Basic formation mechanisms of Lake Doroninskoye soda water,East Siberia,Russia
2018-08-30SvetlanaBorzenkoLeonidZamanaLarisaUsmanova
Svetlana V.Borzenko•Leonid V.Zamana•Larisa I.Usmanova
Abstract The primary scientific goal of studying salt lakes is to better understand the formation of small continental type hydrogeochemical systems.Many scientists have attributed the metamorphism of the chemical composition of salt lakes to the evaporative concentration of water.However,the formation of soda water is inconsistent with this hypothesis.Thus,analyzing intrabasinal biochemical processes and water—rocks interactions during the evaporative concentration of water allows us to understand the major mechanisms of the formation and evolution of water compositions.Therefore,the aim of this paper is to identify the key processes involved in the formation of the chemical composition of the water in Lake Doroninskoye. An analysis of the distribution of major components shows that Na+,,,and Cl-are dominant in this water.High concentrations of these elements are the result of evaporative water concentration.Calcium,magnesium,and potassium are not accumulated because the water is saturated in minerals containing these elements.The main barrier to the growth of the sulfate content of water is sulfate reduction.This process also contributes to the additional reproduction of carbon dioxide,which reacts with the products of the hydrolysis of aluminosilicates OH-to formand,thus further contributing to the natural processes of soda formation.
Keywords Saline lake·Sulfate reduction·Thermodynamic equilibrium
1 Introduction
There are many continental-type salt lakes in the territory of East Transbaikalia and its adjacent areas of Mongolia and China.Their salinity is caused by the evaporative concentration of lake water in arid climates,in which the directional transformation of chemical composition occurs with chemical changes(i.e.,from carbonate to sulfate to chloride-type)(Valyashko 1962;Posokhov 1981;Shvartsev 1982;Zheng 2014).However,the actual distribution of the chemical components in the water of these lakes with increasing salinity is different than the sequence mentioned above.Chloride and carbonate salts of sodium are prevalent in this chemical composition;sulfates are found in small amounts,and they are rarely included in the name of a water chemical type,only as the second anion in order of importance(Zamana and Borzenko 2010).Lake Doroninskoye is an example of this,as it is the only known meromictic soda water body in Transbaikalia and within the entire Siberian region,according to the scientific literature(Vlasov et al.1961;Borzenko et al.2015).It differs from typical holomictic(i.e.,homogeneous in depth)steppe lakes due to its expressed meromixis(stratification),and it represents a rare type of salt soda lake(Namsaraev 2009).The scientific community knows of only a few such water bodies in the world,and they are located in the United States and the African rift zone and were formed under the influence of more recent post-volcanic processes.These include Mono Lake(California)(Sorokin et al.2002;Humayoun et al.2003),Big Soda Lake(Cloern et al.1983)and Soap Lake,Nevada(Gorlenko 2007),as well as the small Lake Sonachi(Mac Intyre and Melack 1982)in Kenya.These lakes mainly differ from Lake Doroninskoye in their origin;Lake Doroninskoye is located in continental sediments and has never been affected by post-volcanic processes.
Currently,the study of stratified soda lakes is of great importance because the unique chemical composition of their water and the diversity of their bacterial life indicate that such bodies of water existed during the early stages of the global origin of Earth’s ecosystem(Zavarzin and Žilina 2000).However,many important aspects of the formation of this type of lake are not well understood.The genesis of soda waters is considered to be a central problem in modern theoretical hydrogeochemistry(Shvartsev 2004).Therefore,the soda lake Doroninskoye can serve as a kind of a model for us to study the mechanisms and magnitude of the impact of various factors on the chemical composition and salinity of the lake water.
2 Objectives and research methods
Lake Doroninskoye is located in Eastern Siberia,150 km to the west of the city of Chita.It is located at the bottom of the Chita-Ingoda intermountain basin.The surrounding mountain ranges are mainly composed of granites and gneisses,and the basin is filled with Mesozoic sandstone and siltstone rocks and loose Quaternary sediments.The lake district belongs to the steppe zone,and it has a typical arid climate.The average annual precipitation is 340 mm;the minimum precipitation value was recorded in 2007(180 mm).The amount of evaporation that occurs from the water surface and soil is 1.5–2 times higher than the observed amount of rainfall.The undrained water body has a water area of approximately 4.5 km2and a depth of 6.5 m.The lake bottom sediments comprise silt with a capacity of up to 7.5 m.The precipitate of pelitic fractions has a smectite-kaolinite-hydromica composition,and it contains iron hydrosulfides with an admixture of dolomite and carbonate-ankerite groups(Serebrennikova and Jurgenson 2010).
Sampling was conducted on Lake Doroninskoye during all seasons from 2003 to 2016.These samples were collected using a Plexiglas bathometer at the center of the lake,from the top down through 0.25–1.0 m in the vertical direction of the water column.During this entire period,369 water samples were collected.Water samples collected for hydrogen sulfide analysis and oxygen analysis were taken from the bathometer through the siphon tube and then transferred into two dark bottles;these bottles were blown clean with carbon dioxide prior to the addition of water.To save unstable components,such as oxygen and hydrogen sulfide,they were preserved( fixed)at the sampling locations.
The chemical analysis of water samples was carried out using conventional methods(Fomin 1995;Novikov et al.1990).The concentrations of calcium and magnesium were determined using the atomic absorption method in a nitrous acetylene flame on a SOLAAR 6 M spectrophotometer.The flame-emission method was used to determine the sodium and calcium amounts.The potentiometric method,with the application of ion-selective electrodes,was used to determine pH,Eh,O2,and Cl-.Titration was used to determine the contents of CO2,,and.Sulfate ions,in the form of barium sulfate,were determined using the turbidimetric method.Hydrogen sulfide H2S,hydrosulfide HS-,and elemental sulfur S0were precipitated from 100 ml of each water sample using zinc acetate on ‘‘blue ribbon’’ filters.Thiosulfatesand sulfiteswere isolated using silver nitrate from the remainder of the filtrate(Aubrey et al.2010).The photometric method was first used to determine all forms of sulfur;then,they were transformed into hydrogen sulfide(Volkov and Zhabina 1990).At first,hydrogen sulfide was distilled from precipitate in an argon environment;then,elemental sulfur was extracted and reduced with chromium chloride II to hydrogen sulfide in an acidic environment.Considering the complexity associated with dividing thiosulfatesand sulfites,they were determined together(during second precipitation)and expressed in terms of thiosulfates(thiosulfate sulfur,S0S4+).
Our method allows us to determine the concentration of the total S0in the water,including truly dissolved sulfur,suspended sulfur,colloidal sulphur,and elemental sulfur in the compositions of polysulfide ions.It should also be noted that polysulfide ion sulfurwas determined by analysing hydrogen sulfide and hydrosulfide sulfur(Buseyev and Simonova 1975).
Hydrogen sulfide was analyzed by treating a weighed quantity of bottom sediments with diluted hydrochloric acid;then,the mixture was heated,and evolved hydrogen sulfide was distilled in a tank filled with zinc acetate.After stripping the hydrogen sulfide,elemental sulfur and thiosulfate were jointly determined by reducing them with chromium chloride II to hydrogen sulfide using a photometric end-point,as in the case with water.The detection limit of sulfur using this determination method is 5 mg/L.
The determination of dissolved organic carbon(DOC)was carried out using an Elementar Liquid DOC carbon analyser(Germany)with an infrared radiation detector.The high-temperature catalytic oxidation method was used,in which carbon compounds were decomposed to CO2at a temperature of 800°C.The accuracy rate of this method is less than 28%when the DOC content ranges from 1 to 5 mg/L,and it is 14%when the DOC content ranges from 50 to 250 mg/L.
The simulation of equilibria in the ‘‘water-minerals’’system was carried out using the physical–chemical code in the PHREEQC(Parkhurst and Appelo 2013)and HG32(Bukaty 2002)software.This algorithm is based on the principle of chemical thermodynamic constants,which allows us to estimate the degree of water saturation with respect to different sulfur-containing minerals.The process then permits us to determine the ability of water to dissolve or precipitate solid phases that are in equilibrium with diverse species in solution.
In addition to the chemical analyses of water samples that were performed in 2007–2009,microbiological studies were conducted by the Laboratory of Aquatic Ecosystems of the Institute of Natural Resources,Ecology and Cryology SB RAS(Chita),the Institute of General and Experimental Biology SB RAS(Ulan-Ude),and the Institute of Microbiology of the Russian Academy of Sciences(Moscow).
Determinations of the sulfur isotopic compositions of dissolved sulfate ions and hydrogen sulfide,as well as the carbon isotopic compositions of dissolved carbonates,were performed using a MAT 253 mass spectrometer(Thermo Fisher Scientific,Germany).The 1σ (‰)analytical error of sulfur was±0.2 and that of carbon was±0.1.The resulting sulfur isotopic compositions were given relative to CDT,and the resulting carbon isotopic compositions were given relative to PDB.
To process the data obtained here,the method of mathematical statistics(Excel)was applied to determine the maximum,minimum,and average contents of all components of lake water.
3 Results and discussion
3.1 Chemical composition and main salt sources of lake water
According to the water classification(Alekin 1970)used here,the water of Lake Doroninskoye is soda-type water.Its general chemical composition can be expressed by the formula(+70 Cl-29/Na+98(eq.%).Its pH ranges from 9.57 to 10.49.
Due to stratification,the hydrochemical characteristics of the lake vary significantly with depth.In the upper oxygen layer,which ranges in depth from 3.5 to 5.0 m,TDS varies from 10,277 to 35,849 mg/L,depending on the season(Table 1).At greater depths,the oxygen layer is replaced by a hydrogen sulfide layer,in which the water salinity ranges from 25,500–35,247 mg/L and the hydrogen sulfide concentration is 370 mg/L.In this layer,the oxidation–reduction potential (Eh) values become negative.
In both layers,synchronous changes in salinity andand HCO3-concentrations can be traced to seasonal dynamics;increases in their contents were recorded in autumn and spring.Cl-concentrations generally increased from the end of summer and peak in February and March(Table 1),which reflects their dependence on seasonal climate phenomena,such as rainfall and ice formation.
Many studies have shown regular increases in the average annual salinity values and concentrations of major ions,in which their maximum values occur at the peak of the dry season(2007).Sulfates are an exception to this trend,as their contents decreased during this period(Table 2).
In terms of inter-seasonal and inter-annual dynamics,the increase in carbonate content outstrips those of chlorides and sulfates because the former’s values do not correspond to their natural concentrations in water produced by evaporation,as they should accumulate in approximately equal amounts but they do not.Consequently,there are additional mechanisms that control their contents.To determine these mechanisms,it is necessary to first define the contributions of minerals into the lake.
For our calculations,we used data on the chemical composition of the saline recharge(Table 3)and their proportions within the water input.These proportions are determined based on the ratio of the corresponding water input(in mm)relative to the total amount,which is equal to the potential natural water loss from the surface of the lake(490 mm).These proportions are defined as follows:rainfal—340/490=0.694; underground water—120/490=0.245;and surface runoff—30/490=0.061.
The annual amounts of chemical components entering the lake per 1 dm2of its water area(Q)and its total salt contents(Q1)are shown in Table 4.These calculations show that groundwater provides the majority of the chemical components that enter the lake,and with the exception of chloride,most of these components are introduced by rainfall,so surface run-off is of secondary importance.The average underground water composition according to our testing data is given in Table 3;water samples that were unsaturated in calcite were included in these calculations.
The underground water within the study area is mostly related to typical leaching waters;they are hydrocarbonate in composition,mainly contain calcium,and exhibit salinity values of up to 0.7 g/L and a pH of 7.7(Borzenko 2012).These waters have a HCO3-Ca-Na chemical composition.They are mainly formed by the dissolution of water-bearing minerals in rocks.Within underground water,there are waters with high salinity(from 0.8 g/L)and pH(from 7.6)values;these can be referred to as the waters of the initial stage of salinization(Shvartsev and Wang 2006).
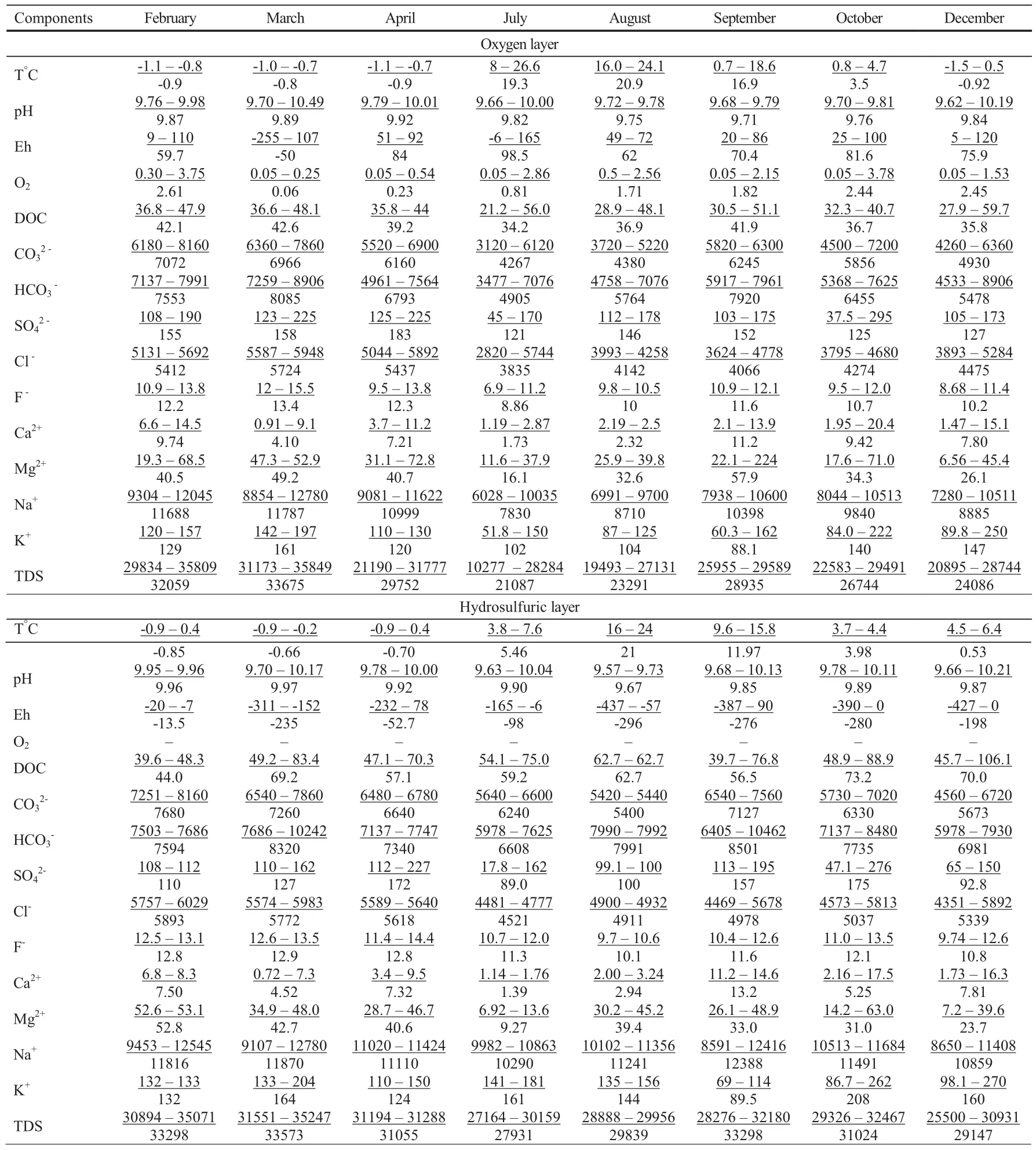
Table 1 Hydrochemical compositions in annual(2003-2016 г.)study of Lake Doroninskoye
The chemical composition of the water of Lake Doroninskoye is substantially different than the average composition of the water involved in its saline recharge(Table 5).Obviously,chlorine and sodium fully reflect the process of the evaporative concentration of the solution;this is demonstrated by the Na+/Cl-ratios observed in two comparable compositions(i.e.,2.3 and 2.2).The slightdecrease in this ratio in lake water indicates the loss of sodium with increasing salinity.Carbonates accumulate in the lake in smaller amounts than chlorine and sodium,but they are still dominant.For example,the ratios of(+)/Cl-and(+)/Na+in lake water are 2.7 and 1.27,whereas in the original solution they are 24 and 11,respectively.The most dramatic changes are found in the ratios of cations,particularly calcium and sodium.Calcium is dominant in the initial composition,and the Na+/Ca2+ratio(0.58)of the lake water indicates the substantial predominance of sodium(1385).A similar situation,although with different ratios(0.52 and 35,respectively),occurs between chlorine and sulfates.However,within the considered range of salinity,these components must have accumulated in approximately equal proportions(Shvartsev et al.2014).This situation thus requires special consideration.

Table 2 Chemical compositions(mg/L)of lake waters
3.2 Thermodynamic modelling
To explain these disparities,the thermodynamic modelling of changes in the water composition due to water evaporation based on the formation of secondary mineral phases was performed using the PHREEQC program.This calculation used the chemical composition of water Q1(Table 5).Cl-was used as an indicator of the degree of evaporation,as it is the most conservative component that does not interact with the formation of secondary minerals within the studied range of water salinity.
The results of thermodynamic modelling indicate that all of the chlorine can be explained as the result of evaporative concentration.Sodium,with few exceptions,is also concentrated in the solution.The high potassium contents in the calculated composition demonstrate only an incomplete account of the secondary K-containing minerals in the model,as their number in the program database is limited.The reasons for the low calcium and magnesium contents are attributed to the calculation that demonstrates that they are mainly bound with calcite,dolomite and magnesite.The calculated sulfate content is higher than that in the lake water,and the amounts of carbonate and hydrogen carbonate are lower(Tables 5 and 6).
The accumulation of carbonates in the lake may be due to the fact that the initial concentrations of the feed waters are subject to the inequality+>-rCa2++rMg2+(in which ‘‘r’’indicates an equivalent form of concentration).In this case,the amount of carbonate components was slightly higher than the amount of alkaline earth elements(Table 5),which explains the high carbonate content in the residual solution.However,these contents are higher in water than they are in the modelling results.The hydrochemical balance indicates that all of the carbonates in the lake water input,except for those contributed from underground and surface runoff,are formed through the salinity of dissolved organic matter(COD),most of which is used for the bacterial reduction of sulfates.The processes of sulfate reduction and their role in the enrichment of water by ions of carbonic acid will be discussed later.
From a modern standpoint(Shvartsev et al.2007),the‘‘water–rock’’system is one of equilibrium and non-equilibrium.The dissolution of primary rock minerals,whichare usually not in equilibrium with water under conditions of leaching,is accompanied by the formation of secondary minerals;they then achieve equilibrium with water with the accumulation of chemical elements.To determine the role that hydrogenic mineral formation plays in defining the chemical composition of the lake water,hydrogeochemical data were analyzed using the PHREEQC software(Fig.1).

Table 3 Average chemical composition of waters(mg/L)participating in the saline recharge of Lake Doroninskoye(Borzenko 2012)

Table 4 Components of the saline recharge of Lake Doroninskoye(%of total component amount)
First,we considered the equilibrium of water with calcite;its saturation,as was shown by Shvartsev(1998),occurs when the salinity of natural water is approximately 0.6 mg/L and its pH value is 7.4.According to our calculations,underground water with a salinity of more than 0.8 g/L and a pH of greater than 7.6 is in equilibrium with calcite(Fig.2a).Following the formation of calcite,the Ca contents in waters do not increase.Clay minerals remove most of the K and Mg,and some of the Ca,from the solution(Fig.2g,h,i).However,all underground water is notin equilibrium with endogenousminerals (e.g.,anorthite,K-feldspar,albite,forsterite);therefore,when dissolving,they are the main sources of chemical components(Shvartsev 1982).Sodium,as the main cation,is removed from aluminosilicate minerals,and their dissolution occurs by the following reaction:
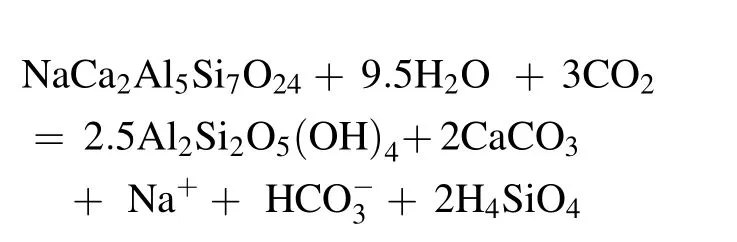
Despite the fact that in some cases,sodium is in equilibrium with Na-montmorillonite(Fig.2f),it is bound to a lesser extent than the other cations so therefore,it accumulates more intensively in water.The concentration ofincreases along with it.The chemical type of the underground water that forms under these conditions is soda-type water.Obviously,the soda formation process begins in underground water and continues in the lake,where fresh water gradually transforms into a mineralized solution that is enriched by saline components.

Table 5 Average chemical composition of waters(mg/L)and the concentration degree

Table 6 The remaining part of water in the calculated solution(by HG32 program)
The water of Lake Doroninskoye is in equilibrium with calcite(Fig.2a).The calculation performed using the HG32 program showed that it is in equilibrium with other carbonate minerals,particularly with dolomite CaMg(CO3)2,nesquehonite MgCO3·3H2O,siderite FeCO3,and gaylussite Na2Ca(CO3)2·5H2O.This water is not in equilibrium with endogenous minerals(e.g.,anorthite,K-feldspar,forsterite),although it is in equilibrium with analcime and in some cases,albite(Fig.2f,g,h,i).There are no samples that are in equilibrium with gypsum CaSO4·2H2O(Fig.2b)because the activity of sulfate ionsis below that of the eutectic.For sulfides,the saturation of pyrite FeS2and amorphous hydrotroilite FeS.2H2O has been observed.
The accuracy of these thermodynamic calculations is supported by the presence of iron sulfides in the sediments of the lake,in which the proportion of the latter accounts for approximately 2%of the total compositions of the sediments.All of the carbonates listed above,except for gaylussite,were found at different depths(using X-ray phase and chemical analyses)in the sediment traps that were installed during the ice period.Gaylussite was found in the sediments during the summer-autumn period(Serebrennikova and Jurgenson 2010;Borzenko 2014).The amounts of albite and quartz in these traps varied widely,comprising 4%–48% of the total compositions of hydrogenous material;their contents depended on the silicon content of the water.Hydrohalite NaCl·2H2O and soda Na2CO3·10H2O were only formed in winter in the cryopeg zone(i.e.,in waters with negative temperatures)at depths ranging from the under-ice layer to 4–5 m.However,they dissolved due to sedimentation in the area of the thermocline,which increased the concentrations of major ions(Na+,,Cl-)and thus increased the water salinity near the bottom layer.

Fig.1 Location map of Lake Doroninskoye
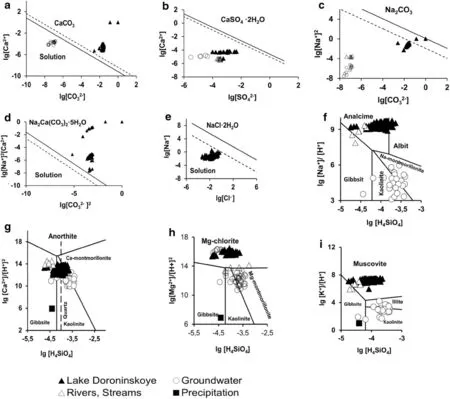
Fig.2 Systems:a CaCO3—water,b CaSO4—water,c Na2CO3—water,d Na2Ca(CO3)25H2O—water,e NaCl—water,f H2O–Al2O3–Na2O–CO2–SiO2(at 25 °C),g H2O–Al2O3–CaO–CO2–SiO2,h H2O–Al2O3-MgO–CO2-SiO2,i H2O–Al2O3–К2O–CO2–SiO2,with plotting of water composition of Lake Doroninskoye and district waters.1–25 °C,2–0 °C
Obviously,the supersaturation of calcite,dolomite,nesquehonite,and other minerals in water caused the contents of Ca2+and Mg2+to stay constant,and their maximum values in the lake to be 20.4 and 224 mg/L,respectively.Potassium actively participated in the formation of secondary minerals by forming mica(Fig.2i)and clay minerals;thus,its content in the water did not exceed 270 mg/L.
Chlorine had no geochemical barriers during this stage of the evaporative concentration of waters;thus,its content increased with increasing water salinity.Because Cl-is the result of evaporative concentration,Na+should accumulate in the same amount.However,the value of the Na+/Cl-ratio in a calculated composition(Q1)actually was 2.3,whereas that in the water of Lake Doroninskoye was 2.2.The reduced values of the Na+/Cl-ratio can be explained by sodium loss as a result of its involvement in hydrogenous mineral formation;compared with other cations,thisdegree of involvement is considerably lower.This was demonstrated not only by the Na+/Cl-ratio but also by the amounts of analcime(10-3mg/L),albite(10-6mg/L)and gaylussite(10-8mg/L)that precipitated in water.Therefore,sodium was accumulated in the water of the lake.

Table 7 Concentration of sulfur species(mg/L)in Lake Doroninskoye
3.3 Geochemical transformations of sulfur
Previous studies have shown that sulfide is ubiquitous in water(Borzenko and Zamana 2011).Due to the high alkalinity of the lake water,hydrogen sulfide occurred in its dissociated form,mainly(99%)in the form of hydrosulfide HS-(S2-)(Zamana and Borzenko 2010).The maximum HS-content was observed in winter,which was caused by the increased activity of sulfate-reducing bacteria(Zaharyuk 2010;Gorlenko et al.2010).In autumn,the HS-content decreased due to the reduced intensity of sulfate reduction.The minimum S2-value was observed in the beginning of under-ice vegetation,and it was accompanied by a significant increase in the O2content that was dissolved in water(Tables 1 and 7).
There is a reverse correlation between O2and HS-both over time and with depth in the oxygen layer(with a correlation ratio r=-0.87).The reason for the coexistence of these geochemically incongruent components is the speed ratio;on the one hand,oxygen is input into water from the atmosphere and its production in the water column,whereas on the other hand,hydrogen sulfide forms during the process of sulfate reduction caused by oxygentolerant bacteria,as was discovered in the 1980s(Hardy and Hamilton 1981).
The vertical distribution of S2-shows the development of the process of bacterial sulfate reduction in the water column of the lake.This is confirmed by the random HS-distribution in the upper hydrochemical zone,the presence of a substantial increase in its content in the chemocline,and its subsequent decline to the bottom.
In addition to sulfate sulfur and hydrogen sulfide,water contains thiosulfate S0S4+()and elemental S0,which are intermediate products in the redox cycle betweensulfur and H2S.The contents of these forms of sulfur,as well as that of HS-,exhibit changes in depth and throughout the seasonal cycle(Tables 7,8;Fig.3).
The dynamics of S0S4+content are mainly determined by the reduction process,as consistent changes in S2-and S0S4+concentrations appeared more often(r=0.84);however,the nature of this connection is twofold(i.e.,reducing and oxidizing).During periods of ice cover,the intensity of microbiological processes decreases(Matyugina and Belkova 2015).In addition,increases in the S0S4+content due to decreasing HS-and S0concentrations indicate a growing role in its formation of chemical oxidation(Zamana and Borzenko 2011).
Elemental sulfur is formed as a result of the oxidation of bacterial hydrogen sulfide caused by chemical means or the activity of thionic bacteria(Volkov 1990).Because sulfur is an intermediate product in oxidation reactions,it does not accumulate in lake water in significant quantities.It is present in at least two forms,namely,polysulfide,which is indirectly confirmed by its close correlation with HS-(r=0.66)in the summer,and elemental sulfur,which is reflected in the observed increase in S0concentrations that are significantly higher than those of S2-,in October(Fig.3).
The observed dynamics in the distribution of the reduced forms of sulfur reflect the parallel bacterial reduction processes of sulfates occurring in the lake and the oxidation processes of generated hydrogen sulfide.In thissystem,we can observe the ubiquitous presence of all studied sulfur derivatives.However,the rate of the bacterial production of hydrogen sulfide is such that it cannot be oxidized,and it occurs throughout the water column of the lake.

Table 8 Contents of reduced sulfur species и isotopic composition of sulfur in the seasonal cycle
The S6+()contents observed in the water exhibit the uneven redistribution of its contents in the water column;under anaerobic conditions,in the majority of cases,they reduce in proportion due to increases in the hydrogen sulfide content.The occurrence of the active process of sulfate reduction in the chemocline area is confirmed by the presence of significant isotopic effects,as sulfates are enriched in the heavy isotope δ34S(up to 28.2 ‰),and hydrosulfides are enriched in the light isotope(up to 7.4‰)(Table 8).
The low contents of sulfates in the lake can clearly be explained by their bacterial reduction.While there is evidence of the oxidation of hydrogen sulfide,some of the sulfates have been irreversibly reduced to hydrogen sulfide;these sulfates mainly react with iron to form hydrotroilite FeS·2H2O,which precipitates from a solution.All of these processes are indicative of the directional desulfation of the lake water.

Fig.3 Vertical variations of contents(%)of reduced sulfur compounds versus content of=S2-+S0+S0S4+in brine in the central station of Lake Doroninskoe in annual cycle:a 02.03.2007,b 02.09.2007,c 16.10.2007,d 28.02.2008
The role of sulfate reduction is not limited to the transformation of sulfur species,as electron transport is provided by carbon oxidation as a component of the dissolved organic substances of the water column.Based on the relationship between S and CO2,as the products of sulfate reduction that were produced at a rate of 29.8 mg S/L per day,CO2production may reach up to 41 mg/L per day(Borzenko et al.2014).The development of sulfate sulfur-oxidizing,and chemo-and phototrophic microorganisms in the lake allows a noticeable amount of not only sulfur but also carbon to be transformed.The relationship between the sulfate reduction and the formation of carbonates is clearly visible in the distribution of the isotopic composition of carbon and in the forms of sulfur during one of the sampling periods,as they show good agreement(Table 9 and Fig.4).In the chemocline area,where the concentration of hydrogen sulfide is higher,the carbon of+has a lighter isotopic composition(δ13C to-0.17‰).This is possible because moreand its derivativeions were formed due to the CO2produced as a result of the bacterial decomposition of the organic matter contained in water;the DOC values indicate high concentrations of CO2(Table 1).Relative toatmospheric CO2(δ13CPDB– 5.0‰–8.0‰),using carbon dioxide fractionation coefficients in this system for hydrocarbonates and carbonates of 1.014 and 1.012(Galimov 1968),respectively,the δ13C values are at least 3.4‰richer in light isotopes compared to the equilibrium value(Zamana and Borzenko 2014).This also suggests the involvement of biogenic carbon in the formation of carbonates dissolved in the lake.In some cases,the weighting of the carbon of dissolved carbonates in the near-bottom water layer is most likely associated with methanogenesis,the presence of which is confirmed by the presence of methanogenic bacteria(Gorlenko et al.2010).The presence of OH-indicates that hydrolysis reactions occurred in the aluminosilicate minerals of the basic rocks in the water catchment area of the lake.

Table 9 Contents of reduced(S2-)and oxidized(S6+)sulfur and δ13C dissolved carbonates in the lake water in September 2008

Fig.4 Distribution of S6+,S2-,δ13C values of dissolved carbonates in the same period of testing
4 Conclusions
The accumulation of Na+and HCO3-()during the present step of the evolution of the lake is possible because these components do not encounter significant restrictions in the form of secondary minerals.Addressing thermodynamic equilibrium through the lens of water–rock interactions has shown that the calcium and magnesium contents in water are limited by the saturation of lake waters,mainly by calcite,dolomite,nesquehonite and gaylussite.Potassium is bound by clays.Sulfate reduction restricts the accumulation of sulfate ions in the lake and contributes to the additional reproduction of carbon dioxide,which in turn reacts with the hydrolysis products of aluminosilicate OH-to form hydrocarbonate and carbonate ions.
Thus, intrabasinal hydrobiological processes and hydrogenous mineral formation,along with evaporative concentration,control the chemical composition of Lake Doroninskoye.Evaporite sedimentation limits the accumulation of many components in water,but it concentrates sodium and carbonates,i.e.,soda.Bacterial production further contributes to the natural processes of the formation of soda water.
AcknowledgementsThis study was financially supported by the Russian Science FoundationThe interaction mechanisms,equilibrium state and evolution trend of the salt waters and brines—basic and ultrabasic rocks systems(on the example of Siberian platform areas)(RSF Project No.17-17-01158).
杂志排行
Acta Geochimica的其它文章
- The vanadium isotopic composition of L ordinary chondrites
- Variations of tr ace elements under hydrological conditions in the Min River,Eastern Tibetan Plateau
- Effects of carbon anhydrase on utilization of bicarbonate in microalgae:a case study in Lake Hongfeng
- Enrichment characteristics and risk assessment of Hg in bird feathers from Caohai wetland in Guizhou Province,China
- Carbazoles and benzocarbazoles confirm migration of leaked petroleum through caprocks and overlaying formations of Valhall Well 2/8-8 in the North Sea
- Ore genesis of Badi copper deposit,northwest Yunnan Province,China:evidence from geology, fluid inclusions,and sulfur,hydrogen and oxygen isotopes
