Effects of carbon anhydrase on utilization of bicarbonate in microalgae:a case study in Lake Hongfeng
2018-08-30HaitaoLiYanyouWuLihuaZhao
Haitao Li•Yanyou Wu•Lihua Zhao
Abstract A bidirectional labeling method was established to distinguish the proportions of and CO2utilization pathways of microalgae in Lake Hongfeng.The method was based on microalgae cultured in a medium by adding equal concentrations of NaH13CO3with different δ13C values simultaneously.The inorganic carbon sources were quantified according to the stable carbon isotope composition in the treated microalgae.The effects of extracellular carbonic anhydrase(CAex)on the and CO2utilization pathways were distinguished using acetazolamide,a potent membrane-impermeable carbonic anhydrase inhibitor.The results show utilization of the added was only 8%of the total carbon sources in karst lake.The proportion of theutilization pathway was 52%of total inorganic carbon assimilation.Therefore,in the natural water of the karst area,the microalgae used less bicarbonate that preexisted in the aqueous medium than CO2derived from the atmosphere.CAex increased the utilization of inorganic carbon from the atmosphere.The microalgae with CAex had greater carbon sequestration capacity in this karst area.
Keywords Microalgae·Carbonic anhydrase·
1 Introduction
High pH and high concentration of bicarbonate are two typical characteristics of karst lakes.The main component of karst is MgCa(CO3)2,a highly soluble rock.The proportion of CO2in total dissolved inorganic carbon(DIC)is less than 1%in high pH conditions(Riebesell et al.1993).Thus,the CO2in aquatic media that can be directly utilized by photosynthesis in microalgae is limited(Talling 1976).Several microalgae have adapted by forming carbon-concentrating mechanisms(CCMs)to increase CO2concentrations to meet their photosynthetic demands(Colman et al.2002;Giordano et al.2005).Another strategy is to utilize bicarbonate(Colman et al.2002).Carbonic anhydrase(CA)may play the key role in these carbon assimilation systems.
CA(EC 4.2.1.1),a zinc-containing metalloenzyme,catalyzes the reversible interconversion betweenand CO2.CA is one of the most important enzymes in physiological processes and significantly accelerates the photosynthetic assimilation of inorganic carbon(Ci)(Badger and Price 1994;Sültemeyer 1998).CA is widely distributed and multiple types exist in microalgae.One of the most important CAs is extracellular CA(CAex),which may be involved in CCMs and inutilization(Williams and Turpin 1987;Badger and Price 1994;Elzenga et al.2000;Mondal et al.2016).
Stable carbon isotope(δ13C)analysis is an important tool to identify various Ci sources(Fry and Sherr 1984;Bade et al.2006;Chen et al.2009).Different Ci sources and assimilation mechanisms cause variations in δ13C fractionation.Thein the uncatalyzed pathway produces approximately 10‰ of δ13C fractionation(Mook et al.1974),whileassimilation catalyzed by CAex produces only 1.1‰of δ13C fractionation(Marlier and O’Leary 1984).An approximately 9‰ discrimination of carbon isotope has been found between thecatalyzed by CAex and that uncatalyzed(Wu et al.2012).
Acetazolamide(AZ)is a potent membrane-impermeable CA inhibitor that selectively inhibits CAex activity(Moroney et al.1985).The addition of AZ enables determination of the effect of CAex on Ci utilization.
Several studies have investigated mechanisms of Ci utilization in microalgal species(Axelsson et al.1995;Moazami-Goudarzi and Colman 2011;Moulin et al.2011;Smith-Harding et al.2017).However,the conventional technique cannot quantify the proportions of DIC sources and their microalgal pathways in karst lakes(Xie and Wu 2017).This is the aim of this study.
To this end,microalgae from Lake Hongfeng were cultured in different concentrations of NaHCO3and AZ.The proportion of Ci sources and pathways were determined by comparing their δ13C compositions under separate experiments adding two labeled δ13C bicarbonates.We then estimated the contribution of microalgal CAex to Ci sources and utilization pathways in the karst lake.
2 Materials and methods
2.1 Research site
Lake Hongfeng(106°19′to 106°28′E,26°26′to 26°35′N)is in central Guizhou Province in the core of the southwest karst area of China.The concentration ofin Lake Hongfeng is 1.0–2.5 mmol/L,and the pH is 8.1 ± 0.4(Wu et al.2008).
2.2 Microalgae incubation
The microalgal samples were obtained from Lake Hongfeng.All samples were incubated at 25.0± 1.0°C under a 150 μmol m-2s-1light intensity with a 12/12 h day/night cycle.The pH was adjusted to 8.10 by adding NaOH.AZ was obtained from Sigma-Aldrich Co.(St.Louis,USA).
The microalgae were grown in the lake water in Erlenmeyer flasks after filtration through a 45-μm glass microfiber filter.Treatments are listed in Table 1.The cultures were treated for 5 days.
2.3 Measurement of the microalgal growth
Microalgal protein was analyzed using the method of Coomassie Brilliant Blue(Sedmak and Grossberg 1977).A volume of 5–10 ml of microalgae was centrifuged,and then resoluted.The optical density was tested using a spectrophotometer(Labtech UV-2000,Boston,USA)at595 nm(OD595).The protein content is expressed as ug/L based on the aqueous medium.

Table 1 Experimental treatments of the microalgae
2.4 Measurement of δ13C in microalgae
The microalgae materials were freeze-dried and then converted to CO2at 850°C in a quartz tube with copper oxide to provide oxygen for combustion.The extracted CO2from the samples was purified as follows.
Water and oxygen were removed from the gas stream using two traps.The first was an alcohol–liquid nitrogen mixture to separate the water vapor,and the second was liquid nitrogen to condense the CO2.After this double distillation,the isolated CO2was collected into a sample tube.The CO2sample was analyzed with an isotope ratio mass spectrometer(Finnigan MAT 252,Bremen,Germany).All isotopic compositions(δ13C)are expressed as per mille(‰)and compared with the Pee Dee Belemnite(PDB)standard[see Eq.(1)].The analytical precision was± 0.1‰.
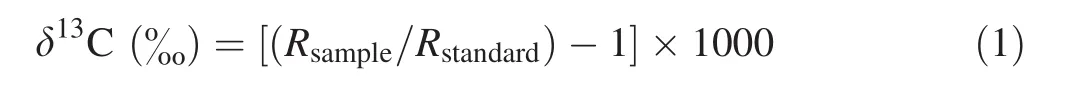
whereRsampleandRstandardare the ratios of heavy to light isotope (13C/12C)of the sample and the standard,respectively.
2.5 Distinguishing the different carbon sources and metabolic pathways
This study chose an open system to simulate natural conditions.In this type of system,Ci in the liquid medium and the atmosphere is in dynamic balance.We created a bidirectional labeling method that simultaneously cultured microalgae in NaHCO3of different δ13C to address this problem.
There is an assumption that the proportion of added Ci utilization is the same under the same concentration ofat the same time regardless of which labeledwas added.This is the theoretical basis of the bidirectional labeling method.
In general,algae can utilize DIC from the atmosphere and added.Therefore,the δ13C of the algae was fit for the bivariate isotope-mixture model that can be expressed as:
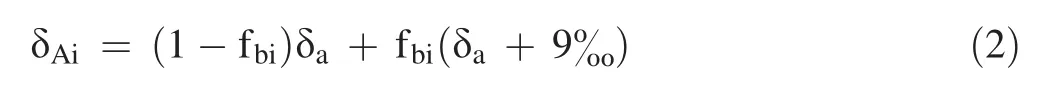
where δTiis the δ13C value of the algae cultured in the same concentration ofwith different δ13C values;fBiis the proportion of the utilization of DIC from the addedin the total carbon sources used by the microalgae;and δAiand δBiare the δ13C values of the algae after using DIC from atmospheric CO2or from the added,respectively,as their sole carbon sources.
In this experiment,the microalgae could utilize both CO2andas carbon sources.Approximately 9‰carbon isotope discrimination has been observed between CO2andutilization pathways(Wu et al.2012).Therefore,δAiand δBican be expressed as follows:

where fbiis the proportion of thepathway;δais the δ13C value of the algae after using DIC in the sole form of the CO2utilization pathway from the atmospheric source;and δaiis the δ13C value of the algae after using DIC in the sole form of the CO2utilization pathway of the addedsource.
Based on Eqs.(3)and(4),Eq.(2)can be expressed as:
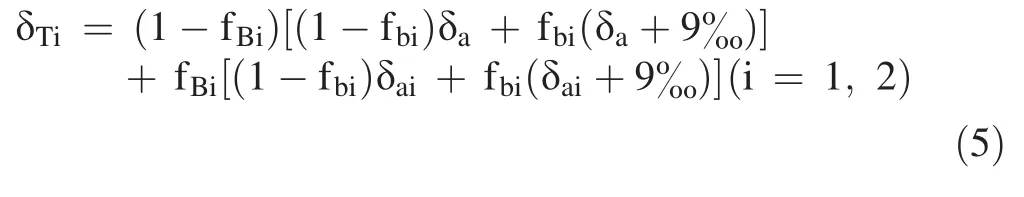
Equation(5)can be simplified to:

For the two labeled types of NaHCO3in our experiments,Eq.(6)can be rewritten as:

There are some important facts that we should note in Eqs.(7)and(8).The first is that the proportion of added Ci utilization is the same at the same concentration ofregardless of which labeled NaHCO3was added.Therefore,fB1=fB2=fB.The second is that the proportion of thepathway is the same at the same concentration ofregardless of the labeled δ13C of NaHCO3.Therefore,fb1=fb2=fb.Although δa1and δa2cannot be obtained,the difference between them is simply the difference between δ13C values of the first and second labeled NaHCO3in the medium.Therefore,the(δa1- δa2)can be replaced with(δC1- δC2).Therefore,fBcan be expressed as follows:

where δC1and δC2are the δ13C values of the first and second labeled NaHCO3in the medium,respectively.From Eq.(9),it can be concluded that the proportion of the utilization of DIC from the addedin the total carbon sources used by the microalgae(fB)was dependent only on the δ13C values of the algae harvested and the labeled NaHCO3added,regardless of DIC form and origin.
The(δai- δa)in Eq.(6)can be replaced with(δCi--δC0).A new equation can then be formulated as:

Therefore,Eq.(6)can be rewritten as:

The proportion of the HCO3-pathway(fb)can then be calculated as:

When fbi=0,Eq.(12)can be rewritten as:

From Eqs.(9),(12),and(13),the proportion of thepathway by the microalgae(fb)can be calculated.It was dependent only on the δ13C values of the algae harvested and the labeled NaHCO3added.
To analyze the complete picture of Ci utilization,the bidirectional labeling method(NaH13CO3with different δ13C values added)can solve the difficulties of the time course of parameters(concentrations and isotopic data in incubation).
2.6 Statistical analysis
All experiments were conducted in triplicate.Data are expressed as mean±standard error.
3 Results
3.1 Microalgae biomass
The content of protein in the treated microalgae increased in parallel with the NaHCO3added(Table 2).However,it decreased with increasing concentrations of AZ added.Under the same concentration of NaHCO3,microalgae growth was severely restricted by AZ.Compared to the control,the average effect of AZ on the microalgal protein content was69% at1.0 mmol/L AZ and35% at10.0 mmol/L AZ.Among all treatments,the maximal growth rate treatment(20.0 mmol/L NaHCO3without AZ)was 4.12-fold higher than the minimal treatment(1.0 mmol/L NaHCO3with 10.0 mmol/L AZ).

Table 2 The protein contents(μg/L)and percentages of microalgae under bicarbonate and AZ treatments
3.2 Stable carbon isotope composition of the microalgae
The δ13C of the DIC was-11.0‰ ± 0.4‰ which is positive relative to the δ13C of the added NaHCO3(-17.4‰ or-28.4‰).In the end,the δ13C value of the microalgae decreased as the amount of NaHCO3increased;it also decreased as AZ increased(for the same concentration of NaHCO3)(Table 3).The δ13C of the microalgae cultured in the treatment was affected by the added NaHCO3with different δ13C values.In general,the more negative the NaH13CO3added,the more negative the δ13C of the microalgae harvested for the same concentration of NaHCO3added.
3.3 Variation in carbon sources during different concentrations of NaHCO3and acetazolamide
Based on Eq.(9),we calculated the proportion of the utilization of DIC from the added NaHCO3(fB).The fBincreased with increasing NaHCO3concentration whether AZ was present or not(Table 4).In the treatment at 20.0 mmol/L NaHCO3,fBincreased with increasing concentration of AZ added.
Table 4 The proportion of the utilization of DIC from the addedto the total carbon sources under bicarbonate and AZ treatments

Table 4 The proportion of the utilization of DIC from the addedto the total carbon sources under bicarbonate and AZ treatments
aNaHCO3or AZ concentration added in the culture medium separatelyThe δ13C values of the two kinds of NaHCO3added are-17.4‰ or-28.4‰ separately(‰,PDB)
[NaHCO3][AZ]ammol/L 0 1.0 10.0 1.0 0.06±0.03 0.06±0.04 0.03±0.03 2.5 0.08±0.02 0.08±0.05 0.09±0.03 5.0 0.09±0.04 0.17±0.05 0.12±0.05 20.0 0.20±0.06 0.35±0.09 0.40±0.09
3.4 Variation in carbon pathways under different concentrations of NaHCO3and acetazolamide
Based on Eq.(12),the proportion of thepathway(fb)in microalgae was calculated.It decreased with increasing NaHCO3concentration in the treatment without AZ(Table 5).The fbalso decreased with increasing AZ concentration at the same concentration of added NaHCO3.In the treatment with 10.0 mmol/L AZ,fbvalues were all very small(fb≤0.12).
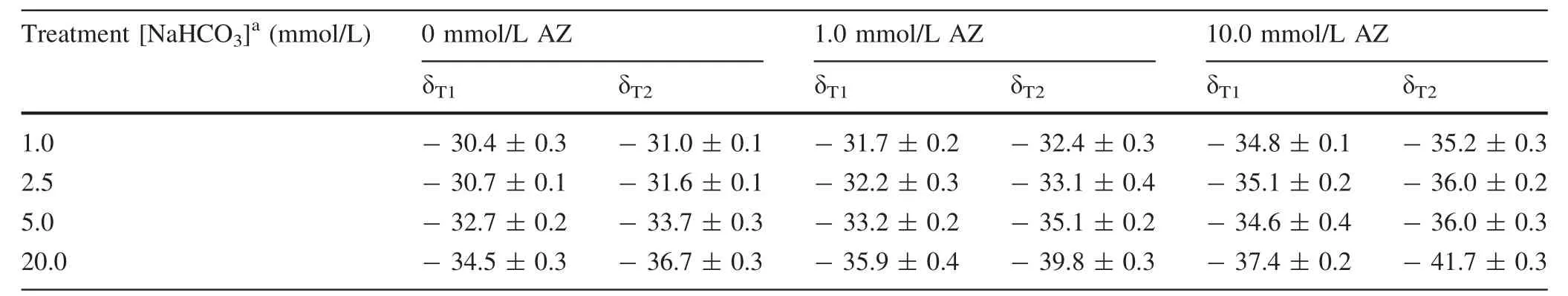
Table 3 δ13C of the microalgae cultured under bicarbonate and AZ treatments
Table 5 The proportion of thepathways under bicarbonate and AZ treatments
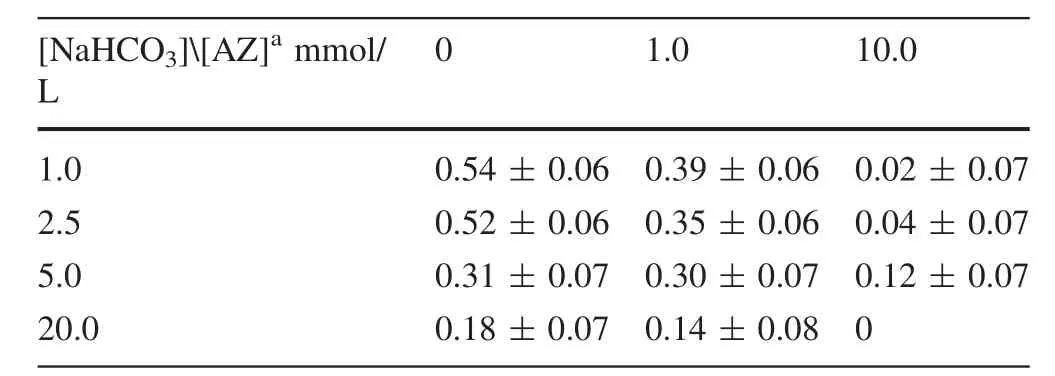
Table 5 The proportion of thepathways under bicarbonate and AZ treatments
aNaHCO3or AZ concentration added in the culture medium separatelyThe δ13C values of the two kinds of NaHCO3added are-17.4‰ or-28.4‰ separately(‰,PDB)
[NaHCO3][AZ]ammol/L 0 1.0 10.0 1.0 0.54±0.06 0.39±0.06 0.02±0.07 2.5 0.52±0.06 0.35±0.06 0.04±0.07 5.0 0.31±0.07 0.30±0.07 0.12±0.07 20.0 0.18±0.07 0.14±0.08 0
4 Discussion
4.1 The effect of bicarbonate and acetazolamide on microalgae growth and carbon isotopes
Simultaneously,the stable carbon isotope composition in algae reflects the utilization of DIC(Chen et al.2009).In this study,the more negative the added NaH13CO3,the more negative the δ13C detected in microalgae for the same concentration of added NaHCO3.Stable carbon isotope composition in the microalgae andconcentration in the medium were negatively correlated,and especially so when NaH13CO3(-28.4‰)was added.This demonstrates that the utilization of DIC can alter the δ13C value in microalgae.Moreover,the stable carbon isotope composition without AZ was significantly different from that with AZ.The δ13C value in microalgae with AZ was more negatively altered than that without AZ.The δ13C in microalgae was the most negative in the presence of 10.0 mmol/L AZ.This suggests that CAex can alter the utilization of DIC,and accelerate the rapid interconversion betweenand CO2,because the slow(uncatalyzed)interconversion of CO2would bring approximately 10‰stable carbon isotope fractionation(Mook et al.1974).
4.2 The effect of bicarbonate and acetazolamide on microalgae carbon utilization

Fig.1 Proposed model of dissolved inorganic carbon utilization by algae in karst lake.Note CA,carbon anhydrase.CAex,the extracellular carbon anhydrase.AE,anion exchange
The pH of karst lakes in southwest China is generally approximately 8.1.CO2in the water is limited—usually less than 1%of total DIC(Riebesell et al.1993).Under these conditions,bicarbonate is the main form of DIC(Fig.1).However,the rate of direct bicarbonate utilization by anion exchange is slow in microalgae(Fig.1).Compared with the direct utilization of CO2and bicarbonate by microalgae without CAex,the major pathway converting CO2from bicarbonate is rapidly catalyzed by CAex(Fig.1).CAex accelerated photosynthetic Ci assimilation,promoted the conversion of bicarbonate to CO2for algal physiological needs,and constantly assimilated CO2from the atmosphere into the water.Ultimately,we found that the algae used atmospheric CO2as the main DIC source via the bicarbonate pathway under the catalysis of CAex(Fig.1).
In the natural water of karst areas,microalgae have adjusted their Ci metabolism strategy to adapt to the environment.This study found that microalgal utilization of the bicarbonate that preexisted in the water at the karst area was very small whether AZ was added or not.In Lake Hongfeng,the dominant family is Chlorophyceae,which has high CA activity(Wu et al.2008).When AZ was added in the medium,CAex activity and growth of the dominant microalgae species were inhibited.As 2.5 mmol/L NaHCO3was added—which is similar to natural conditions in karst areas—both growth and carbon sequestration capacity of the microalgae were largely suppressed by 10.0 mmol/L AZ(37%compared to that without AZ).This shows that microalgae with CAex have greater carbon sequestration capacity in karst lakes.
5 Conclusion
The bidirectional labeling method presented in this study is an effective way to quantify the proportions of Ci sources and their utilization pathways in microalgae.It can help delineate the mechanism of Ci utilization in microalgae under different conditions.In the natural water of karst areas,microalgae used less bicarbonate preexisting in the aqueous medium than CO2derived from the atmosphere.CAex generally increased the utilization of Ci from the atmosphere.The microalgae with CAex had greater carbon sequestration capacity in the lake.
AcknowledgementsThis work was supported by the National Natural Sciences Foundation of China(U1612441),Foundation of Guizhou Province([2014]2131)and Doctor Foundation of Guizhou Normal University(0514014).
杂志排行
Acta Geochimica的其它文章
- The vanadium isotopic composition of L ordinary chondrites
- Variations of tr ace elements under hydrological conditions in the Min River,Eastern Tibetan Plateau
- Enrichment characteristics and risk assessment of Hg in bird feathers from Caohai wetland in Guizhou Province,China
- Carbazoles and benzocarbazoles confirm migration of leaked petroleum through caprocks and overlaying formations of Valhall Well 2/8-8 in the North Sea
- Basic formation mechanisms of Lake Doroninskoye soda water,East Siberia,Russia
- Ore genesis of Badi copper deposit,northwest Yunnan Province,China:evidence from geology, fluid inclusions,and sulfur,hydrogen and oxygen isotopes
