Detection of tyrosine,trace metals and nutrients in cow dung:the environmental significance in soil and water environments
2018-08-30KhanMostofaLonglongLiCongqiangLiu
Khan M.G.Mostofa•Longlong Li•Congqiang Liu
Abstract This study examined the dissolved organic matter(DOM)components of cow dung using a combination of fluorescence(excitation–emission matrix,EEM)spectroscopy and parallel factor(PARAFAC)modelling along with eleven trace metals using ICP-MS and nutrients(and )using an AA3 auto analyser.EEM–PARAFAC analysis demonstrated that cow dung predominantly contained only one fluorescent DOM component with two fluorescence peaks(Ex/Em=275/311 nm and Ex/Em=220/311 nm),which could be denoted as tyrosine by comparison with its standard.Occurrence of tyrosine can be further confirmed by the FTIR spectra.Trace metals analysis revealed that Na,K and Mg were significantly higher than Ca,Fe,Mn,Zn Sr,Cu,Ni and Co.Theconcentrations were substantially higher than.These results thus indicate that the dissolved components of the cow dung could be useful for better understanding its future uses in various important purposes.
Keywords Cow dung ·Excitation–emission matrix(EEM)spectroscopy·Parallel factor(PARAFAC)modelling·Tyrosine·Trace metals
1 Introduction
Cattle(cow or ox)are undoubtedly considered the largest of all types of animals in terms of sheer numbers that can convert particulate organic matter(POM;e.g.,grasses,plant materials,raw or dry straw and aquatic plants),when taken in as food,into dissolved organic matter(DOM)and other dissolved components.Such changes of POM into different dissolved fractions are primarily caused by microorganisms in the cattle,yielding what is termed ‘‘cow dung.’’Cow dung is widely used for many purposes.For example,charcoals produced from cow dung are currently used in different applications,including for removing organic or inorganic metallic pollution(Kadota and Niimi 2004;Ojedokun and Bello 2016),for producing methane for cooking purposes(Alfa et al.2014),and for serving as a natural organic manure for agricultural purposes,which has long been described in human history(Ghosh et al.2004).In addition,composting is one of the most effective methods for reusing the organic wastes and cow dung together with other types of live stocks’manure,which are currently being used to amend soil(Karak et al.2014;Li et al.2011).Cow dung is generally preserved in an open field,which can generally cause components to leach into the surrounding surface water and soil ecosystems.Despite how important cow dung is in its many applications and uses,the dissolved components in cow dung have not been examined extensively.The characteristics of key DOM components have been extensively investigated due to technological advances influorescence(excitation–emission matrix,EEM)spectroscopy.EEM offers an advantage over 3D-scan methodology by providing new information regarding the fluorescence properties of DOM in water,soil,sediments,snow,and aerosol samples(Yamashita and Tanoue 2003a;Mostofa et al.2013).Currently,parallel factor(PARAFAC)modelling candisintegrate EEM spectra into independent fluorescence components,thereby providing a unique solution to identify the fluorescent DOM(FDOM)components(Stedmon et al.2003;Mostofa et al.2010).PARAFAC modelling,in combination with EEM spectra,has been used to identify the humic substances(fulvic acids and humic acids)in soil(Zhang et al.2017),humic-like substances in sediment pore water(Tfaily et al.2015;Chen et al.2016a,b;Mostofa et al.2017),sewerage-impacted fulvic acid-like substances and protein-like substances in downstream river(Mostofa et al.2010,2013;Wells et al.2017),marine humic-like substances(Kowalczuk et al.2009;Yamashita et al.2010)and atmospheric aerosols(Chen et al.2016a,b).Currently,EEM–PARAFAC modelling has also been widely applied to detect the humic-like substances of phytoplankton origin(Stedmon et al.2007;Zhang et al.2009),extracellular polymeric substances(EPS)along with their photo- flocculation mechanisms(Shammi et al.2017a,b)as well as metal-DOM interactions in surface water(Yamashita and Jaffé2008).EEM–PARAFAC modelling can therefore be used as an important analytical tool to detect the main DOM components in the cow dung.
For a better understanding of its uses in many regards,this study explored the detection of the FDOM components in cow dung using a combination of EEM and PARAFAC modelling for the first time.In addition,this study also determined the contents of important trace metals and nutrients(and)in cow dung.
2 Materials and methods
2.1 Materials
We collected six samples from five different locations,including Xinjiang Uygur Autonomous Region,Inner Mongolia Autonomous Region,Tianjin City,Guizhou Province and Hubei Province in China due to the variation of plant material in different geographical locations.Each sample(~ 500–1000 g)was collected immediately from fresh bulk cow dung after excretion and then kept in a black polyethylene container.To avoid any kind of further degradation,cow dung samples were immediately stored in a freezer(–20 °C)and then analyzed within 7 days.Cow dung samples(10 g/L)were dissolved in 250-mL deionised water in conical flasks,which were then processed using an ultrasonic system for better dissolution.Then,the solutions were centrifuged at a rate of 5000 r/min for 10 min; finally,they were filtered with 0.45 μm GF/F filters.The filtrate solutions were kept in brown glass bottles that were refrigerated while awaiting further analyses.To extract tyrosine from the cow dung solutions,a freeze-drying method was used.
2.2 Analytical procedures
The electric conductivity(EC)and pH were measured using orion star A329 pH meter(Thermo scientific,USA).EEM spectra were collected with a fluorescence spectrometer(F-7000,Hitachi,Japan).Excitation was scanned from 220 to 400 nm by steps of 5 nm,and emission wavelengths were recorded from 280 to 500 nm at 1 nm intervals with a scanning speed at 1200 nm/min(Mostofa et al.2010)Fluorescence intensity(FI)of samples was corrected with a Milli-Q water blank.PARAFAC model was performed in MATLAB using the N-way Toolbox for MATLAB using methods described in previous studies(Stedmon et al.2003).The data EEMs of the samples were modeled with excitation wavelength ranging from 220 to 400 nm by every 5 nm and emission wavelength from 280 to 500 nm by every 1 nm in this study.Milli-Q water blank was subtracted from every sample before running in the PARAFAC model.A total of 18 EEM spectra from triplicates of six cow dung samples were used for PARAFAC modelling.The variability explained by the PARAFAC analysis was 88%for raw cow dung samples.
Fourier transform infrared(FTIR)spectra were measured using an FTIR Spectrometer(Thermo Nicolet 6700,USA).Liquid samples were evenly applied on the surface of KBr tablets,and then the infrared absorbance was measured.Same solution(10 g/L)of the aforementioned cow dung samples was used to analyze the trace elements.The Octopole Reaction System(ORS3)in the ICP-MS was operated in a single mode,using helium cell gas,which provided a reliable and effective cell method to remove all polyatomic interferences,regardless of the analyte or matrix composition.Calibration was carried out from 0.1 to 500 ppb for all metals studied.Triplicate measurements for each cow dung sample were conducted to obtain the standard deviation.
3 Results and discussion
3.1 Detection of tyrosine in the cow dung and its environmental significance

Fig.1 Fluorescent component of samples(a)and an aqueous solution of standard tyrosine(b)identified using EEM–PARAFAC modelling of the sample’s EEM spectra
The EEM–PARAFAC results demonstrated that only one fluorescence component occurred in the cow dung samples,which displayed two fluorescence peaks:peak T at Ex/Em=275/311 nm and peak TUVat Ex/Em=220/311 nm(Fig.1a).This fluorescent component was identified as tyrosine by comparing with standard tyrosine,which is composed of two similar fluorescence peaks(Peak T at Ex/Em=270/311 nm andPeakTUVatEx/Em=225/311 nm)(Fig.1b).Similar results have been reported in earlier studies(Yamashita and Tanoue 2003a;Mostofa et al.2013).Among the three aromatic amino acids,tryptophan and phenylalanine standards were detected via fluorescence at distinct wavelength regions,showing peak T at Ex/Em=280/340 nm and peak TUV=225/340 nm for tryptophan and showing peaksforphenylalanineat 250/292 nm and 220/292 nm,respectively(Yamashita and Tanoue 2003a;Mostofa et al.2013).Correspondingly,the fluorescence intensity for peak T varied from 167.6±7.6 to 294.2±13.4 a.u.(arbitrary unit)/g cow dung,while standard tyrosine in pure Milli-Q water solution yielded a fluorescence intensity of approximately 30–32 a.u./mg for 25-and 50-mg standard samples.By considering the concentration of fluorescence intensity,a rough estimation demonstrated that the total content of tyrosine varied approximately from 5.6±0.25 to 9.8±0.45 g/kg cow dung in the studied samples.Considering the high amounts of tyrosine in cow dung,it could be advantageous to extract pure tyrosine from cow dung at an industrial scale,which warrants further studies(Table 1).
The presence of tyrosine in the cow dung samples could be further verified by FTIR spectra along with standard tyrosine spectra(Fig.2).In Fig.2,FTIR spectra for standard tyrosine were entirely similar to those in cow dung samples,thereby indicating the occurrence of tyrosine in cow dung.FTIR spectra showed three distinct peaks,such as the in plane and out of plane O–H bending vibrations at the range of 880–399 cm-1,C=O of COO-group at 1700–1600 cm-1,and both O–H(hydrogen-bonded)and N–H stretching at 3446–3200 cm-1(Contreras et al.2011;Bhat and Ahmad 2016).In addition,the occurrence of tyrosine[HO–C645–CH2–CH(NH2)–COOH]in cow dung may microbially producealong with CH4production from an amino-carboxylic acid group present in the tyrosine molecularstructure [HO–C645–CH2–CH(NH2)–COOH],which is labile for microbial degradation(Khan et al.2013;Yamashita and Tanoue 2003b;Mostofa et al.2013).It can be noted that dissolved amino acids comprise the largest identified component,approximately 15%,of the bulk dissolved organic nitrogen(DON)pool(Yamashita and Tanoue 2003b).DON is considered one of the major forms of nitrogen in both freshwater and marine environments and is an important source of nitrogen for heterotrophic bacteria(Voss et al.2013).
It is well-known that methanogenesis occurs in cow dung under anaerobic conditions which produce methane or biogas(Kelleher et al.2000;Salam et al.2015;Alfa et al.2014).Such CH4formation could result from microbial breakdown of high contents of tyrosine,which were identified in cow dung.The reaction mechanism for CH4generation from tyrosine can be depicted below:

These reaction productions are evidenced from earlier studies(Conrad 1999;Kelleher et al.2000;Al Seadi et al.2008;Zupančičand Grilc 2012).Detection of high contents ofin cow dung in this study also confirmed this reaction mechanism.
Moreover,a significant increase in soil nutrients and their subsequent increasing leaching rate into water were observed after the incorporation of both biogas slurry and biochar from cow dung(Wang et al.2006;Lu et al.2012;Guo et al.2014).Such increasing effects could be linked with occurrences of high contents of tyrosine in cow dung.These results therefore indicate that dissolved components of cow dung are substantially suitable for typical sources in surface waters,any type of plantation in soil,the amending of soil,the production of charcoals,the production of biogas-CH4,and the removal of metallic pollution,possibly through complexation of metals with tyrosine aminofunctional groups.Complexation of tyrosine with metals could be a focus of further research.

Table 1 Concentrations of various metals analysed in six cow dung samples using the Octopole Reaction System (ORS3)in the ICP-MS
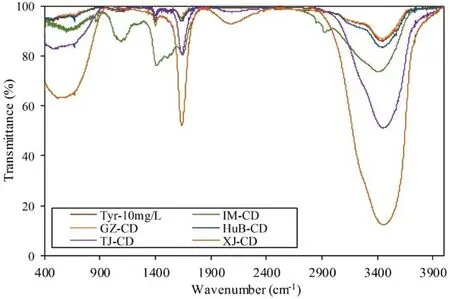
Fig.2 Fourier transform infrared(FTIR)spectra of samples along with standard tyrosine in Milli-Q pure water(10 mg/L)along with cow dung(CD)samples collected from different sites
3.2 Variation in pH,EC,trace metals and nutrients in cow dung
The pH ranged from 6.26 to 7.83 for all raw cow dung samples.Such variation in pH may be partly linked with the predominant occurrence of tyrosine in the cow dung,showing a strong correlation with the fluorescence intensity of peak T with pH(r2= –0.64,p<0.001,n=6).This could occur due to microbial degradation of tyrosine,which can produceand other products.In addition,pH values in cow dung(6.26–7.83)could be similar to good characteristics of soil,which often range from slightly acidic to moderately alkaline(6.1–8.4)in soil environments(Maeda et al.2016;Liebig et al.2017;Ying et al.2017).It suggests that the cow dung pH has the potential to improve soil quality and its properties.The electric conductivity(EC)for all six cow dung samples varied from 125.8 to 252.1 μs/cm.EC was observed to be the highest(245 ± 6.8 μs/cm) for Tianjin and the lowest(176 ± 19.3 μs/cm)for Guizhou cow dung samples.Such variation in EC in different cow dung samples may result from the occurrences of differences in dissolved components in the respective cow dung samples.
The results of the trace metals demonstrate that the concentrations ranged from 30.4 to 246.5 mg/kg(mean=104.4±73.3 mg/kg)forNa,from 65.9 to 240.2 mg/kg(mean 159.1±56.9 mg/kg)for K,from 85.8 to 165.4 mg/kg(mean 132.4±34.5 mg/kg)for Mg,from 10.3 to 24.8 mg/kg(mean 16.9±4.9 mg/kg)for Ca,from 1.2 to 3.9 mg/kg(mean 2.99±1.1 mg/kg)for Fe,from 0.9 to 8.4 mg/kg(mean 3.4±3.5 mg/kg)for Mn,from 0.5 to 4.6 mg/kg(mean 2.5±1.4 mg/kg)for Zn,from 0.7 to 5.0 mg/kg)(mean 2.4±1.5 mg/kg)for Sr in the cow dung samples studied.Concentrations of Cu,Ni and Co were relatively low,ranging from 0.3 to 0.8 mg/kg(mean 0.51±0.2 mg/kg)for Cu,from 0 to 0.13 mg/kg for Ni and from 0 to 0.033 mg/g for Co.Among the metals studied,Na,K and Mg significantly outperformed the other metals,although most of the trace elements are below the standard of the world limit as well as different agricultural soils(Coskun et al.2006;Ahsan et al.2009;Lidman et al.2017),but their contents could be supportive for low deficit soils.Some of these trace metals are considered to be essential elements for plant development,particularly in agricultural-and plantation-based areas wherein cow dung acts as organic fertilizer.
Nutrients were mostly measured asandwhich are key components of N-fertilizer.The concentrations ofandranged from 68.4±19.0 to 330.6±11.7 mg/kg(mean=170.5.±99.0 mg/kg)and from 9.5±0.9 to 10.2±1.3 mg/kg(mean=9.7±0.3 mg/kg),respectively,across all cow dung samples(Fig.3).These results indicate that high concentrations ofandare highly labile and the key component for better acting as an organic fertilizer for agriculture and any other plantation purposes in soil environments.Correspondingly,relatively high contents ofcompared to those ofare susceptible to subsequent production offromthrough nitrification,whilemay originate from microbial degradation of organic nitrogen in tyrosine,which is labile for microbial degradation.
4 Conclusions
The results are summarized below:
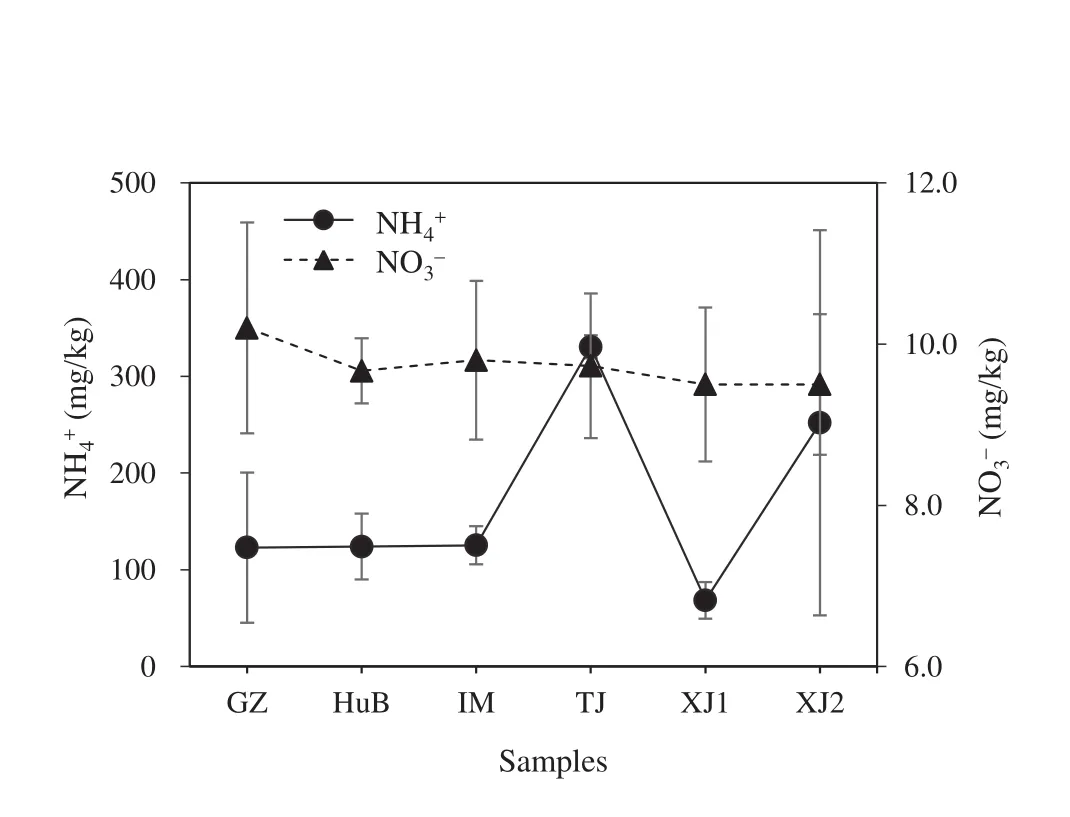
Fig.3 Variation inand concentrations in the cow dung samples.Data represent the mean(±standard deviation,SD)of three independent measurements for the same cow dung sample
1. Cow dung samples mainly contained only tyrosine,which was identified using a combination of EEM–PARAFAC modelling as well as by the FTIR spectra.
2. By considering the concentration of fluorescence intensity between cow dung sample sand standard tyrosine,a rough estimation demonstrated that the total content of tyrosine varied approximately from 5.6±0.25 to 9.8±0.45 g/kg in six cow dung samples.
4. Relatively high contents ofcompared to those ofare susceptible to subsequent production offromthrough nitrification whilemay originate from microbial degradation of organic nitrogen in tyrosine.
5. Trace metals analysis revealed that Na,K and Mg were significantly higher than Ca,Fe,Mn,Zn Sr,Cu,Ni and Co.
6. Detection of tyrosine using a combination of EEM–PARAFAC modelling and its high content in the cow dung is an important paradigm for determining its better use in various purposes.
AcknowledgementsThis study was financially supported by the Key Construction Program of the National 985 Project,Tianjin University,China and the National Key R and D Program of China(2016YFA0601000).
Compliance with ethical standards
Conflict of interestThe authors declare that they have no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- The vanadium isotopic composition of L ordinary chondrites
- Variations of tr ace elements under hydrological conditions in the Min River,Eastern Tibetan Plateau
- Effects of carbon anhydrase on utilization of bicarbonate in microalgae:a case study in Lake Hongfeng
- Enrichment characteristics and risk assessment of Hg in bird feathers from Caohai wetland in Guizhou Province,China
- Carbazoles and benzocarbazoles confirm migration of leaked petroleum through caprocks and overlaying formations of Valhall Well 2/8-8 in the North Sea
- Basic formation mechanisms of Lake Doroninskoye soda water,East Siberia,Russia
