Synthesis and Crystal Structures of New Sulfido/oxo-molybdenum Complexes(Et4N)2[MoO(S4)(bdtX2)]and(Et4N)2[Mo2O2(μ2-S)2(bdtX2)](X=H and Cl)
2018-08-09HUMengjieCUIYujieQIANTingtingXINZhifengJIAAiquanZHANGQianfeng
HU Mengjie,CUI Yujie,QIAN Tingting,XIN Zhifeng,JIAAiquan,ZHANG Qianfeng
(InstituteofMolecularEngineeringandAppliedChemistry,AnhuiUniversityofTechnology,Ma’anshan243002,China)
Abstract:Substitution of(Et4N)2[MoIVO(S4)2]in acetonitrile with mole ratio(1∶1)1,2-benzenedithiol(H2bdt)and 3,6-dichloro-1,2-benzenedithiol(H2bdtCl2)gave mono(dithiolene)oxo-molybdenum(IV)complexes(Et4N)2[MoO(S4)(bdtX2)](X=H,1;X=Cl,2).Treatment of 1 and 2 with additional equimolar amount of 1,2-arenedithiol in warm acetonitrile afforded bis(µ-sulfido)dimolybdenum(V)species(Et4N)2[Mo2O2(μ2-S)2(bdtX2)2](X=H,3;X=Cl,4).All four complexes were characterized spectroscopically with UV-3000 spectrophotometer,of which complexes 2,3 and 4 have been further determined with single-crystal X-ray diffraction.Results show the synisomers are formed referring to the position of the apical oxo ligands with respect to the Mo2S2plane.
Key words:sulfido-molybdenum;oxo-molybdenum;dithiolene;synthesis;structure
The bioinorganic significance of oxo-dithiolene molybdenum complexes is well-known,because they could serveasmolecularmodelsfortheactivesitesofseveralenzymefamilies,suchasthedimethylsulfoxidereductases[1-3].In recent years,much attention has been directed toward the syntheses of oxo/sulfido mono-,bi-and tri-molybdenum-dithiolenecomplexestogetherwiththeirspectroscopicandelectronicstructurestudies[4-6].SinceDraganjacetal.reported the isolation of complex(Et4N)2[MoO(S4)2]containing two tetrasulfido ligands from hydrolysis of its sulfur analogue(Et4N)2[MoS(S4)2]in N,N-dimethyl formamide or acetonitrile[7],a series of oxo/sulfido molybde-num-dithiolene complexes were synthesized by using of(Et4N)2[MoO(S4)2]as the starting material.For examples,the reaction of[MoO(S4)2]2−with dibenzoyl acetylene resulted in the formation of a dithiolene complex ion,[MoO(S2C2(COPh)2)2]2−[8];and treatment of(Et4N)2[MoO(S4)2]with dimethyl acetylenedicarboxylate(DMA)afforded dithiolene complex(Et4N)2[MoO(S2C2(CO2Me)2)[9].It was proposed that the reactions proceeded by electrophilic attack of DMA on the Mo-S2sites,with subsequent DMA insertion into these chromophores,indicating the tetrasulfido ligand being a lablile leaving group[9].On the other hand,1,2-dithiols,such as toluene-3,4-dithiol and 1,2-benzenedithiol,are typical and commercially available dithiolene ligands included in the 1,2-dithiolene complexes,which have provided numerous opportunities in solid-state chemistry as molecular conductors or as nonlinear optical materials[10-12].Therefore,development of new synthetic procedures for more dithiolene oxo-/sulfido-Mo complexes is necessary to expand the fields of bioinorganic chemistry as well as material science.Herein we report new synthetic approaches to the mono(dithiolene)oxo-molybdenum(IV)complexes(Et4N)2[MoO(S4)(bdtX2)]and bis(µ-sulfido)bi(dithiolene)oxo-molybdenum(V)complexes(Et4N)2[Mo2O2(μ2-S)2(bdtX2)2]from reactions of(Et4N)2[MoO(S4)2]and 1,2-arenedithiol.
1 Experimental
All syntheses were performed in oven-dried glassware under a purified nitrogen atmosphere using standard Schlenk techniques.The solvents were purified by conventional methods and degassed prior to use.1,2-Benzenedithiolate(bdt)and 3,6-dichloro-1,2-benzenedithiolate(bdtCl2)were purchased from Alfa Aesar Ltd and used as supplied.(Et4N)2[MoO(S4)2]was prepared by the literature procedure[9].All elemental analyses were carried out using a Perkin-Elmer 2400 CHN analyzer.Electronic absorption spectra were obtained on a Shimadzu UV-3000 spectrophotometer.Infrared spectra were recorded on a Digilab FTS-40 spectrophotometer with use of pressed KBr pellets.
1.1 Synthesis of(Et4N)2[MoO(S4)(bdtH2)](1)
To a solution of(Et4N)2[MoO(S4)2](250 mg,0.398 mmol)in acetonitrile(30 mL)and DMF(1 mL)was added a solution of 1,2-benzenedithiol(H2bdt)(56 mg,0.398 mmol)in toluene(5 mL)at room temperature.The solution was stirred at 50℃for 2 h and a yellow precipitate was formed,which proved to be elemental sulfur.After filtration,the filtrate was removed in vacuo and washed with 5 mL diethyl ether for three times.The residue was dissolved in 10 mL acetonitrile and layered with 10 mL of diethyl ether and allowed to stand at ca.4℃.Red brown needleshaped micro-crystals of complex 1 were obtained after five days,and were isolated by filtration,washed with diethyl ether,and dried in vacuo.Yield:178 mg(70%).-Anal.for C22H44N2OS6Mo:calcd.C 41.23,H 6.92,N 4.37%;found C 41.27,H,6.90,N 4.40%.-1H NMR(DMSO-d6,anionic part,ppm):δ6.90(m,2H,Ph),7.53(m,2H,Ph).-IR(KBr,cm-1):ν 489(m),761(s),781(m),925(m),1 225(m),1 444(vs),2 990(m).
1.2 Synthesis of(Et4N)2[MoO(S4)(bdtCl2)](2)
Complex 2 was prepared by the same method for complex 1,except that H2bdtCl2was used instead of H2bdt.Yield:192 mg(68%).-Anal.for C22H42Cl2N2OS6Mo:calcd.C 37.27,H 5.96,N 3.95%;found C 37.21,H,5.92,N 3.99%.-1H NMR(DMSO-d6,anionic part,ppm):δ 7.08(m,2H,Ph).-IR(KBr,cm-1):ν 489(m),785(m),933(m),1 220(m),1 444(vs),2 989(m).
1.3 Synthesis of(Et4N)2[Mo2O2(μ2-S)2(bdt)2](3)
H2bdt(28 mg.0.2 mmol)was added to an acetonitrile solution(30 mL)of complex 1(127 mg,0.2 mmol),and the solution was stirred for 6 h at 40℃.The resulting solution was concentrated to ca.10 mL and then stood for 1 h.After elemental sulfur precipitated and was removed by filtration,the filtrate was removed in vacuo and washed with 5 mL diethyl ether for three times.The residue was dissolved in 10 mL acetonitrile and layered with 15 mL of Et2O,after three days,a brown crystals precipitated,which was collected by filtration and dried in the air.Yield:84mg(51%).-Anal.forC28H48N2O2S6Mo2:calcd.C40.52,H5.82,N3.41%;foundC40.50,H,5.84,N3.43%.-1H NMR(DMSO-d6,anionic part,ppm):δ 6.79(m,4H,Ph),7.45(m,4H,Ph).-IR(KBr,cm-1):ν 546(m),799(m),968(m),1 190(m),1 489(s),1 493(m),2 956(m).-UV-vis(CH3CN,nm):λmax355(s),465(w).
1.4 Synthesis of(Et4N)2[Mo2O2(μ2-S)2(bdtCl2)2](4)
Complex 4 was prepared by the same method for complex 3,except that H2bdtCl2was used instead of H2bdt.Yield:192 mg(68%).-Anal.for C28H44Cl4Mo2N2S6O2:calcd.C 33.58,H 4.48,N 2.78%;found C 33.61,H,4.46,N 2.76%.-1H NMR(DMSO-d6,anionic part,ppm):δ 7.28(m,4H,Ph).-IR(KBr,cm-1):ν 549(m),789(m),989(m),1 089(m),1 270(m),1 474(s),1 498(m),2 985(m).-UV-vis(CH3CN,nm):λmax355(s),425(w).
1.5 X-Ray crystallography
Crystallographic data and experimental details for(Et4N)2[MoO(S4)(bdtCl2)](2),(Et4N)2[Mo2O2(μ2-S)2(bdt)](3)and(Et4N)2[Mo2O2(μ2-S)2(bdtCl2)](4)are summarized in Tab.1.The selected bond lengths and bond angles are listed in Tab.2.Intensity data were collected on a Bruker SMART APEX 2000 CCD diffractometer using graphite-monochromated MoK radiation(λ=0.710 73 Å)at 296(2)K.The collected frames were processed with the software SAINT.The data were corrected for absorption using the program SADABS.The structures were solved by Direct Methods and refined by full-matrix least-squares on F2using the SHELXTL software package.All non-hydrogen atoms were refined anisotropically except the[Et4N]+cations in complex 2 due to the obviously disordered.The positions of all hydrogen atoms were generated geometricallyassigned isotropic displacement parameters,and allowed to ride on their respective parent carbon or nitrogen atoms before the final cycle of least-squares refinement.The largest peak in the final difference maps had height of 1.131 e·Å-3for 2 are in the vicinity of the molybdenum atom.
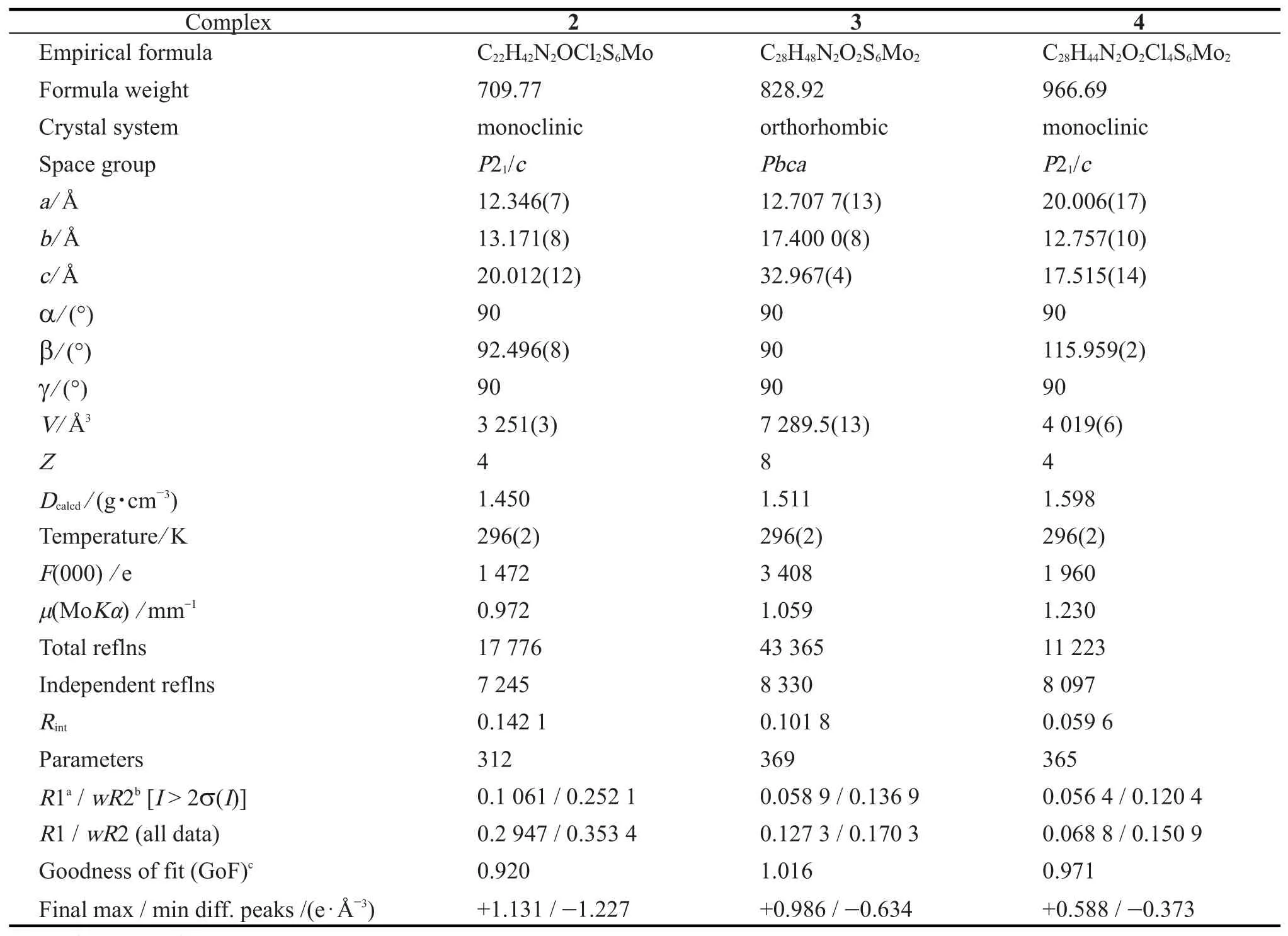
Tab.1 Crystallographic data and experimental details for(Et4N)2[MoO(S4)(bdtCl2)](2),(Et4N)2[Mo2O2(μ2-S)2(bdtH2)](3)and(Et4N)2[Mo2O2(μ2-S)2(bdtCl2)](4)
2 Results and Discussion
Previously,Sugimoto reported that mono(dithiolene)oxo-molybdenum complexes[MoIVO(S4)(bdtX2)]2-could be prepared by two methods:the substitution reaction of a tetrasulfido ligand in[MoO(S4)2]2-with the corresponding dithiol and the hydrolysis reaction of the corresponding mono(dithiolene)sulfido-molybdenum complexes[MoS(S4)(bdtX2)]2-with water and phenol[13].Afterwards,Haumann and Schulzke reported the structure of complex(NEt4)2[MoO(xdt)(S4)](H2xdt=4,5-dimethylbenzene-1,2-dithiol)obtained by reaction of(NEt4)2[MoO(S4)2]and equivalent H2xdt in acetonitrile at room temperature[14].Guided by these results,we took the reactions of(Et4N)2[MoIVO(S4)2]withequivalent1,2-benzenedithiol(H2bdt)and3,6-dichloro-1,2-benzenedithiol(H2bdtCl2)inCH3CN andobtainedtheexpectedproducts(Et4N)2[MoO(S4)(bdtX2)](X=H,1;X=Cl,2)inmoderateyields(Fig.1).Sugimoto also stated that mono(dithiolene)oxo-molybdenum complex(Et4N)2[MoO(S4)(bdt)]could be converted to be bis(dithiolene)MoIVO complexes in the presence of additional dithiol[13].However,treatment of mono(dithiolene)oxomolybdenum(IV)complexes(Et4N)2[MoO(S4)(bdtX2)](1and2)withadditionalequimolardithiolsinthispaperled to isolation of bis(µ-sulfido)dimolybdenum(V)species(Et4N)2[Mo2O2(μ2-S)2(bdtX2)2](X=H,3;X=Cl,4)in moderate yields.The syn-isomers are formed in both complexes 3 and 4 referring to the position of the apical oxo ligands with respect to the Mo2S2plane.It is noted that Schulzke has reported the structure of complex(Et4N)2[Mo2O2(μ2-S)2(bdt)2]usingbenzene-1,2-dithiolandslightlyexcessof(Et4N)2[MoO2S2]inacetonitrileatroomtemperature in a relatively low yield(17%)[15].Moreover,Holm has synthesized the anti-isomer(Et4N)2[Mo2O2(μ2-S)2(bdt)2]uti-lizing MoVIcomplex[MoO3(bdt)]2-and H2S[16].
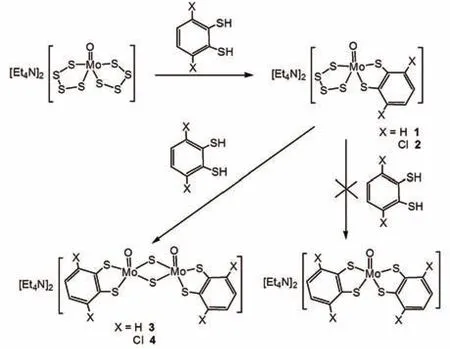
Fig.1 Synthetic reactions of sulfido/oxomolybdenum complexes 1-4
The structures of complexes 2,3 and 4 were established by X-ray crystallography.Actually,it was not easy to obtain high-quality crystals of complex 2 for single-crystal X-ray diffraction analysis.Complex 2 crystallizes in space group P21/c with Z=4,with one[MoO(S4)(bdtCl2)]2-anion and two[NEt4]+cations,as shown in Fig.2(a).The molybdenum atom is coordinated by a terminal oxo O(1),two sulfur atoms S(1)and S(4)of a tetrasulfido group,and two sulfur atoms S(5)and S(6)of dianionic bdtCl2ligand.The bond length ofis 1.824(2)Å,slightly longer than that in the related complex(NEt4)(PPh4)[MoO(S4)(bdtCl2)](1.732(4)Å)with a different cation[13].Thebond lengths range from 2.329 8(16)to 2.4193(14)Ǻ,compared well to those in(NEt4)(PPh4)[MoO(S4)(bdtCl2)](2.335(2)-2.405(2)Å)[13].Thebond angles ranging from 105.96(10)oto 108.90(7)o,similar to those in(NEt4)(PPh4)-[MoO(S4)(bdtCl2)](106.9o-110.6o)[13].For the tetrasulfido group,the S1,S2,S3,and S4 atoms form a zigzag structure in which the torsion angle defined byis about 20°,which is smaller than those in the related complexes[13-14].
The dinuclear molybdenum complex 3 crystallizes in space group Pbca with Z=8,and its anion structure is shown in Fig.2(b).Thedistance is 2.878 9(8)Å in complex 3,similar to that in the syn-isomer(NEt4)2[MoO(S4)(bdt)](2.890 5(4)Å)obtained by an alternative synthetic method reported by Schulzke[15]and the antiisomer,i.e.,centrosymmetric dimmer(NEt4)2[MoO(S4)(bdt)](2.901 8(13)Å)reported by Holm[16].The average bond length ofis 1.750(4)Å in complex 3,compared well to that reported by Schulzke(1.741 5 Å)[15],but obviously shorter than that in complex 2 (Et4N)2[MoO(S4)(bdtCl2)](1.824(2)Å),possiblyduetothehigheroxidationstateofmolybdenum in complex 3.The averagein complex 3 are 2.328 1 and 2.406 6 Å,respectively,which are in good agreement to the corresponding parametersof2.3346and2.411ÅreportedbySchulzke(1.741 5 Å)[15].
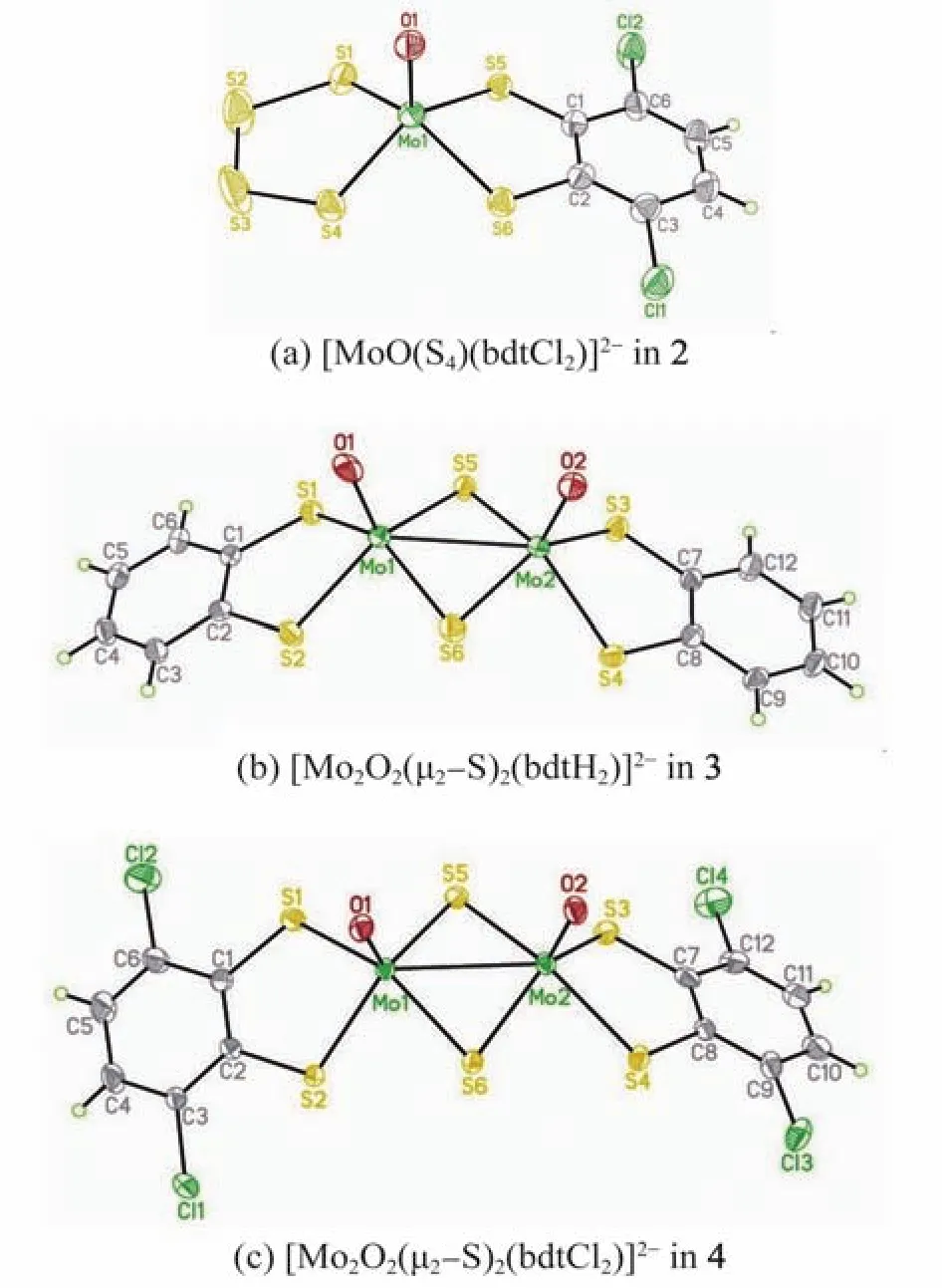
Fig.2 ORTEPdiagrams of complexes showing the atom labeling scheme and 40%thermal ellipsoids
The dinuclear molybdenum complex 4 crystallizes in space group P21/c with Z=4,and its anionic part is shown in Fig.2(c).The anion consists of two[MoO(bdtCl2)]+units bridged by two sulfide groups.In complex 4,the two terminal oxo groups are located in syn configuration as in complex 3.The Mo Mo distance is 2.876(2)Å in complex 4,almost the same to that in complex 3(2.878 9(8)Å).The average Mo Oterminalbond length of 1.692(5)Å is a little shorter than that in complex 3(1.750(4)Å).The meanbond length of 2.414Åis slightly longer than the meanbond length of 2.332Åin complex 4.The bond angle of(150.81(7)°)is compared to that of(134.75(9)°),and the stereochemistry of Mo2 is almost the same as that of Mo1,indicating the molybdenum atoms both possess a square-pyramidal geometry as in its sulfur analogue(Et4N)2[Mo2S2(μ2-S)2(bdtCl2)2][13].
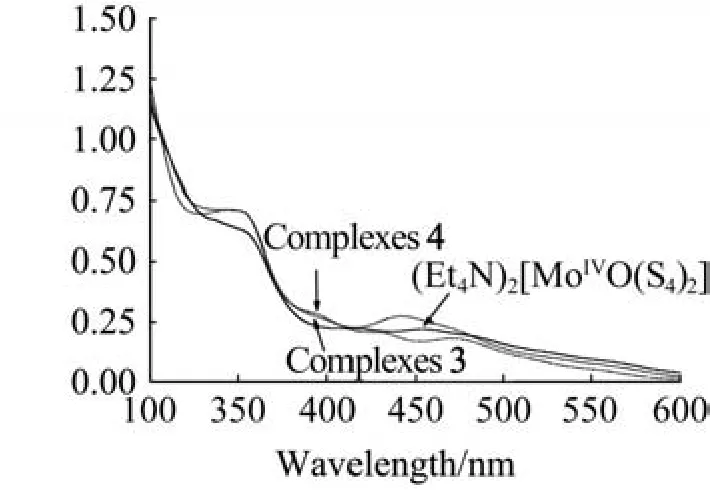
Fig.3 UV-Vis absorption spectrum of complexes in acetonitrile
Complexes 1-4 were also characterized by the proton NMR data,IR,UV-vis and elemental analyses.For example,the ν(Mo=O)stretching value of complex 4(IR:989 cm-1)compared well to those of(Et4N)2[MoO(S4)2](IR:932 cm-1)[17]and(Et4N)2[Mo2O2(μ2-S)2(bdt)2](IR:966 cm-1)[15].The electronic spectra of dimeric complexes 3 and 4 together with the starting material(Et4N)2[MoIVO(S4)2]are characterized(Fig.3)bytwoabsorptionsatarangeof355-465nm,comparable with that of related oxo-molybdenum complex(Et4N)2[MoO(bdt)(bdtCl2)](330 and 423 nm)[13].Bands at around355nmareprobablydominatedbytheS↔Mochargetransfers,which are blue-shifted relative to that of the complex[(edt)2Mo2O(µ3-S)(µ-S)2Cu2(Py)4]at 336 nm(edt=ethanedithiolate,Py=pyridine)[18].
3 Conclusion
Mono(dithiolene)oxo-molybdenum(IV)complexes(Et4N)2[MoO(S4)(bdtX2)]andbis(µ-sulfido)oxo(dithiolene)molybdenum(V)species(Et4N)2[Mo2O2(μ2-S)2(bdtX2)2]were successfully isolated from oxo-bis(tetrasulfido)molybdenum(IV)complex(Et4N)2[MoO(S4)2]and 1,2-arenedithiols in moderate yields,which will be used as effective synthons for further construction of new biologically heterobimetallic clusters.
