植物营养元素运输载体的功能及其调控机制研究进展
2018-07-05陈迪潘伟槐周哉材严旭潘建伟3
陈迪,潘伟槐,周哉材,严旭,潘建伟3*
(1.浙江师范大学化学与生命科学学院,浙江金华321004;2.绍兴文理学院生命科学学院,浙江绍兴312000;3.兰州大学生命科学学院,兰州730000)
氮(N)、磷(P)、钾(K)和锌(Zn)等是植物生长发育的重要必需元素。N是植物所需的最重要的大量元素之一,土壤中的硝酸盐(NO3-)和铵盐(NH4+)是植物吸收利用氮源的主要形式。植物根的表皮和皮层细胞通过不同的转运蛋白从土壤中吸收NO3-,然后通过代谢还原成铵盐,由铵直接合成氨基酸,供植物利用[1]。P是能量转移和各类代谢等的重要调控元素。K参与植物细胞的许多生理过程,如酶激活、气孔运动和pH的稳态等。Zn是植物生长发育所必需的微量元素,参与糖类、脂类和核酸等各类代谢的调控。
由于植物营养是作物高产、稳产的重要生理基础,目前对植物细胞如何吸收利用N、P、K和Zn等营养元素的分子机制已有较为深入的研究,尤其是对这些营养元素的转运蛋白本身的调控研究已取得长足进展,并且是近几年作物营养研究领域的热点之一。本文在扼要介绍N、P、K和Zn等转运蛋白理化特性的基础上,着重介绍了最近几年有关这些转运蛋白的生物学功能及其作用机制的最新进展,为作物养分高效利用和品质遗传改良提供新的研究思路和策略。
1 植物氮转运蛋白及其吸收运输机制
已知高等植物有4类转运蛋白具有NO3-的转运活性,包括 NRT1/PTR(nitrate transporter 1/peptide transporter,硝酸盐转运蛋白1/寡肽转运蛋白)或NPF(NRT1/PTR family,NRT1/PTR家族)、NRT2、CLC(chloride channel family,氯离子通道家族)和SLAC/SLAH(slow anion channels,慢速阴离子通道蛋白)。高等植物为适应不同浓度的NO3-环境,分别进化出2类NO3-转运蛋白系统:高亲和转运系统(high-affinity transport system,HATS)和低亲和转运系统(low-affinity transport system,LATS)。HATS由 NRT2/NNP(NRT2/nitrate-nitrite-porter)家族成员组成,当外界NO3-浓度低至微摩尔水平时,其对NO3-的吸收有积极作用;相反,LATS由NPF家族成员主导,在毫摩尔浓度时吸收NO3-。这2类转运蛋白系统的转运活性均需要细胞提供能量和胞外质子梯度[2]。
在N不足的情况下,NRT2在植物NO3-/H+同向吸收过程中起着关键性调控作用,但需要伴侣蛋白NAR2的参与(图1)。水稻基因组编码5个OsNRT2家族成员,其中OsNRT2.3a和OsNRT2.3b由OsNRT2.3通过选择性剪接机制形成,在不同组织中发挥功能。水稻OsNRT2.1、OsNRT2.2和OsNRT2.3a介导NO3-的吸收,但依赖于伴侣蛋白OsNAR2.1的参与、根的发育状态和植株体内N的水平[8]。此外,其他物种的NRT2成员也已经被鉴定(表1)。
第3类转运体是氯离子通道蛋白CLC,具有转运NO3-的功能。拟南芥和水稻基因组均编码7个CLC家族成员(表1)。有研究表明,CLCa和CLCb是定位于液泡膜上的2NO3-/1H+逆向转运蛋白,在液泡NO3-的积累中发挥重要的作用(图1)。而定位于液泡膜上的CLCg的具体功能目前尚不清楚。当前,对烟草和大豆CLC功能的研究还较落后(表1)。
第4类NO3-转运体是SLAC/SLAH。拟南芥的SLAC/SLAH家族共有5个成员(表1),这些基因的蛋白产物均定位在质膜上。AtSLAC1和AtSLAH3在保卫细胞中编码S型阴离子通道,能直接调控脱落酸(abscisic acid,ABA)信号,促使Cl-和NO3-从保卫细胞中释放,刺激气孔关闭。AtSLAH2在根中柱细胞中表达,介导NO3-的输出,与从根到茎中NO3-的运输有关。功能分析表明,SLAH2是NO3-的特异性通道,其活性受CBL1和CIPK21/23互作蛋白调控。而SLAH3的活性受CDPK21(calciumdependent protein kinase,钙离子依赖性蛋白激酶)磷酸化激活(图1)[25]。目前,在水稻中仅鉴定出2个SLAC基因(表 1),其中 SLAC1受蛋白激酶OsSAPK8(stress-activated protein kinase,应激活化蛋白激酶)的正调控。这些研究结果表明,SLAC/SLAH在不同组织部位中介导NO3-的运输。

表1 不同植物物种已被鉴定的N转运蛋白类型和种类Table 1 Types and numbers of identified N transporter in different plant species
植物NH4+的运输主要由铵转运蛋白(ammonium transporters,AMT)介导。拟南芥共有6个AMT转运蛋白(表1)。最近的研究发现,AMT1;1和1;2受CBL1-CIPK23的磷酸化调控,从而抑制NH4+的转运活性[26](图1)。水稻共有10个AMT类转运蛋白,分为 4个亚类:OsAMT1、OsAMT2、OsAMT3和OsAMT4。其中OsAMT1是高亲和转运蛋白,而OsAMT2~AMT4介导低亲和NH4+的转运[23],但至今有关OsAMT2~AMT4的报道较少。最近研究表明,转录因子OsDOF18(DNA binding with one finger 18)正调控OsAMT1;1、1;3和2;1的表达,从而促进植物细胞对NH4+的吸收[27]。此外,在小麦和玉米中也已鉴定出若干个AMT蛋白(表1),但其具体的作用机制仍有待研究。
2 植物磷转运蛋白及其吸收运输机制
已知植物磷转运蛋白(phosphate transporter,PHT)主要分为5个家族:PHT1、PHT2、PHT3、PHT4和PHT5。
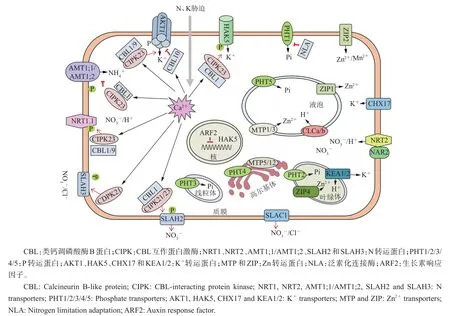
图1 植物N、P、K和Zn转运蛋白及其调控机制[25-26,28-31]Fig.1 Transporters and their action mechanisms of N,P,K and Zn in plants[25-26,28-31]
PHT1主要定位于质膜上(图1)。从表2可知,拟南芥共有9个PHT1成员,在其他物种中也已经被鉴定到多个PHT1成员。已知拟南芥RING类型 E3泛素化连接酶NLA(nitrogen limitation adaptation)能介导PHT1的降解,从而降低PHT1的质膜丰富度和Pi的吸收[28](图1)。水稻共有13个PHT1成员,多数功能已经被鉴定(表2)。有研究显示,水稻OsNLA通过与OsPHT1.2和OsPHT1.8互作,促进其降解,从而抑制Pi的过度积累。在Pi充足的条件下,水稻酪蛋白激酶ⅡCK2磷酸化OsPHT,抑制OsPHT与磷酸盐转运蛋白运输促进因子(phosphate transporter traffic facilitator 1,PHF1)的互作,从而阻止OsPHT从内质网到质膜的运输,导致其在内质网(endoplasmic reticulum,ER)积累[32]。有研究表明,低Pi能促进小麦MYB转录因子TaPHR1(phosphate starvation response 1)与 TaPHT1基因启动子结合,从而上调TaPHT1的转录[33]。相反,拟南芥转录因子MYB62经低Pi诱导下调AtPHT1;1和AtPHT1;4的转录[34],并且抑制WRKY75表达也会导致AtPHT1;1和AtPHT1;4转录下调[35]。同样,大麦转录因子TabHLH1上调促进烟草NtPHT1表达,从而提高植株对低Pi的耐受性[36]。此外,其他多个物种的PHT1功能也陆续被鉴定(表1)。以上研究结果表明,PHT1转录调控和转录后调控均为介导磷吸收的重要调控机制。
PHT2转运蛋白定位于叶绿体内膜上(图1)。拟南芥和水稻均只有1个PHT2成员(表2),均在叶中表达且受低Pi诱导。目前,PHT2的具体作用机制仍有待进一步研究。
PHT3转运蛋白定位于线粒体内膜上(图1)。从表2中可以看出,拟南芥有3个AtPHT3成员,在水稻、小麦等物种中也存在多个PHT3同源蛋白。PHT4转运蛋白位于高尔基体上(图1),参与调控叶的形成、植物防御、耐盐性等过程。拟南芥共有6个AtPHT4成员,主要在根和叶中表达,其他物种也存在多个PHT4转运蛋白(表2)。但PHT3和PHT4的具体功能至今仍知之甚少。
随着新媒体的快速发展,微信群成为家校沟通的重要渠道。管理班级微信群,与其要求家长在群里不能做什么,还不如与家长商讨能做什么,以及怎么做。开学初,我借助家长会,与各科老师以及家长充分探讨,最终确定了班级微信群每天“群聊”的话题。同时,这也被当作家长的一项“作业”来完成。
PHT5蛋白,又被称为SPX-MFS蛋白(SYG1/PHO81/XPR1),定位于液泡膜上(图1)。表2显示:拟南芥PHT5家族共由3个成员组成,过表达AtPHT5均能引起植株生长缓慢和液泡Pi的积累,表明PHT5成员具有功能丰余性;水稻SPX-MFS家族有4个成员,Pi饥饿会抑制OsSPX-MFS1的表达,但促进OsSPX-MFS2的表达,不过它们均受OsmiR827的负调控[49]。这些研究结果证实了PHT5能调控胞内Pi的稳态,对维持植物细胞的正常生长分化具有重要的生物学功能。

表2 不同物种已被鉴定的P转运蛋白的类型和数量Table 2 Types and numbers of identified P transporters in different plant species
3 植物钾转运蛋白及其吸收运输机制
与硝态氮吸收机制相似,植物细胞内也同样存在高亲和与低亲和的K+吸收系统,它们分别应对低浓度和高浓度的K+环境。关于K+通道蛋白家族的研究,近几年国内已经有较好的综述[50],本节着重介绍K+转运蛋白的功能及其作用机制。
植物细胞共有4类K+转运蛋白家族:KT/HAK/KUP(K+transporter/high-affinity K+transporter/K+uptake permease,K+转运蛋白/高亲和K+转运蛋白/K+吸收通透酶)、HKT(high-affinity K+transporter,高亲和 K+转运蛋白)、CHX(cation/hydrogen exchanger,阳离子/H+反向转运蛋白)和KEA(K+/H+efflux antiporters,K+/H+反向转运蛋白)。从表3中可以看出,拟南芥KT/HAK/KUP转运蛋白共有13个成员,水稻OsHAK家族共有27个成员。此外,在其他物种中也存在多个同源蛋白。KT/HAK/KUP基因几乎在所有植物组织或器官中均有表达,表明该转运蛋白家族对植物器官的生长发育和维持细胞中K+的平衡具有非常重要的作用。
HKT转运蛋白是K+/Na+同向转运体。HKT可分为亚族Ⅰ和Ⅱ,其中亚族Ⅰ存在于单子叶和双子叶植物中,主要由Na+转运蛋白组成,亚族Ⅱ似乎只存在于单子叶植物中,由Na+和K+转运蛋白组成。由表3可知,拟南芥HKT家族仅有1个成员,而水稻HKT家族由9个成员组成,主要介导Na+的转运。
已知HAK5和AKT1是拟南芥中2大主要的K+吸收系统。从图1中可以看出:在低K+条件下,拟南芥生长素响应因子(auxin response factor 2,ARF2)能与HAK5启动子结合从而抑制HAK5的表达[31];CBL1与CIPK9互作能促进HAK5的磷酸化和K+的吸收;拟南芥AKT1介导的K+吸收表现为双亲和特性,在K+不足的情况下,CBL1/9能分别与CIPK23互作,并招募CIPK23至细胞质膜,进而磷酸化AKT1,导致由低亲和吸收转变为高亲和;而CBL10作为负调控因子直接与AKT1互作,抑制AKT1介导的K+胞质转运[51];水稻OsAKT1是盐敏感型K+吸收的主要通道,OsCBL1-OsCIPK23复合物能提高OsAKT1介导的K+吸收[52],表明OsAKT1介导的K+转运过程受OsCBL1-OsCIPK23的正调控。
CHX属于CPA(cation/proton antiporters,阳离子/质子反向转运蛋白)超家族。表3显示,拟南芥共有28个成员,其中研究最广泛的CHX17介导质膜K+的转运[53](图1)。关于CHX的功能在水稻和野生大豆中也有报道,但其具体作用机制有待进一步研究。
植物KEA家族是K+/H+转运体。拟南芥有6个KEA成员,它们均在维管组织中表达(表3)。叶绿体有3个K+输出反向转运体KEA1~KEA3,其中KEA1和KEA2位于叶绿体内膜上,调控叶绿体K+/H+的稳态(图1),均与光合作用相关[54]。有关水稻、玉米和高粱等作物的KEA作用机制仍需进行深入研究。

表3 不同物种已被鉴定的K转运蛋白的类型和数量Table 3 Types and numbers of identified K transporters in different plant species
4 植物锌转运蛋白及其吸收运输机制
Zn转运蛋白可分为ZIP、CDF(cation diffusion facilitator,阳离子扩散促进子家族)和P1B-ATP酶型家族HMA等蛋白家族(表4)。ZIP转运蛋白在植物和动物中功能相当保守,在Zn2+的吸收和转运过程中不可或缺。拟南芥ZIP家族共有16个成员,其中研究最清楚的是AtZIP1和AtZIP2,分别定位在液泡膜和质膜上,介导液泡Zn2+的输出和质膜Zn2+/Mn2+的吸收[71]。水稻ZIP家族有17个成员,主要参与 Zn2+转运[72]。

表4 不同物种已被鉴定的Zn转运蛋白的类型和数量Table 4 Types and numbers of identified Zn transporters in different plant species
CDF转运蛋白,又称金属耐受性蛋白(metal tolerance proteins,MTP)。大多数CDF是Mn2+/H+(Zn2+)逆向运输蛋白,介导过度金属阳离子从胞质到胞外或细胞器的转运。CDF通常定位于质膜或细胞器膜上,参与金属解毒、金属蛋白的组装和分泌囊泡的包装。拟南芥CDF家族有12个成员,而水稻有10个成员,按功能可分为3类:Zn-CDF、Zn/Fe-CDF和Mn-CDF。由图1可知,AtMTP1和AtMTP3定位于液泡膜上,介导Zn2+从胞质到液泡的转运,以维持胞质低Zn2+浓度;AtMTP5和AtMTP12位于高尔基体,介导Zn2+从胞质到高尔基体的转运[77]。
P1B-ATP酶型家族HMA在重金属离子的胞内分配与解毒过程中起重要作用,可分为2个亚类:Ⅰ型(Cu+/Ag+)和Ⅱ型(Zn2+/Cd2+/Pb2+/Co2+)。拟南芥HMA亚族有8个HMA成员,其中AtHMA1~AtHMA4属于Ⅱ型,分别介导不同组织中Zn2+的转运,而其余属于Ⅰ型。水稻共有9个HMA成员,其中OsHMA1~OsHMA3属于Ⅱ型,其余属于Ⅰ型。大麦和小麦等作物中的HMA蛋白也有报道。
5 植物营养元素转运蛋白的内吞调控机制
尽管营养元素对植物生长发育是必需的,但过多的吸收与积累也会对植物细胞造成毒害;因此,对营养元素吸收和转运进行精确调控是植物生长发育的重要调控机制之一。质膜内吞(endocytosis)是真核细胞吸收胞外营养物质和传递胞内外信号的重要途径,也是调控脂质、受体和转运蛋白质膜丰度与降解的重要手段。因此,深入剖析质膜内吞调控机制对理解植物生长发育调控机制具有重要的科学意义。
已知硼和铁转运蛋白的质膜内吞是植物细胞吸收硼和铁及其分布的重要机制[78-81]。在低硼条件下,拟南芥硼输入载体NIP5;1(nodulin 26-like intrinsic protein 5;1,类Nod26膜内在蛋白)和硼输出载体BOR1(boron transporter 1,硼转运蛋白)在根尖细胞中分别定位于细胞外侧质膜和内侧质膜上,这种亚细胞定位对硼的吸收与转运极为重要。硼外源处理能快速诱导BOR1质膜内吞和液泡降解,但不影响NIP5;1亚细胞定位[81];网格蛋白接头蛋白复合体AP-2亚基AP-2µ功能缺失将抑制NIP5;1质膜内吞和侧向极性定位[80]。植物细胞铁的吸收在很大程度上依赖于铁输入载体IRT1(iron-regulated transporter 1,铁离子转运蛋白),而化学或遗传损害网格蛋白或网格蛋白介导的内吞(clathrin-mediated endocytosis,CME)功能促进了IRT1的质膜定位,尤其在质膜外侧定位[78-79]。这些研究结果表明,网格蛋白及其介导的内吞对维持转运蛋白的质膜极性定位具有重要功能。
目前,对N、P、K和Zn等转运蛋白的内吞研究相对较少。布雷菲德菌素A(brefeldin A,BFA)是植物细胞外吐(exocytosis)或再回收(recycling)途径的抑制剂,是植物细胞质膜内吞分析的重要工具。已知K+转运蛋白CHX17定位于质膜上,其表达受K+饥饿诱导。BFA处理会引起AtCHX17质膜信号下降,但在BFA小体中的信号上升[82]。AtPHT1;1是质膜和内质网定位的Pi转运蛋白,BFA处理同样诱导PHT1;1质膜信号下降而BFA小体信号上升[83]。这些研究结果表明,CHX17和PHT1;1质膜丰度受内吞途径的调控。
6 展望
植物营养元素的吸收与转运机制是近几年植物营养生理学研究领域的重要热点之一。至今对植物N、P、K和Zn等转运蛋白的功能研究已经取得了一定进展,尤其对这些蛋白家族基因在不同生理条件下的响应机制已经有了较好的了解。然而,关于这些转运蛋白的具体分子作用机制或调控机制仍有待于进一步探索。目前亟待解决的科学问题有:1)对这些转运蛋白晶体结构的解析将是理解转运蛋白分子作用机制的核心科学问题,其晶体结构能否解析成功将成为本研究领域的一个突破/瓶颈;2)弄清这些转运蛋白的分泌、膜定位和液泡降解等过程的细节问题将是理解转运蛋白功能调控的核心内容;3)将植物响应外界的信号通路整合成全面的信号网通路将成为研究转运蛋白功能机制的突破性进展;4)剖析不同转运蛋白之间的协调作用;5)分析这些转运蛋白在植物逆境胁迫过程中的生物学功能,尤其是在逆境条件下,对作物产量性状的贡献。这些科学问题的解决将对作物产量生产(包括作物高产、稳产和品质育种等)具有重要的理论和现实指导意义。
:
[1] LI T Y,LIAO K,XU X F,et al.Wheat ammonium transporter(AMT)gene family:Diversity and possible role in hostpathogen interaction with stem rust.Frontiers in Plant Science,2017,8:1637.
[2] LAW C J,MALONEY P C,WANG D N.Ins and outs of major facilitator superfamily antiporters.Annual Review of Microbiology,2008,62:289-305.
[3] CHIBA Y,SHIMIZU T,MIYAKAWA S,et al.Identification ofArabidopsisthaliana NRT1/PTR FAMILY (NPF)proteins capable of transporting plant hormones.Journal of Plant Research,2015,128(4):679-686.
[4] LÉRAN S,EDEL K H,PERVENT M,et al.Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2,a phosphatase inactivated by the stress hormone abscisic acid.Science Signaling,2015,8(375):ra43.
[5] FAN X R,FENG H M,TAN Y W,et al.A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen.Journal of Integrative Plant Biology,2016,58(6):590-599.
[6] XIA X D,FAN X R,WEI J,et al.Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and longdistance transport.Journal of Experimental Botany,2015,66(1):317-331.
[7] LIN C M,KOH S,STACEY G,et al.Cloning and functional characterization of a constitutively expressed nitrate transporter gene,OsNRT1,from rice.Plant Physiology,2000,122(2):379-388.
[8] KOTUR Z,GLASS A D M.A 150 kDa plasma membrane complexofAtNRT2.5andAtNAR2.1isthemajor contributor to constitutive high-affinity nitrate influx in Arabidopsis thaliana.Plant,Cell&Environment,2015,38(8):1490-1502.
[9]PELLIZZARO A,ALIBERT B,PLANCHET E,et al.Nitrate transporters:An overview in legumes.Planta,2017,246(2):585-595.
[10]FAN X R,NAZ M,FAN X R,et al.Plant nitrate transporters:From gene function to application.Journal of Experimental Botany,2017,68(10):2463-2475.
[11]BUCHNER P,HAWKESFORD M J.Complex phylogeny and gene expression patterns of members of the NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family(NPF)in wheat.Journal of Experimental Botany,2014,65(19):5697-5710.
[12]BAI H,EURING D,VOLMER K,et al.The nitrate transporter(NRT)gene family in poplar.PLoS One,2013,8(8):e72126.
[13]SEYOSHI K,ISHIKAWA S,ABDEL-LATIF H I S.Nitrate transport in barley//Nitrogen Assimilation in Plants.Kerala,India:Research Signpost,2010.
[14]VON WITTGENSTEIN N J J B,LE C H,HAWKINS B J,et al.Evolutionary classification of ammonium,nitrate,and peptide transporters in land plants.BMC Evolutionary Biology,2014,14:11.
[15]GUO T C,XUAN H M,YANG Y Y,et al.Transcription analysis of genes encoding the wheat root transporter NRT1 and NRT2 families during nitrogen starvation.Journal of Plant Growth Regulation,2014,33(4):837-848.
[16]孟森.林木细根氮素吸收动态及氮转运蛋白基因表达.陕西,杨凌:西北农林科技大学,2016.MENG S.Dynamics of nitrogen uptake and gene expression of nitrogen transporter by forest roots.Yangling,Shaanxi:Northwest A&F University,2016.(in Chinese with English abstract)
[17]NGUYEN C T,AGORIO A,JOSSIER M,etal.Characterization of the chloride channel-like,AtCLCg,involved in chloride tolerance in Arabidopsis thaliana.Plant Cell Physiology,2016,57(4):764-775.
[18]LI X J,YU B J,CUI Y Q,et al.Melatonin application confers enhanced salt tolerance by regulating Na+and Claccumulation in rice.Plant Growth Regulation,2017,83(3):441-454.
[19]WONG T H,LI M W,YAO X Q,et al.The GmCLC1 protein from soybean functions as a chloride ion transporter.Journal of Plant Physiology,2013,170(1):101-104.
[20]LURIN C,GÜCLÜ J,CHENICLET C,et al.CLC-Nt1,a putative chloride channel protein of tobacco,co-localizes with mitochondrial membrane markers.The Biochemical Journal,2000,348(2):291-295.
[21]QIU J E,HENDERSON S W,TESTER M,et al.SLAH1,a homologue of the slow type anion channel SLAC1,modulates shoot Cl-accumulation and salt tolerance in Arabidopsis thaliana.Journal of Experimental Botany,2016,67(15):4495-4505.
[22]FAN X L,WU J M,CHEN T Y,et al.Loss-of-function mutation of rice SLAC7 decreases chloroplast stability and induces a photoprotection mechanism in rice.Journal of Integrative Plant Biology,2015,57(12):1063-1077.
[23]YE X X,SUN Y J,LIU P P,et al.Evolutionary analysis of AMT (ammonium transporters)family in Arabidopsis thaliana and Oryza sativa.Molecular Soil Biology,2016,7(11):1-7.
[24]GU R L,DUAN F Y,AN X,et al.Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize(Zea mays L.).Plant Cell Physiology,2013,54(9):1515-1524.
[25]GEIGER D,MAIERHOFER T,AL-RASHEID K A S,et al.Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1.Science Signaling,2011,4(173):ra32.
[26]STRAUB T,LUDEWIG U,NEUHÄUSER B.The kinase CIPK23 inhibitsammonium transportin Arabidopsis thaliana.The Plant Cell,2017,29(2):409-422.
[27]WU Y F,YANG W Z,WEI J H,et al.Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots.Molecules Cells,2017,40(3):178-185.
[28]LIN W Y,HUANG T K,CHIOU T J.Nitrogen limitation adaptation,a target of microRNA827,mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis inArabidopsis.The Plant Cell,2013,25(10):4061-4074.
[29]MA Q,TANG R J,ZHENG X J,et al.The calcium sensor CBL7 modulatesplantresponsesto low nitrate in Arabidopsis. Biochemical and Biophysical Research Communications,2015,468(1/2):59-65.
[30]MAO J J,MANIK S M N,SHI S J,et al.Mechanisms and physiological roles of the CBL-CIPK networking system in Arabidopsis thaliana.Genes,2016,7(9):62.
[31]ZHAO S,ZHANG M L,MA T L,et al.Phosphorylation of ARF2 relieves its repression of transcription of the K+transporter gene HAK5 in response to low potassium stress.The Plant Cell,2016,28(12):3005-3019.
[32]CHEN J Y,WANG Y F,WANG F,et al.The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels.The Plant Cell,2015,27(3):711-723.
[33]WANG J,SUN J H,MIAO J,et al.A phosphate starvation response regulator Ta-PHR1 is involved in phosphate signalling and increases grain yield in wheat.Annals of Botany,2013,111(6):1139-1153.
[34]DEVAIAH B N,MADHUVANTHI R,KARTHIKEYAN A S,et al.Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor inArabidopsis.Molecular Plant,2009,2(1):43-58.
[35]DEVAIAH B N,KARTHIKEYAN A S,RAGHOTHAMA K G.WRKY75 transcription factorisa modulatorof phosphate acquisition and root development in Arabidopsis.Plant Physiology,2007,143(4):1789-1801.
[36]DING WW,WANGYX,FANG W B,et al.TaZAT8,a C2H2-ZFP type transcription factor gene in wheat,plays critical roles in mediating tolerance to Pi deprivation through regulating P acquisition,ROS homeostasis and root system establishment.Plant Physiology,2016,158(3):297-311.
[37]AYADI A,DAVID P,ARRIGHI J F,et al.Reducing the genetic redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 transporters to study phosphate uptake and signaling.Plant Physiology,2015,167(4):1511-1526.
[38]PASZKOWSKI U,KROKEN C,ROUX V,et al.Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscularmycorrhizal symbiosis.Proceedings ofthe NationalAcademy of Sciences of the USA,2002,99(20):13324-13329.
[39]MLODZINSKA E,ZBOINSKA M.Phosphate uptake and allocation:A closer look at Arabidopsis thaliana L.and Oryza sativa L.Frontiers in Plant Science,2016,7:1198.
[40]LIU F,XU Y J,JIANG H H,et al.Systematic identification,evolution and expression analysis of the Zea mays PHT1 gene family reveals several new members involved in root colonization by arbuscular mycorrhizal fungi.International Journal of Molecular Sciences,2016,17(6):930.
[41]SISAPHAITHONG T,KONDO D,MATSUNAGA H,et al.Expression of plant genes for arbuscular mycorrhizainducible phosphate transporters and fungal vesicle formation in sorghum,barley,and wheat roots.Bioscience,Biotechnology,and Biochemistry,2012,76(12):2364-2367.
[42]ZHANG C X,MENG S,LI M J,et al.Genomic identification and expression analysis of the phosphate transporter gene family in poplar.Frontiers in Plant Science,2016,7:1398.
[43]CHEN A Q,CHEN X,WANG H M,et al.Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato.BMC Plant Biology,2014,14:61.
[44]VERSAW W K,HARRISON M J.A chloroplast phosphate transporter,PHT2;1,influences allocation of phosphate within the plant and phosphate-starvation responses.The Plant Cell,2002,14(8):1751-1766.
[45]史书林,王丹凤,颜彦,等.水稻磷转运蛋白OsPHT2;1在提高磷素利用率方面的作用.中国水稻科学,2013,27(5):457-465.SHI S L,WANG D F,YAN Y,et al.Function of phosphate transporter OsPHT2;1 in improving phosphate utilization in rice.Chinese Journal of Rice Science,2013,27(5):457-465.(in Chinese with English abstract)
[46]SHUKLA V,KAUR M,AGGARWAL S,et al.Tissue specific transcript profiling of wheat phosphate transporter genes and its association with phosphate allocation in grains.Scientific Reports,2016,6:39293.
[47]WANG D L Y,LÜ S L,JIANG P,et al.Roles,regulation,and agricultural application of plant phosphate transporters.Frontiers in Plant Science,2017,8:817.
[48]VELASCO V M E,MANSBRIDGE J,BREMNER S,et al.Acclimation of the crucifer Eutrema salsugineumto phosphate limitation is associated with constitutively high expression of phosphate-starvation genes.Plant,Cell&Environment,2016,39(8):1818-1834.
[49]LIN S I,SANTI C,JOBET E,et al.Complex regulation of two target genes encoding SPX-MFS proteins by rice miR827 in response to phosphate starvation.Plant and Cell Physiology,2010,51(12):2119-2131.
[50]伍国强,水清照,冯瑞军.植物K+通道AKT1的研究进展.植物学报,2017,52(2):225-234.WU G Q,SHUI Q Z,FENG R J.Research advance of K+channel AKT1 in plants.Chinese Bulletin of Botany,2017,52(2):225-234.(in Chinese with English abstract)
[51]REN X L,QI G N,FENG H Q,et al.Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+homeostasis in Arabidopsis.The Plant Journal,2013,74(2):258-266.
[52]LI J,LONG Y,QI G N,et al.The Os-AKT1 channel is critical for K+uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex.The Plant Cell,2014,26(8):3387-3402.
[53]WANG Y,WU W H.Regulation of potassium transport and signaling in plants.Current Opinion in Plant Biology,2017,39:123-128.
[54]DANA S,HERDEAN A,LUNDIN B,et al.Each of the chloroplast potassium efflux antiporters affects photosynthesis and growth of fully developedArabidopsis rosettes under shortday photoperiod.Physiologia Plantarum,2016,158(4):483-491.
[55]GOMEZ-PORRAS J L,RIAÑO-PACHÓN D M,BENITO B,et al.Phylogenetic analysis of K+transporters in bryophytes,lycophytes,and flowering plants indicates a specialization of vascular plants.Frontiers in Plant Science,2012,3:167.
[56]HE C Y,CUI K,DUAN A G,et al.Genome-wide and molecular evolution analysis of the poplar KT/HAK/KUP potassium transporter gene family.Ecology and Evolution,2012,2(8):1996-2004.
[57]YANG T Y,ZHANG S,HU Y B,et al.The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels.Plant Physiology,2014,166(2):945-959.
[58]HYUN T K,RIM Y,KIM E,et al.Genome-wide and molecular evolution analyses of the KT/HAK/KUP family in tomato(Solanum lycopersicum L.).Genes&Genomics,2014,36(3):365-374.
[59]ZHANG Z B,ZHANG J W,CHEN Y J,et al.Genome-wide analysis and identification of HAK potassium transporter gene family in maize(Zea mays L.).Molecular Biology Reports,2012,39(8):8465-8473.
[60]晁毛妮,温青玉,张晋玉,等.大豆KUP/HAK/KT钾转运体基因家族的鉴定与表达分析.西北植物学报,2017,37(2):239-249.CHAO M N,WEN Q Y,ZHANG J Y,et al.Identification and expression analysisofKUP/HAK/KT potassium transporter gene family in soybean[Glycine max(L.)Merr.].Acta Botanica Boreali-Occidentalia Sinica,2017,37(2):239-249.(in Chinese with English abstract)
[61]ZHANG C,LI H J,WANG J Y,et al.The rice high-affinity K+transporter OsHKT2;4 mediates Mg2+homeostasis under high-Mg2+conditions in transgenic Arabidopsis.Frontiers in Plant Science,2017,8:1823.
[62]PLATTEN J D,COTSAFTIS O,BERTHOMIEU P,et al.Nomenclature for HKT transporters,key determinants of plant salinity tolerance.Trends in Plant Science,2006,11(8):372-374.
[63]REN Z J,LIU Y,KANG D,et al.Two alternative splicing variants of maize HKT1;1 confer salt tolerance in transgenic tobacco plants.Plant Cell,Tissue and Organ Culture,2015,123(3):569-578.
[64]WANG T T,LIU Z J,LIU Z Q,et al.SbHKT1;4,a member of the high-affinity potassium transporter gene family from Sorghum bicolor,functions to maintain optimal Na+/K+balance under Na+stress.Journal of Integrative Plant Biology,2014,56(3):315-332.
[65]GIERTH M,MASER P.Potassium transporters in plants:Involvement in K+acquisition,redistribution and homeostasis.FEBS Letters,2007,581(12):2348-2356.
[66]CHEN Y,MA J K,MILLER A J,et al.OsCHX14 is involved in the K+homeostasis in rice(Oryza sativa)flowers.Plant Cell Physiology,2016,57(7):1530-1543.
[67]JIA B W,SUN M Z,DUANMU H Z,et al.GsCHX19.3,a member of cation/H+exchanger superfamily from wild soybean contributes to high salinity and carbonate alkaline tolerance.Scientific Reports,2017,7:9423.
[68]ZHENG S,PANT,FAN LG,et al.AnovelAtKEAgene family,homolog of bacterial K+/H+antiporters,plays potential roles in K+homeostasis and osmotic adjustment in Arabidopsis.PLoS One,2013,8(11):e81463.
[69]CHEN H T,CHEN X,WU B Y,et al.Whole-genome identification and expression analysis of K+efflux antiporter(KEA)and Na+/H+antiporter(NHX)families under abiotic stress in soybean.Journal of Integrative Agriculture,2015,14(6):1171-1183.
[70]韩蕾,宋志忠,王莉,等.7种植物K+/H+逆向转运蛋白的生物信息学分析.基因组学与应用生物学,2011,30(3):372-378.HAN L,SONG Z Z,WANG L,et al.Bioinformatics analysis of 7 plants’K+/H+antiporters.Genomics and Applied Biology,2011,30(3):372-378.(in Chinese with English abstract)
[71]MILNER M J,SEAMON J,CRAFT E,et al.Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis.Journal of Experimental Botany,2013,64(1):369-381.
[72]ISHIMARU Y,BASHIR K,NISHIZAWA N K.Zn uptake and translocation in rice plants.Rice,2011,4(1):21-27.
[73]KOLAJ-ROBIN O,RUSSELL D,HAYES K A,et al.Cation diffusion facilitator family:Structure and function.FEBS Letters,2015,589(12):1283-1295.
[74]DENG F L,YAMAJI N,XIA J X,et al.A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice.Plant Physiology,2013,163(3):1353-1362.
[75]KAZNINA N M,TITOV A F,TOPCHIEVA L V,et al.The content of HvHMA2 and HvHMA3 transcripts in barley plants treated with cadmium.Russian Journal of Plant Physiology,2014,61(3):355-359.
[76]TAN J J,WANG J W,CHAI T Y,et al.Functional analyses of TaHMA2,a P1B-type ATPase in wheat.Plant Biotechnology Journal,2013,11(4):420-431.
[77]TANAKA N,FUjIWARAT,TOMIOKA R,etal.Characterization of the histidine-rich loop of Arabidopsis vacuolar membrane zinc transporter AtMTP1 as a sensor of zinc level in the cytosol.Plant and Cell Physiology,2015,56(3):510-519.
[78]BARBERON M,ZELAZNYE,ROBERTS,etal.Monoubiquitin-dependent endocytosis of the IRON-REGUL ATED TRANSPORTER 1(IRT1)transporter controls iron uptake in plants.Proceedings of the National Academy of Sciences of the USA,2011,108(32):12985-12986.
[79]BARBERON M,DUBEAUX G,KOLB C,et al.Polarization of IRON-REGULATED TRANSPORTER 1(IRT1)to the plant-soil interface plays crucial role in metal homeostasis.Proceedings of the National Academy of Sciences of the USA,2014,111(22):8293-8298.
[80]WANG S L,YOSHINARI A,SHIMADA T,et al.Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates boron transport in Arabidopsis roots.The Plant Cell,2017,29(4):824-842.
[81]TAKANO J,TANAKA M,TOYODA A,et al.Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways.Proceedings of the National Academy of Sciences of the USA,2010,107(11):5220-5225.
[82]CHANROJ S,PADMANABAN S,CZERNY D D,et al.K+transporter AtCHX17 with its hydrophilic C tail localizes to membranes of the secretory/endocytic system:Role in reproduction and seed set.Molecular Plant,2013,6(4):1226-1246.
[83]BAYLE V,ARRIGHI J F,CREFFA,et al.Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation.The Plant Cell,2011,23(4):1523-1535.
