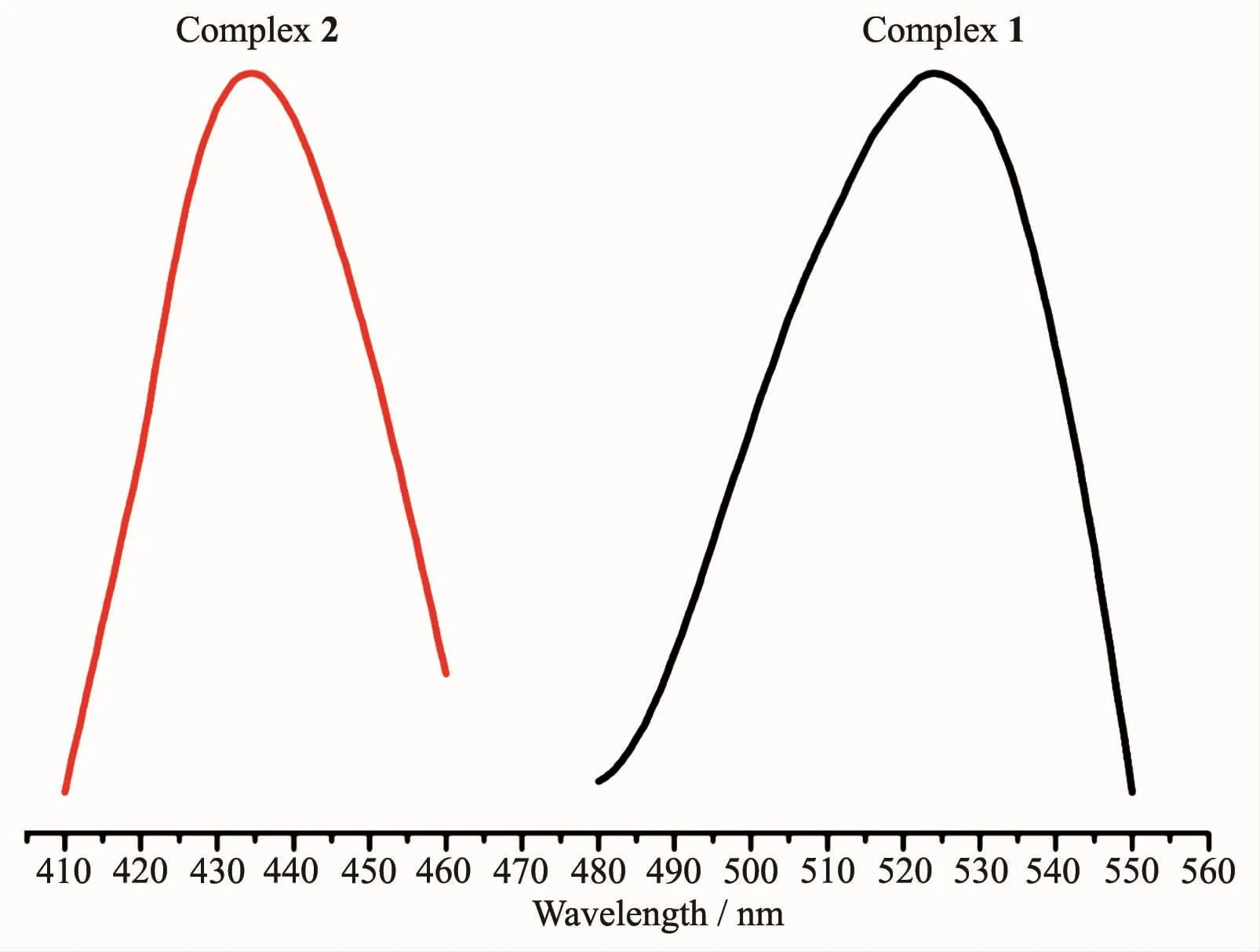
Fig.3 Luminescent spectra of 1 and 2 in the solid state at 298 K
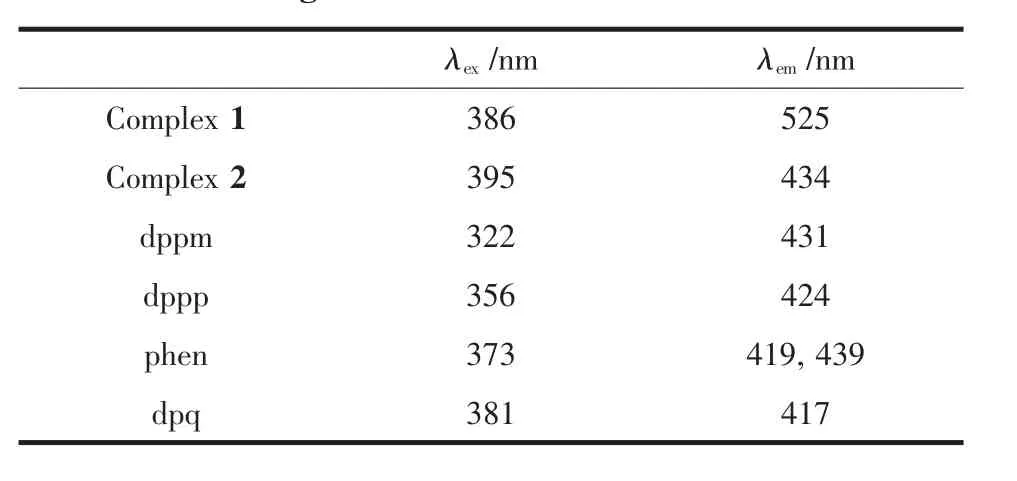
Table 4 Fluorescent data of complexes 1 and 2 and the ligands
2.6 Terahertz(THz)absorption spectra
The room temperature terahertz(THz)absorption spectra of the AgCF3SO3,dppm,dppp,phen,dpq and complexes 1~2 were measured in the range of 0.2~3.0 THz.All the above compounds have characteristic resonance peaks,which may be explained by the fact that in polar molecules the dipoles rotate and vibrate,resulting in strong absorption and chromatic dispersion.The peaks found for them are as follows:AgCF3SO30.69,0.84,1.00,1.16,1.30 THz;dppp 0.27,0.37,0.42,0.56,0.66,1.07,1.27,1.38 THz;phen 1.05,1.58,1.76,2.17,2.51,2.75 THz;dpq 0.64,1.29,1.70,2.34,2.69 THz;complex 1 0.35,0.53,0.76,0.94,1.17,1.53,1.70,1.87,2.28,2.52,2.69 THz and complex 2 0.24,0.53,0.76,0.93,1.67,1.35,1.52,1.70,1.88,2.29,2.46 THz (Fig.4).Comparing the THz absorption spectra of the products with those of the reactants,most peaks of the ligands and AgCF3SO3disappeared or moved in the complexes.New peaks appear in the new complexes indicating that the THz absorption spectra are associated with the coordination of silverガ ions and the ligands.Although the correspondence between the crystal structures and observed spectra does not allow a definitive characterization,it is possible to make tentative assignments of many of the observed features in the terahertz region for the samples.The results are a supplement to the THz spectroscopic properties of Ag complexes containing nitrogen and phosphorous ligands.
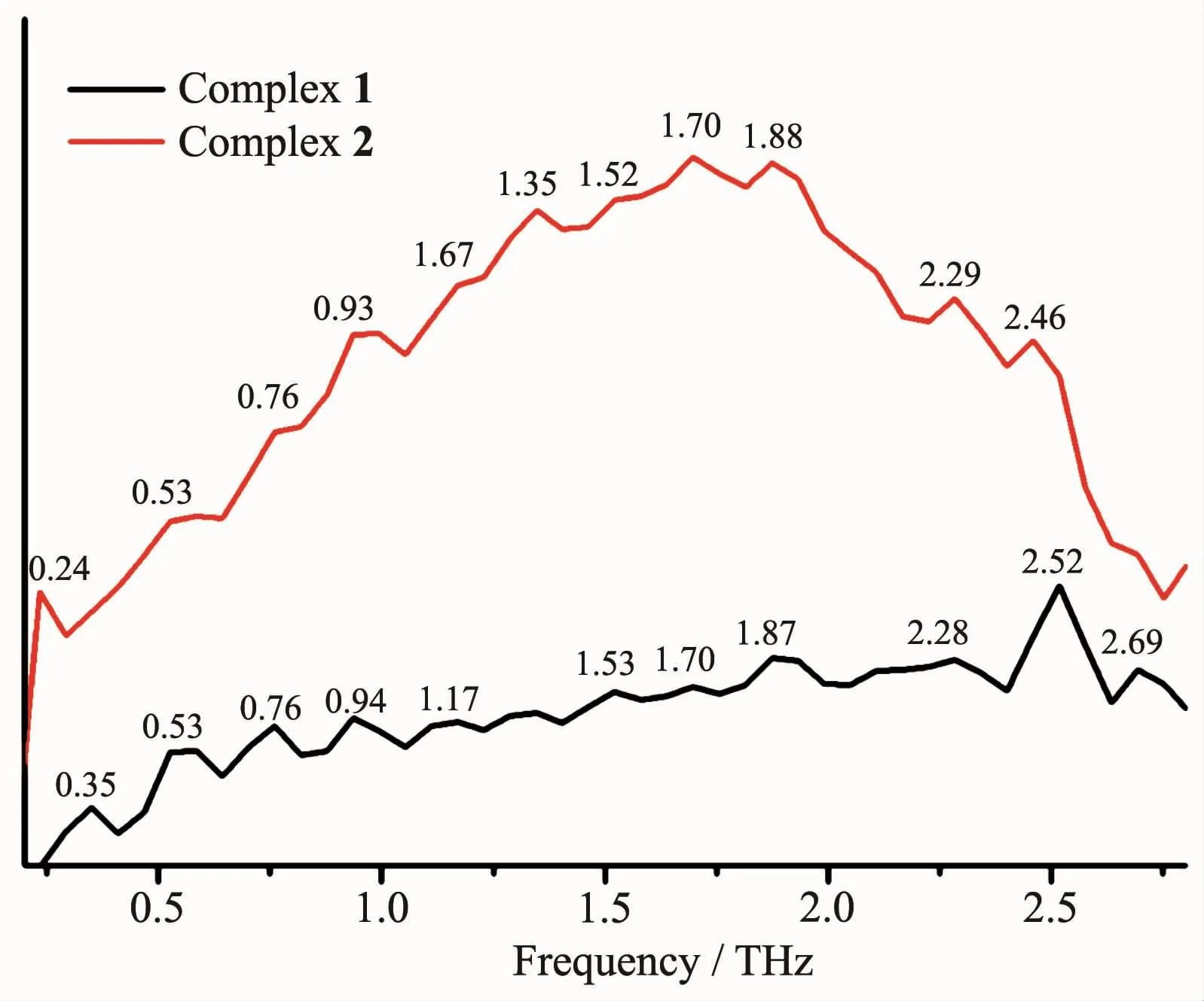
Fig.4 Terahertz spectra of complexes 1 and 2 in the range of 0.2~2.8 THz
