Variations of tr ace elements under hydrological conditions in the Min River,Eastern Tibetan Plateau
2018-08-30XuetaoZhuYunchaoLangJunZhongHuDingHuijunHeZhifengYanSiliangLi
Xuetao Zhu•Yun-chao Lang•Jun Zhong•Hu Ding•Huijun He•Zhifeng Yan•Si-liang Li
Abstract In order to better understand the relative importance of hydrologic variation and anthropogenic disturbance and their complex interactions within the trace elemental geochemical cycle,water samples were collected monthly over 1 year in the Min River,eastern Tibetan Plateau,and analyzed for trace element composition.The dissolved trace elements exhibited different relationships with increasing discharge compared with major elements.The elements analyzed can be divided into three groups according to their behavior in response to changing discharge:(1)elements that showed weak positive correlation with discharge,e.g.Cu,V,and Ba;(2)elements that exhibited weak negative correlation with discharge,including Rb,Sr,Pb,Sb,Zn,Cr,Cd,and U;and(3)elements that displayed no significant correlation with variation in discharge,e.g.Ti,Fe,Co,Ni,and As.Cu was strongly affected by anthropogenic activities and flushed into the river with increasing discharge.Ba has a strong solubility in the terrestrial environment,dissolved quickly,and was released into the river.The positive relationship between V concentration and discharge may be attributed to secondary reactions,such as precipitation and adsorption on oxides and aluminosilicate clays.Conservative behavior had an impact on the geochemical behavior of Sr and Rb across hydrologic variation.Pb,Zn,Sb,Cd,and Cr underwent a mild dilution effect connected with anthropogenic activities.The chemostatic behavior of U was regulated by carbonate dissolution and biological uptake.In addition,higher temperatures enhanced biotic activities,affecting the concentrations of Fe and Ni.The relationship between power law slopes and coefficient of variation for discharge and solute concentration suggests that concentrations of trace elements vary significantly with increasing discharge compared with major elements.Silicate mineral weathering had less effect on the fluvial solutes with increasing discharge.Mining activity may exert an additional control on concentration–discharge dynamics of anthropogenic trace elements.
Keywords Trace elements ·Concentration–discharge relationship·Tibetan Plateau·River
1 Introduction
The study of large rivers provides information about global material transportation(Gaillardet et al.1999).Rivers transport eroded materials in dissolved and solid forms,thereby transporting continental material to the oceans.Element concentration plays an important role in the geochemical response to weathering in the catchment(Gaillardet et al.1999;Rai et al.2010).Fluvial trace elemental compositions are good indicators of specific geochemical processes,including secondary reactions(Shiller and Mao 2000;Stefansson and Gislason 2001)and nonconservative behavior(Gaillardet et al.2014).They are also highly sensitive proxies of human impacts in rivers(Chen et al.2014;Gaillardet et al.2014),especially the rivers originating from the Tibetan Plateau,which have attracted much attention for the critical evidence they provide of plateau uplift and erosion processes(Galy and France-Lanord 2001;Singh 2010;Van Hoang et al.2010).
Trace elements in rivers are mainly derived from rock weathering,mine waste water,and other anthropogenic inputs.Trace element distribution can be modified by several factors including catchment lithology,weathering processes,and adsorption processes(Murnane and Stallard 1990;Palmer and Edmond 1993).Recent research has highlighted how dissolved solutes derived from chemical weathering in catchments are affected by hydrological processes(Godsey et al.2009).For example,Shiller(1997)undertook a monthly sampling campaign and analyzed trace elements, finding that significant seasonal dissolved concentration changes were observed for Ba and U.Moon et al.(2014)showed that prominent uncertainties in catchment-scale silicate weathering rates were due mostly to variations in discharge and cation fractions from silicate substrates.Investigations of 59 diverse United States catchments resulted in a power-law model fitting the concentration–discharge relationship(C–Q)(Godsey et al.2009).Moving forward,much more attention should be paid to temporal variations of solutes to illuminate the relative importance of hydrologic variation and anthropogenic disturbance as well as their complex interactions with the elemental geochemical cycle(Walling 1997).
To date,little attention has been paid to temporal variations of trace elements in rivers,particularly rivers originating from the Tibetan Plateau.By analyzing element concentrations in the Min River,chemical weathering of the river catchment over the most intense area of erosion should be elucidated.The chemical denudation rate of the Min River is higher than the average Yangtze basin rate(Qin et al.2006).In addition,in contrast to the upstream reaches of the river,the downstream reaches show a significant anthropogenic contribution to major dissolved elements.In this study,we examined dissolved major and trace element contents in the Min River.The research objectives were to understand:(1)temporal variations of the major and trace elements on the intra-annual time scale,(2)factors affecting the covariation of concentration and discharge,and(3)the underlying biogeochemical processes that affect trace element contents.
2 Methods
2.1 Study site
The Min River,which stretches for 735 km,is one of the most important tributaries in the upper reaches of the Yangtze River with a catchment basin area of 13.6×104km2.The Min originates from the eastern margin of the Tibetan Plateau and flows through the densely populated and industrialized Sichuan basin to join the Yangtze River at Yibin(Fig.1).There are two major tributaries of the Min,Dadu River and Qingyi Jiang,whose confluence is in Leshan,in the southwest of the Sichuan basin(CWRC 1999).The section from Songpan to Dujiangyan defines the upper reaches of the Min with a drainage area of 2.3×104km2,and a length of 341 km over which it loses about 900 m in elevation.The middle reaches stretch from Dujiangyan to Leshan with a length of 245 km.This section flows mainly through agricultural and industrial areas in Sichuan,including most of the population in the major cities of Sichuan Province.The downstream reaches of the Min comprise the section from Leshan to Yibin with a length of 155 km.Apart from the source and upper reaches,which are characterized by high elevation and a cold climate,the Min catchment is characterized by a humid subtropical climate,with seasonal variations of air temperature and discharge.Mean annual precipitation in the catchment ranges from 500 to 800 mm on the eastern Tibetan Plateau to 1200–1500 mm in the Sichuan basin.There is an obvious seasonal variation in precipitation,with a rainy period (June–September)accounting for about half of the total annual precipitation(Qin et al.2006).The section above Dujiangyan is on the boundary between the Tibetan Plateau and the Chengdu Plain.The lithology of this region is complicated,but dominated by sandstone,phyllite,granite,and slate,occasionally intercalated with carbonates and volcanic rocks(Qin et al.2006).The catchment below Dujiangyan lies at the edge of the Sichuan platform.Sandstone,shale,and claystone are widely distributed in the river catchment downstream of Dujiangyan.
2.2 Sampling
Twenty water samples were collected monthly from November 2013 to October 2014.In addition to the monthly samples,the high- flow season(June–August)was sampled more intensively(an extra eight samples)to assess the significance of enhanced discharge on trace elements.The sampling site is at the hydrological station of Gaochang,28 km away from the Yangtze River mainstem(Fig.1).Gaochang station is the gauging site for the entireMin River and is the most representative locality to assess geochemical characteristics and anthropogenic impact in the lower Min River catchment(Qin et al.2006).All water samples were collected with a high-purity acid-precleaned 120-mL LDPE(low density polyethylene)bottle near the middle of the river flow using a boat.Water temperature,pH,and electrical conductivity(EC)were measured in the field.Alkalinity was determined by titration with hydrochloric acid within 24 h.Water samples were filtered through a 0.22-μm polytetra fluoroethylene filter within 24 h of sampling.The filtered solution was acidified to pH<2 with ultra-purified HNO3and refrigerated at about 4°C until analysis.Storage bottles were washed with high purity HCl and rinsed with Milli-Q water(18.2 MΩ)before sampling.Discharge data were obtained from the website of the ministry of water resources of China(http://xxfb.hydroinfo.gov.cn/).

Fig.1 a Sketch map showing the location of the Min River catchment in China.b Map of the Min River catchments showing the sampling locations and major cities(the figure in left bottom was the discharge of the catchmen 3t.The dashed line was 3000 m/s,which is divided the whole year into the high flow season and the low- flow season)
2.3 Analytical procedures
The major cations(K+,Na+,Ca2+,and Mg2+)and Si were measured using an inductively coupled plasma optical emission spectrometer(ICP-OES).The anions(,Cl-, and) were determined by ionic chromatography.The reproducibility of replicate analyses was better than 5%for all samples.Most work on the main ions has been published previously(Zhong et al.2017).Trace elements were analyzed using a Thermo Fisher iCAP Qc ICP-MS at the Ocean University of China.The accuracy and precision of the analyzing elements were controlled by adding an internal standard(Rh)and replicate analyses of SLRS-5.The discrepancy between triplicates was 5%and the accuracy and precision of the analytical method were established using international standard BCR-2(Columbia River Basalt).The accuracy and precision with the recommended values were better than 5%.
3 Results
The ranges,means and mean flow weighted concentrations(MFWCs)of trace element concentrations are presented in Table 1.As mentioned above,major element and hydrochemical data were previously reported by Zhong et al.(2017)and are used for discussion purposes alongside the new trace element data presented in this study.
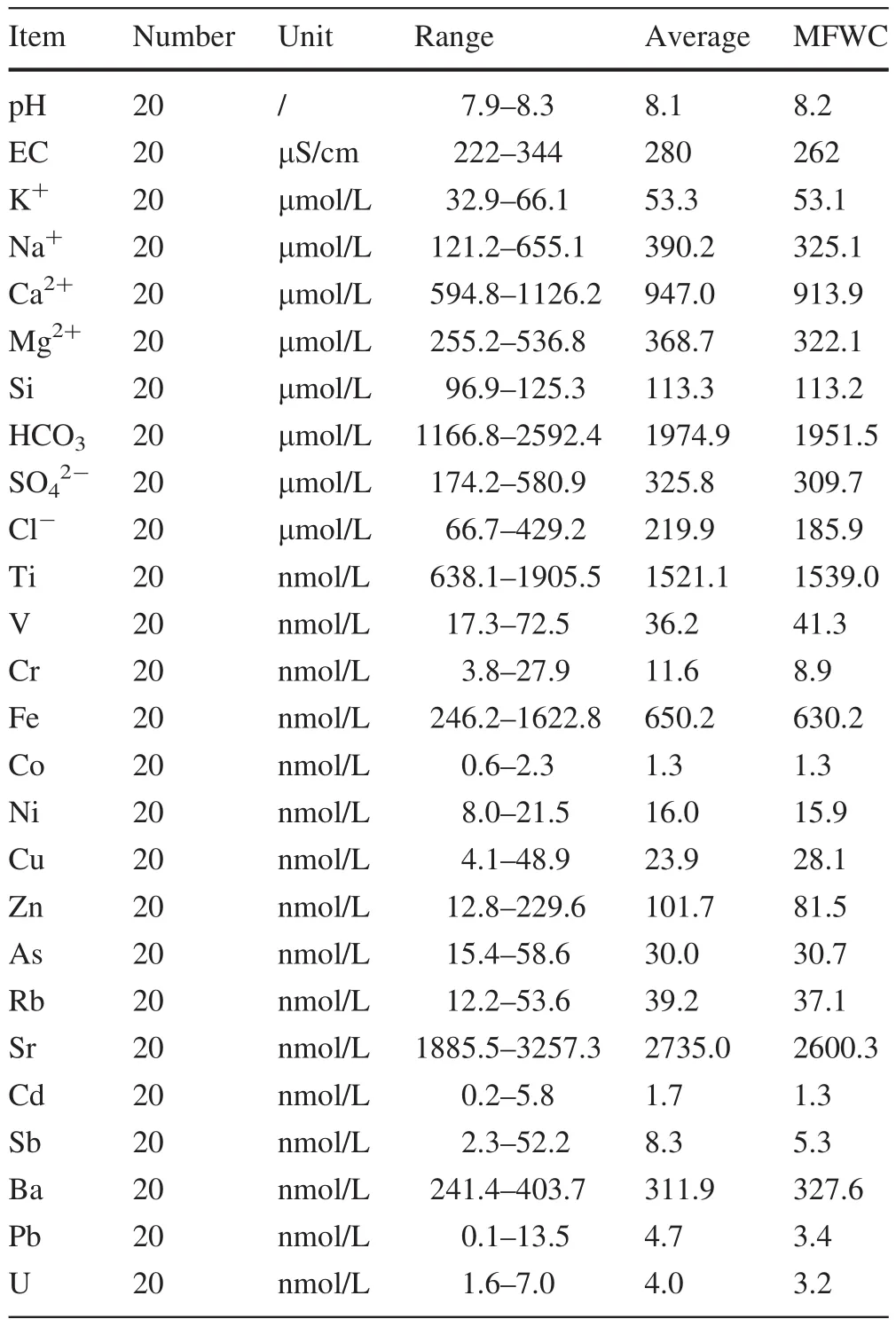
Table 1 The range of basic hydrochemical parameters and interesting elements in Min River
3.1 Major element distribution
The pH,EC,and trace element concentrations of the monthly samples of the Min River water are summarized in Table 1.The MFWC of total dissolved solids(TDS)in the Min River was 228.8 mg/L,about twice the world average of 100 mg/L(Gaillardet et al.1999).High TDS values have previously been found in other Tibetan rivers(Huang et al.2009;Jin et al.2011).The total cationic charge(TZ+=Na++K++2Ca2++2 Mg2+) ranged from 2543 to 3958 μeq/L,with an average of 3036 μeq/L—about 2.7 times the world average for rivers(1123 μeq/L)(Lietal.2014b).The total anionic charge(TZ-=+Cl-++)ranged from 2400 to 3633 μeq/L.We used the Normalized Inorganic Charge Balance(where NICB=(TZ+-TZ-)×100%/TZ+)to estimate overall analytical uncertainty.NICB was characterized by mild inorganic charge imbalance,generally within±5%.The highest discharge occurred in July 2014,and the lowest in May 2014.We divided the year into two periods:the low- flow season(from November 2013 to May 2014)and the high- flow season(from June 2014 to October 2014)according to water discharge data.The dominant ions in the river water were,Ca2+,and Mg2+,together accounting for more than 65%of the total ions.This indicates that water chemistry is dominated by carbonate weathering as previously observed in rivers originating from the Tibetan Plateau(Gaillardet et al.1999;Galy and France-Lanord 1999;Qin et al.2006;Wu et al.2008).As shown in the ternary diagram(Fig.2),Ca2+is the most abundant cation for all these large rivers(Min River,Dadu River,Yangtze River);samples of the Yangtze,Mekong,and Jingsha River were dramatically affected by evaporite dilution(Elbaz-Poulichet et al.1999).The Ca2+content of the Min ranged from 594.8 to 1126.2 μmol/L,with an average of 947.0 μmol/L.Other Himalayan Rivers are also reported to be dominated by Ca2+(Galy and France-Lanord 1999;Noh et al.2009;Li et al.2014a).The most abundant anion in the studied area was,ranging from 1166.8 to 2592.4 μmol/L.The impact of increasing discharge was strong on the major elements Na+and Cl-,which exhibited lower contents in high- flow conditions,demonstrating a distinct dilution effect on these soluble elements(Fig.3).
3.2 Trace element characteristics
As shown in Table 1 and Fig.4,Sr,Ti,and Fe were the main trace elements in the studied river waters and were two orders of magnitude higher than other trace elements.The contents of Sr,Ti,and Fe ranged from 1885.5 to 3257.3,638.1 to 1905.5,and 246.2 to 1622.8 nmol/L,respectively.The MFWC of Sr,Ti,and Fe were 2735.0,1521.1,and 650.2 nmol/L,which are 4.0,149.3,and 0.6 times world average values,both respectively(Gaillardet et al.2014).
Unlike the major elements,the trace elements changed dramatically over the annual cycle,especially in the high flow season,which may be attributed to their specific behavior in the catchment.
4 Discussion
4.1 Concentration–discharge relationship analysis
The covariation between discharge and dissolved solute concentrations can be approximated to a power-law relationship(Moon et al.2014;Torres et al.2015;Baronas et al.2017;Li et al.2017):

Fig.2 Ternary plots of the dissolved major cations(Ca2+,Mg2+,K+and Na+)on an equivalent concentrations(μmol/L)basis in the studied river and other large rivers originated from the Tibetan Plateau in main stream

Fig.3 The temporal variations of major elements concentrations,accompanied with variable discharges

whereCis the concentration of dissolved solutes,Qcorresponds to instantaneous discharge,parametersaandbare constants,with the exponentbthe slope of the best liner fit describing the correlation between concentration and discharge.bapproaching zero indicates chemostatic behavior(concentration of trace element was relatively constant with variable discharge),bapproaching-1 indicates that concentrations vary inversely with discharge,and the dilution effect is the dominant process controlling element concentration changes(Godsey et al.2009).

Fig.4 The concentration(nmol/L)of trace elements and discharge(m3/s)in a whole year
C–Q for major elements has been discussed previously by Zhong et al.(2017).Na+and Cl-concentrations exhibited a strong negative correlation with discharge,indicating that they were affected by the dilution effect to different degrees.Ca2+,Mg2+,andions were mainly derived from weathering of carbonate minerals with a high weathering rate.Furthermore,chemical index of alteration values for limestone and calcite were all above 1,indicating regulation of element concentrations by precipitation.Therefore,these ion species showed a chemostatic–dilutional relationship with increasing discharge.The exponentbforalso varied between-1 and 0,mainly ascribed tobeing derived from different sources from the other ionic species including dissolution of evaporates and pyrite weathering.As shown in Table 1,K+was the lowest concentration major cation in the river(32.94–66.10 μmol/L),probably reflecting ion exchange between soil and water.In contrast to the major elements,trace element concentrations behaved in different ways with increasing discharge(Fig.5),similar to the findings of a previous study offluvial trace elements(Kirchner and Neal 2013).Most trace elements did not correlate significantly with discharge(R2from<0.01 to 0.57).The trace elements analyzed can be divided into three groups according to their behavior in response to changing discharge:(1)elements that showed weak positive correlation with discharge(positivebexponents)(Cu,V,and Ba),(2)elements that exhibited weak negative correlation with discharge(Rb,Sr,Pb,Sb,Zn,Cr,Cd,and U),and(3)elements displaying no significant correlation with variation in discharge(Ti,Fe,Co,Ni,and As).Cu is an indispensable element for building cells and catalyzing biochemical transformation;therefore most pristine Cu in a river would be assimilated by terrestrial organisms and exhibit a strong affinity for sulfides(like pyrite)(Chen et al.2014).As noted above,oxidation of sulfides plays an important role inconcentration of rivers originating from the Tibetan Plateau(Hren et al.2007;Li et al.2014a).Meanwhile,Cu was strongly affected by anthropogenic activities(smelting and mining);concentration of Cu increased significantly in response to pollution of the river.As an alkaline earth metal,Ba has a strong solubility in the terrestrial environment.According to Wu et al.(2013),the bedrock in Min has high concentrations of Ba,compared with UCC(Upper Continental Crust)and PAAS(Post-Archean Australian Shale)and is readily leached into solution(Nesbitt et al.1980;Cullers 1988).With increasing discharge,more Ba in the bedrock would be quickly dissolved and released into the river.Concentrations of Sr were all above 1 μmol/L in the Min River,which is much higher than the world average value of 0.68 μmol/L(Gaillardet et al.2014).Fluvial Sr and Rb are always controlled by chemical weathering of bedrock in research catchments(Goldstein and Jacobsen 1987).As a host mineral of Sr,carbonate weathering is an essential control on Sr concentration.Additionally,previous studies(Qin et al.2006;Zhong et al.2017)have shown that the Saturation Index of Calcite(SIC)was above 0 in the lower Min River near Gaochang Station.Thus,Sr displayed conservative behavior in the Min River with b<0(Fig.5).
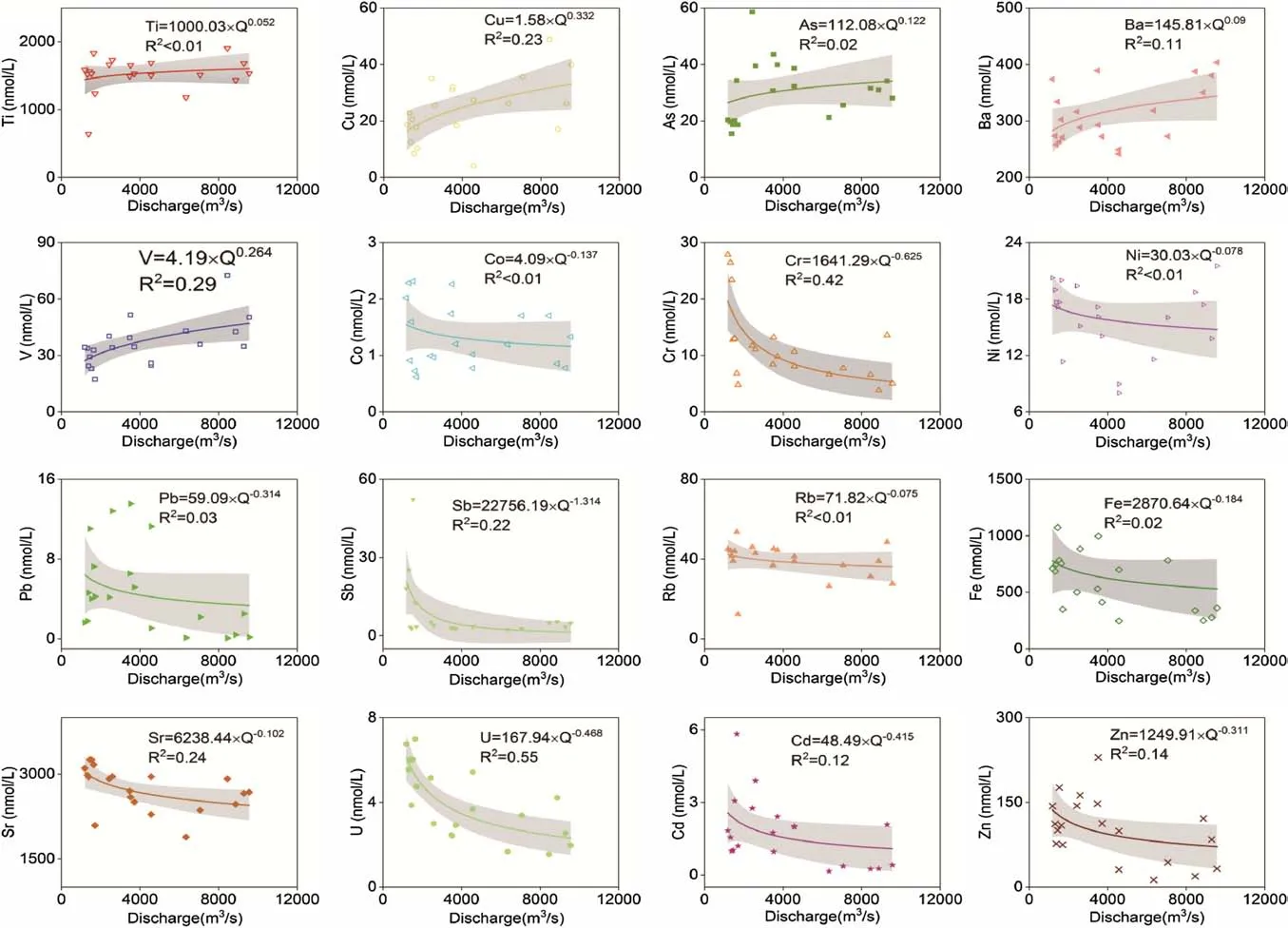
Fig.5 The relationship between concentrations of trace elements and discharge.The solid line was a power-law fit line,the grey zone denotes 95%confidence interval
Pb,Zn,Sb,Cd,and Cr contents in the Min River during the low- flow season were about 1.47-fold,1.44-fold,3.97-fold,2.14-fold,and 1.80-fold higher than those in the highf l ow season,respectively,demonstrating a mild dilution effect.These elements are often attributed to human activity such as smelting,domestic sewage,or mining and therefore they are dominated by ‘‘anthropogenic overprints’’(Chen et al.2014).Correspondingly,theirbvalues were all between-1 and 1.As shown in Fig.5,the power-law slope for V was near 0.Fluvial dissolved V was primarily derived from siliceous rocks(Shiller and Mao 2000)and exhibited similar behavior to the dissolved silica with silicate.Weathering of riverine V-enriched sulfides would also increase V concentrations.With rapid increasing water flow,the interaction between rock and water was strongly impacted by secondary reactions,such as precipitation and adsorption on oxides and aluminosilicate clays.These reactions could potentially have a significant impact on the observed C–Qs.For example,secondary mineral precipitation was sensitive to fluid flow paths and transit times,which were likely to change with discharge.The slopebof U was-0.468(Fig.5).Concentration of U in the river was mainly controlled by weathering of limestone(Palmer and Edmond 1993)and in UO2().Consequently,its behavior should be similar to carbonate-derived ions(Ca2+,Mg2+,and).However,U does not show strongly chemostatic behavior(b=-0.468).This may reflect biological uptake of U(Shiller 1997).Fe,Co,and Ni are insoluble in river water,forming colloids and undergoing precipitation.Fe and Ni are also essential elements for cell growth in organisms.The higher temperatures and increasing discharge in summer enhance biotic activities,increasing adsorption of Fe and Ni.
4.2 Dilutional–chemostatic behavior of trace elements
According to Thompson et al.(2011),the chemostatic behavior of elements can be defined as the ratio of coefficients of variation of concentration and discharge(CVC/CVQ).The equation is defined as:
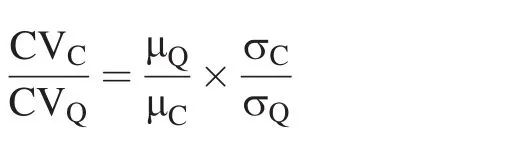
whereCVis the coefficient of variation for different elements,defined as the standard deviation σ divided by its mean value μ.CVC/CVQ≪ 1indicates chemostatic behavior,which is seen as a buffer to change in concentration.Changeable discharge is the uppermost factor to control solute concentrations as CVC/CVQ=1.Relatively low CVC/CVQshould not be over regarded as a chemostatic behavior rule.The term chemostatic does not mean that variable discharge does not exert a control on elemental concentration.Rather,the term implies that concentrations exhibit little change relative to the changing discharge.
All CVC/CVQratios of all trace elements were above 0.65(the maximum was 1.708)(Fig.6).In contrast,the major ions exhibited strongly chemostatic behavior(b≤0.01,CVC/CVQ≤0.02)indicating that their concentrations do not vary significantly with increasing discharge.The distributions of some major elements(such as Na+,Cl-)fall near the dilution line(slope of plot equals-1),representing concentrations easily affected by flow dilution.In addition,a significant dilution effect was observed in U and Cr,which behaved similar to major ions.Ti,As,Rb,and Sr were spread around the central point(b=0,CVC/CVQ=1),showing no significant statistical behavior.Co,Ni,Ba,Cd,Zn,V,Fe,Cu,and Pb were substantially more scattered in the plot than other trace elements,reflecting concentrations that were rarely related to flow rate(termed chemodynamic).Likewise,the C–Q power law slopebin the catchment was flushing-driven(b>0.1)for V,Cu,and As,and dilution-driven(b<-0.1)for Cr,Fe,Co,Zn,Sr,Sb,and Pb.As previously reported(Musolff et al.2015),Ba,V,and Cu displayed strongly chemodynamic behavior(CVC/CVQ≥1)and had positive law slopes.Nevertheless,the correlation coefficients(R2)ofbvalues were insignificant(close to zero)for solutes of Ti,Co,Ni,Rb,As,and Pb.Hence,it could be inferred that some external sources other than the dilution effect significantly contributed to the variations of these elements.

Fig.6 Relationship between coefficients of variation for discharges(CVC)divided solute concentrations(CVQ)and power law slopes(b)for different elements
4.3 Chemical weathering responding to concentration–discharge relationships of trace elements
The concentrations of Ba and V were driven by a flushing effect(b>0)as described above.The V in the river is mainly from silicate weathering,especially of hard rocks(Si+Alk/Si>7.1)(Shiller and Mao 2000).All(Si+Alk)/Si ratios of silicate rocks in this drainage basin are above 7.1.The ratios of Si/(K+Na)could represent differential weathering intensity in different catchments.Dissolved solutes in rivers are mainly derived from gibbsite and kaolinite dissolution when the ratio ranges from 1.0 to 2.0.In our study this ratio was lower than 1.5,suggesting that silicate mineral weathering did not contribute significantly to the fluvial solutes with increasing discharge.As an alkaline earth element,Ba was active in the water–rock interactions and easily mobilized during chemical weathering(Dupréet al.1996;Chen et al.2014).As mentioned above,chemical weathering in the Min River catchment is dominated by carbonate weathering.As a riverine trace element,Ba is related to the dissolution of carbonate and evaporite.We used the relationship between Ba2+/Sr2+and Ca2+/Sr2+to identify the relative contribution from different sources to the dissolved phase in the river water(Fig.7).Ca2+/Ba2+ratios in the high- flow season were higher than those during low flow.This may be connected with lower temperature in the low- flow season,which would decrease the solubility of Ca2+.Zhong et al.(2017)showed Ca2+displayed significantly more stable biogeochemical behavior with increasing discharge.Therefore,Ba had different potential origins with variable discharge(as shown in Fig.8a,b).Aside from the dilution effect(indicator of Na+),Ba increased notably,indicating that Ba may have similar sources to Ti and.As mentioned above,was affected by sulfide oxidation and anthropogenic activities for most rivers originating from the Tibetan Plateau.

Fig.7 The relationship between Ba2+/Sr2+(mol/mol)and Ca2+/Sr2+(mol/mol)in different discharges
The contents of Rb,Sr,Ba,and V exhibited weak correlation with increasing flow,suggesting that these elements were mainly derived from carbonate mineral weathering and not significantly influenced by anthropogenic inputs(Gaillardet et al.2014).Meanwhile,it has been reported that man-induced contents of trace elements in the atmosphere such as Pb and Ni far exceed the contents derived from natural sources(Nriagu and Pacyna 1988),and enter rivers following rains during the high- flow season.The dilution pattern of trace elements suggests these elements mainly originate from silicate weathering due to low dissolution rate of the silicate mineral.The study catchment—especially in upper and middle reaches—hosts significant mining activity including many metallic sulfide deposits(Sun 2010).This may contribute to the increased dissolved solutes with increasing discharge.In addition,some specific minerals like barite and perovskite in the catchment might also influence the concentration of Ba.
5 Conclusions
Analyses of the dissolved trace element chemistry of the Min River catchment exhibited different geochemical characteristics over a 1-year period.Sr,Ti,and Fe were the dominant trace elements in the study region and were two orders higher in concentration than the other elements.In contrast to the major solutes,most trace element concentrations showed a weak correlation with instantaneous discharge(R2from<0.01 to 0.57).According to their different behavior with increasing discharge,we divided the trace elements into three groups:(1)Cu,V,and Ba with weak positive correlation with discharge,(2)Rb,Sr,Pb,Sb,Zn,Cr,Cd and U with a weak negative correlation with discharge,and(3)Ti,Fe,Co,Ni,and As whose concentrations displayed no significant correlation with variations of discharge.Biological uptake likely affects concentrations of some elements that are essential to organisms,e.g.Cu and Zn.In addition,trace element concentrations are thought to be controlled by catchment lithology,characteristic minerals(like sulfides),secondary reactions,and human activity(smelting and mining).The relationship between power law slopes and CVQand CVCsuggests that concentrations of major elements do not vary significantly with increasing discharge,in contrast to trace elements.According to the relationships between Ba2+/Sr2+and Ca2+/Sr2+,1000Ba2+/Na+and 1000Ti4+/Na+,and 1000Ba2+/Na+and/Na+,Ba was mainly derived from carbonate mineral weathering and not significantly influenced by anthropogenic inputs.Furthermore,some trace elements,e.g.Pb and Ni,which can be greatly influenced by anthropogenic contribution,showed a strong response to rainfall events,suggesting that their transport to the river by run-off can exert a major influence during the high- flow season.The dilution pattern of trace elements suggests these elements mainly originate from silicate weathering due to slow dissolution rate of silicate minerals.The studied catchment,especially in its upper and middle reaches,is subject to mining activity including many metallic sulfide deposits.This would also affect riverine trace element concentrations.

AcknowledgementsThis work was financially supported by the National Key R&D Program of China(Grant No.2016YFA0601002),and National Natural Science Foundation of China(Grant Nos.41372376,41422303,41571130072,41561134017).We thank Rob M.Ellam for his help in polishing English,which largely improved the manuscript.
杂志排行
Acta Geochimica的其它文章
- The vanadium isotopic composition of L ordinary chondrites
- Effects of carbon anhydrase on utilization of bicarbonate in microalgae:a case study in Lake Hongfeng
- Enrichment characteristics and risk assessment of Hg in bird feathers from Caohai wetland in Guizhou Province,China
- Carbazoles and benzocarbazoles confirm migration of leaked petroleum through caprocks and overlaying formations of Valhall Well 2/8-8 in the North Sea
- Basic formation mechanisms of Lake Doroninskoye soda water,East Siberia,Russia
- Ore genesis of Badi copper deposit,northwest Yunnan Province,China:evidence from geology, fluid inclusions,and sulfur,hydrogen and oxygen isotopes
