Determination of Hf–Sr–Nd isotopic ratios by MC-ICP-MS using rapid acid digestion after flux-free fusion in geological materials
2018-06-27ZhianBaoChunleiZongLinruFangHonglinYuanKaiyunChenMengningDai
Zhian Bao•Chunlei Zong•Linru Fang•Honglin Yuan•Kaiyun Chen•Mengning Dai
1 Introduction
Laser ablation multi-collector inductively coupled plasma mass spectrometry(LA-MC-ICP-MS)has been applied widely to analyze geological,environmental,material,and archaeological samples due to the technique’s rapid sample preparation process,in situ analysis capability,high spatial resolution,and rapid analysis time(Woodhead et al.2004;Iizuka and Hirata 2005;Yuan et al.2008;Huang et al.2015;Bao et al.2016a;Zhian et al.2016).However,unavoidable isobaric interference(e.g.,87Rb,144Sm,176Yb,and176Lu),molecular interference (e.g.,43Ca44Ca,42Ca44Ca,48Ca38Ar,130Ba14N,and130Ba16O)(Ramos et al.2004;Iizuka and Hirata 2005;Foster and Vance 2006;Muller and Anczkiewicz 2016),and low elemental concentrations mean LA-MC-ICP-MS data must be evaluated carefully to obtain accurate isotopic ratios.Thus,bulk digestion and ion-exchange chemistry are still required for accurate isotopic ratio analyses.The conventional bulk solution analysis can obtain accurate isotopic compositions even at low concentrations in solid,liquid,and gaseous samples.The complete recovery of Sr,Nd,and Hf from intrusive rocks with refractory minerals is a prerequisite for obtaining accurate isotopic compositions.A closed acid digestion bomb has been commonly used for routine isotopic ratio analysis because the boiling point of the reagents is increased by the pressure generated in the closed vessel.The increased temperature and pressure can significantly shorten sample decomposition time and allow the digestion of the refractory phases(Potts and Robinson 2003).In addition,the high-pressure PTFE bomb method has the advantage of a low procedural blank using ultrapure Hf/HNO3/HClO4.However,the disadvantages of bomb dissolution include the greater intensity of labor required,high cost of the bomb-jacket set,long reaction times(5–7 days)(Weis et al.2006;Yang et al.2011;Li et al.2014,2015),and increased danger due to the high pressure produced within the vessel.Another digestion method uses lithium metaborate or Na2O2flux to rapidly dissolve all the refractory minerals in rock powder.However,the threefold weight required of lithium metaborate can be troublesome due to contamination by Nd,Sr,and Hf,which inhibits accurate determination of isotopic composition.The procedural blanks are relatively higher for Nd[370 pg(Kleinhanns et al.2002),1500 pg(Le Fèvre and Pin 2005),~100 pg(large crucible)(Ulfbeck et al.2003),1–3 ng(Tomascak et al.1996)]and Hf[330 pg(Kleinhanns et al.2002),~140 pg(large crucible)(Ulfbeck et al.2003),100±20 pg(large crucible)(Bizzarro et al.2003)],thereby making this method inappropriate for geological samples with low concentrations(<0.5 μg·g-1).Considering the potential blank effect from flux reagents and the long reaction time of bomb dissolution, flux-free fusion combined with rapid acid dissolution is required for the accurate determination of Hf–Sr–Nd isotopic ratios.
Recently,a new method was developed that combines the flux-free fusion technique with rapid Hf/HNO3/HClO4digestion using a high-temperature furnace and a boron nitride(BN)crucible for bulk trace element analyses in silicate rocks by ICP-MS(Bao et al.2016b).The main advantages of the digestion method using a flux-free fusion technique are reduced digestion time,and the lack of requirement for lithium metaborate and high-pressure digestion bomb.Some small undissolved zircon grains still remain in the fused glass,but the Zr and Hf concentrations are identical to the reference values after rapid acid dissolution,thereby guaranteeing that accurate Hf–Sr–Nd isotopic compositions can be determined.
The aim of this study was to accurately determine the Hf–Sr–Nd isotopic compositions in the same rock powder aliquots in the form of a pure Hf–Sr–Nd solution,thereby eliminating the use of lithium metaborate and a highpressure digestion bomb.Rapid dissolution(6 h)by Hf/HNO3/HClO4in a closed screw-top Savillex vial on a hotplate was used to enhance efficiency.We also evaluated the effects of the fusion temperature and fusion time on the isotopic ratios.The flux-free fusion and closed vessel rapid Hf/HNO3/HClO4digestion method was also used successfully to determine Hf–Sr–Nd isotopes in a series of international certified reference materials(CRMs),including those with refractory accessory minerals.
2 Experimental methods
2.1 Chemicals and other materials
Ultrapure water with a resistivity of 18.2 MΩ·cm-1was obtained from a Milli-Q water purification system(Millipore,Bedford,MA,USA).Commercially available hydrofluoric acid(Hf,40%v/v,GR grade),hydrochloric acid(HCl,38%v/v,GR grade),nitric acid(HNO3,68%v/v,GR grade),and perchloric acid(HClO4,70%v/v,GR grade)were further distilled in a sub-boiling distillation system.The Savillex vials were cleaned in hot(130°C)GR-grade HNO3for 2 days and GR-grade HCl for 2 days,followed by 2 days in hot high-purity HCl and 2 days in hot high-purity HNO3,2 days in hot ultrapure water,and a rinse with cold ultrapure water.A BN vessel was designed to melt 600 mg rock powder at high temperature and it then be rapidly quenched in ultrapure water.The BN crucibles were heated at 500°C for 2 min to remove all volatile components before use.Each crucible(900 mm3)was placed in a high-temperature furnace and heated to a stable temperature(measured with a thermocouple)using high-resistance silicon molybdenum bars(purity=99.9%,melting point=2000°C)underan argon gas flow(1.0 L·min-1)to maintain an inert atmosphere.Further details of the BN crucibles and high-temperature furnace can be found in previous studies(Bao et al.2016b;Zhian et al.2016).
Experiments were performed using five extrusive reference materials[BCR-2,BHVO-2,GSR-3(basalt powder),AGV-2(andesite powder),and RGM-2(rhyolite powder)], five intrusive rocks[QLO-1(quartz latite powder),G-2,GSR-1(granite),GSP-2(granodiorite),and W-2(diabase)],which were obtained from the United States Geological Survey(USGS)and Chinese National Research Center for Certified Reference Materials.The CRMs had a wide range of SiO2content—from 44.6%to 73.4%m/m.Previously published values of CRMs’Hf–Sr–Nd isotopic compositions were taken from the GeoReM database(Jochum et al.2005).All CRMs were prepared directly from 200-mesh powders dried at 105°C in an oven for 1 h prior to melting.
Three international standard solutions—NIST NBS-987,JNDi-1,and JMC 475 Hf—were used to monitor the conditions during an analytical session.Conventional cation-exchange resin(AG50W-X8,200–400 mesh)was purchased from BioRad(Richmond,CA,USA).Prepacked extraction chromatography materials(Sr Spec resin and Ln Spec resin,100–150 mesh,1.5 mL)were purchased from Eichrom Industries(Darien,IL,USA).
2.2 Sample digestion
In this study,we aimed to obtain accurate isotopic compositions for the CRMs.Therefore,we describe our fusion process in further detail to provide the reader with sufficient information to evaluate and apply our method(Bao et al.2016b).We fused felsic intrusive rock powders with refractory accessory minerals at 1600°C for 1 min and extrusive rocks at 1400°C for 1 min to obtain fused glasses.All of the chemical preparations were conducted on special class-100 work benches inside a class-1000 clean laboratory.The procedures used for fused glass decomposition in this study were as follows.(1)Approximately 100 mg of fusion fragments was weighed into a 15-mL screw-top Savillexvial.(2)Hf/HNO3/HClO4(1.5 mL/1.5 mL/10 μL)were added to the Savillex vial so the fused glass fragments were completely soaked with acid.(3)The vial was tightly capped and then vibrated ultrasonically for 30 min.(4)The vial was opened and the sample dried on a plate at 140°C by evaporating to incipient dryness.(5)Hf/HNO3(1.5 mL each)were added to the dried sample and the vial screwed and left on a plate at 150°C for 6 h.(6)The vial was opened and evaporated to dryness at 150°C.(7)Next,2 mL HNO3was added to the sample and the residue was converted to the HNO3form,before drying at 150°C.(8)Subsequently,2 mL HCl was added to the dried sample to convert to HCl form and it was dried at 150°C.(9)Finally,the solution collected after digestion with 2 mL 1.7-M HCl and centrifuging for 10 min was prepared for Rb–Sr,Sm–Nd,and Lu–Hf separation.
2.3 Hf,Sr,and Nd separation
Accurate determination of the Hf–Sr–Nd isotopic ratios required high purity samples in order to avoid polyatomic and isobaric interference.Thus,traditional column chromatography was used to separate high purity Hf–Sr–Nd solutions(Fig.1)and to evaluate the influence of the hightemperature fusion procedure.Hf–Sr–Nd were all purified from the same sample solution.The procedures for Hf–Sr–Nd separation were described by Yang et al.(2010),Zong et al.(2012)and Zong(2012).The solution collected after digestion and centrifugation was loaded into a quartz column packed with BioRad AG50W-X8 resin(200–400 mesh),which was pre-conditioned with 11 mL 6-M HCl and 12 mL 1.7-M HCl.Hf was collected with 2.2 mL 1.7-M HCl and prepared for further Ti and Hf purification using Ln Spec resin.Next,10 mL 1.7-M HCl was used to elute Rb.Sr was then collected with 6 mL 3-M HCl,and Y was eluted with 4 mL 3-M HCl.The rare earth elements(REEs)Sm and Nd were collected with 10 mL 6-M HCl and it prepared for further purification using Ln Spec resin.The solution collected containing Ti and Hf was loaded into Ln Spec resin(100–150 mesh,1.5 mL),where the color of the Ln Spec resin changed from orange-red to white,thereby indicating that all of the titanium was separated from Hf using a mixture of 4 M HCl+0.5%H2O2.Finally,Hf was extracted from the column with 3 mL 2-M Hf,and collected in an 8-mL PFA Savillex Vial,before gently evaporating to dryness.This fraction was dissolved in 10 μL concentrated HF,diluted to 5 mL with 2%HNO3,and used for MC-ICP-MS analysis.In order to minimize the isobaric interference of87Rb on87Sr,especially for felsic rocks with high Rb concentrations,the solution collected containing Sr and Rb was gently evaporated to dryness and diluted with 5 mL 8-M HNO3before purification by the third column.The third column packed with Sr Spec resin was pre-conditioned with 10 mL 8-M HNO3before loading the separated solution.Next,20 mL 8-M HNO3was used to wash the resin to remove Rb interference and 10 mL 0.05-M HNO3was used to extract the purified Sr solution.In order to remove the isobaric interference effect due to144Sm on144Nd,the collected REE solution was gently evaporated to dryness and diluted with 0.5 mL 0.25-M HCl before purification by the fourth column.The fourth column packed with Ln Spec resin(100–150 mesh)was pre-conditioned with 10 mL citric acid,10 mL 6-M HCl,10 mL high purity Milli-Q water,and 6 mL 0.25-M HCl,before loading the REE solution.Subsequently,4 mL 0.25-M HCl was used to wash the resin to remove La,Ce,and Pr,and 10 mL 0.25-M HCl was then used to elute the purified Nd solution.Next,10 mL 6-M HCl was used to elute Sm from the column and 10 mL 0.25-M HCl was used to re-equilibrate the Ln Spec resin column.The pure Nd solution collected was evaporated to dryness,dissolved into 2 mL 2%HNO3,and used for Nd analysis.

Fig.1 Hf–Sr–Nd separation scheme used in this study
2.4 Mass spectrometry analytical procedure
Isotopic composition measurements were performed using a Nu II MC-ICP-MS instrument(Nu Instrument,Wrexham,UK)at the State Key Laboratory of Continental Dynamics,Northwest University,Xi’an.The typical instrument parameters and configurations of Faraday cups are summarized in Tables 1 and 2.The purified Sr and Nd solution was taken up with 2 mL 2%HNO3,while the purified Hf solution was adopted with trace ultrapure Hf.The solution was self-aspirated at an uptake rate of 100 μL min-1through a standard PFA nebulizer and desolvated with an AridusTMsystem.Solution standards comprising NIST NBS-987 for Sr,JMC 475 for Hf,and JNDi-1 for Nd were measured during the same MC-ICP-MS runs as the samples.The data were corrected for mass fractionation by normalizing to86Sr/88Sr=0.1194,146Nd/144Nd=0.7219,and179Hf/177Hf=0.7325,according to an exponential law.Hf–Sr–Nd isotope analyses comprised two blocks of 20 cycles per block with an integration time of 10 s per cycle.The wash-out time(180 s)and time for sample transfer(120 s)resulted in an average instrument time of 12 min per sample.The87Sr signal intensity was corrected for potential interference caused by the remaining isobaric overlap effect of87Rb on87Sr by using an87Rb/85Rb value of 0.3857.After column chemistry,the85Rb/88Sr ratios obtained were<2× 10-5for the analysis of fused glasses.Finally,the87Sr/86Sr ratios were determined accurately after correction and they were normalized to SRM 98787Sr/86Sr=0.710248(Howarth and McArthur 1997)for mass fractionation using the standard-sample-standard bracketing(SSB)method.Most of the Sr isotopic data were obtained at an internal precision of≤0.000010(2SE).The144Nd signal intensity was corrected for potential interference caused by the remaining isobaric overlap effect of144Sm on144Nd.After column chemistry,the144Sm/144Nd ratios obtained were<2.5× 10-5for the analysis of fused glasses.Finally,the143Nd/144Nd ratios were determined accurately after correction and they were normalized to JNDi-1143Nd/144Nd=0.512115(Tanaka et al.2000)for mass fractionation using the SSB method.Most of the Nd isotopic data were obtained at an internal precision of≤0.000006(2SE).In addition,the stable145Nd/144Nd ratio(0.348400±13,n=80)obtained after isobaric interference correction agreed with the recommended value of 0.348415 obtained by TIMS(Wasserburg et al.1981),which confirmed the feasibility of the data.173Yb and175Lu were monitored to evaluate isobaric interference of176Lu and176Yb on176Hf.The effects of these types of interference were corrected on-line using the following values for the stable ratios:176Yb/173Yb=0.79323 and176Lu/175Lu=0.026528.The176Hf/177Hf isotopic ratiosdid not differ significantly before or after interference correction because of the low Lu/Hf and Yb/Hf ratios(≤1× 10-5)after column chemistry.In this study,JMC 475 yielded a value of 0.282172±18(2SD,n=70),which agreed well with the recommended value of 0.282163(Blichert-Toft et al.1997).Therefore,all the176Hf/177Hf ratios measured for the CRMs were normalized to the recommended value of 0.282163.In addition,31 analyses of Alfa Hfgave a176Hf/177Hfvalue of 0.282193±11(2SD),which was identical to the values of 0.282192± 6(2σ,n=12)determined by Lu et al.(2007)and 0.282189±19(2SD)by Yang et al.(2011),within 2 s error.The87Sr/86Sr,143Nd/144Nd,and176Hf/177Hf ratios measured for the NBS-987,JNDi-1,and JMC 475 reference solutions during the same analytical sessions as the purified sample solutions were 0.710254±25(2SD,n=60),0.512107±20(2SD,n=56),and 0.282172±18(2SD,n=56),respectively.These isotopic ratios were identical to the previously determined values of 0.710248(Howarth and McArthur 1997)(SRM 987),0.512115±7(Tanaka et al.2000)(JNDI),and 0.282163±9(Blichert-Toft et al.1997)(JMC 475),respectively,within 2 s error.
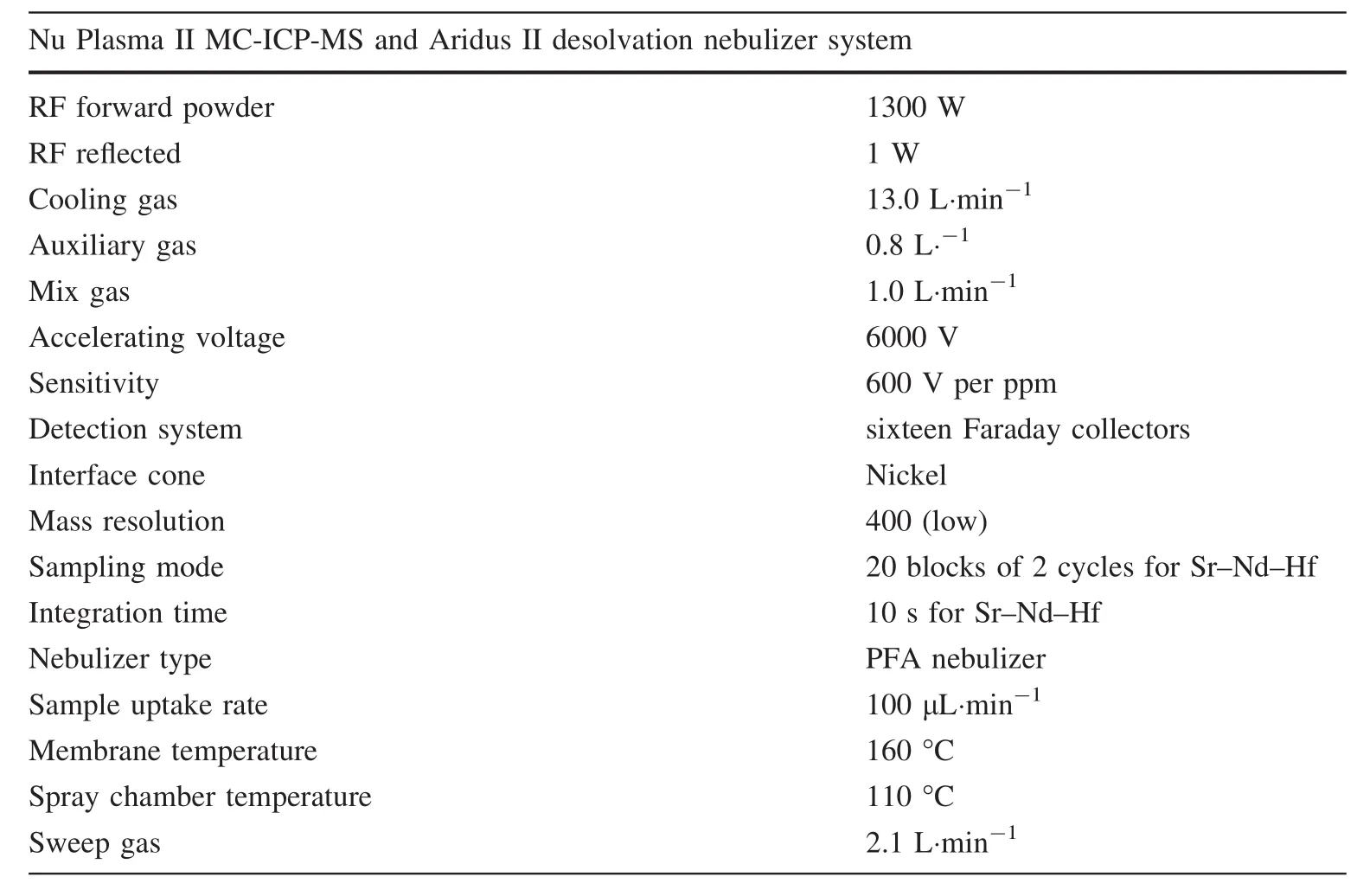
Table 1 Typical instrument operating conditions and data acquisition parameters for Hf–Sr–Nd measurements

Table 2 Faraday cup configurations for Hf–Sr–Nd isotopic measurements
3 Results and discussion
3.1 Effects of fusion temperature and time

Fig.2 Individual87Sr/86Sr,143Nd/144Nd,and176Hf/177Hf analyses for GSP-2 fused at different conditions and analyzed by MC-ICP-MS.For comparison,at the far right side of each figure,the points in white represent reference values obtained by Weis et al.(2006)and the gray area represents two standard deviations for the replicate analyses obtained using MC-ICP-MS.For individual analyses,error bars correspond to the two-sigma error based on the measured isotopic ratio
The granodiorite reference material GSP-2 contained many refractory minerals(e.g.,zircon,rutile,and monazite)and it was used to ascertain the effects of fusion temperature and time on isotopic compositions.An internal normalization approach and the SSB method were used to correct for mass fractionation during analytical sessions.The Hf–Sr–Nd isotopic results obtained for GSP-2 using different fusion conditions are shown in Fig.2.The176Hf/177Hf value ranged from 0.282019 to 0.281978 when the fusion temperature was 1400°C,but it decreased to 0.281959 at 1500°C for 1 min,and then agreed with the reference value of 0.281949±8(Weis et al.2007)when the temperature was 1500 °C for 5 min or 1600 °C.The Hf isotope compositions depended on the recovery of Hf,especially in intrusive rocks with zircon,and the concentration of Hf agreed well with the reference values at 1500 °C for 10 min or 1600 °C(Bao et al.2016b),so fusion at 1500 °C for 10 min or 1600 °C was used to obtain accurate Hf isotopic compositions.The results in Fig.2 indicate that the87Sr/86Sr values obtained after 5 min at 1500 °C or 1600 °C were identical to the results recommended by Weis et al.(2006),within 2 s error.The other87Sr/86Sr values ranged from 0.764755 to 0.765052—lower than the reference,thereby indicating that accessory minerals containing Sr might not have been dissolved in the final solution.The Nd isotopic compositions measured by MC-ICP-MS ranged from 0.511367 to 0.511385,within the range of MC-ICP-MS values(0.511374±11,n=14)obtained by Weis et al.(2006).Our143Nd/144Nd results support the recently published conclusion of Bao et al.(2016b)that Nd can reach 100%–110%recovery by eliminating consideration of fusion temperature and time.Based on the full recovery of trace elements,the Hf–Sr–Nd isotopic compositions obtained by fusion at 1600°C for 1 min agreed with the reference values,where the short heating time suppressed evaporation of the volatile elements and reduced the risk of contamination from the environment.Thus,a high temperature and minimum heating time(1600°C for 1 min)are recommended for the fusion of intrusive rock samples.In addition,1400°C for 1 min is recommended for extrusive rock samples without refractory accessory minerals based on this study.
3.2 Validation of the rapid acid digestion method and final results
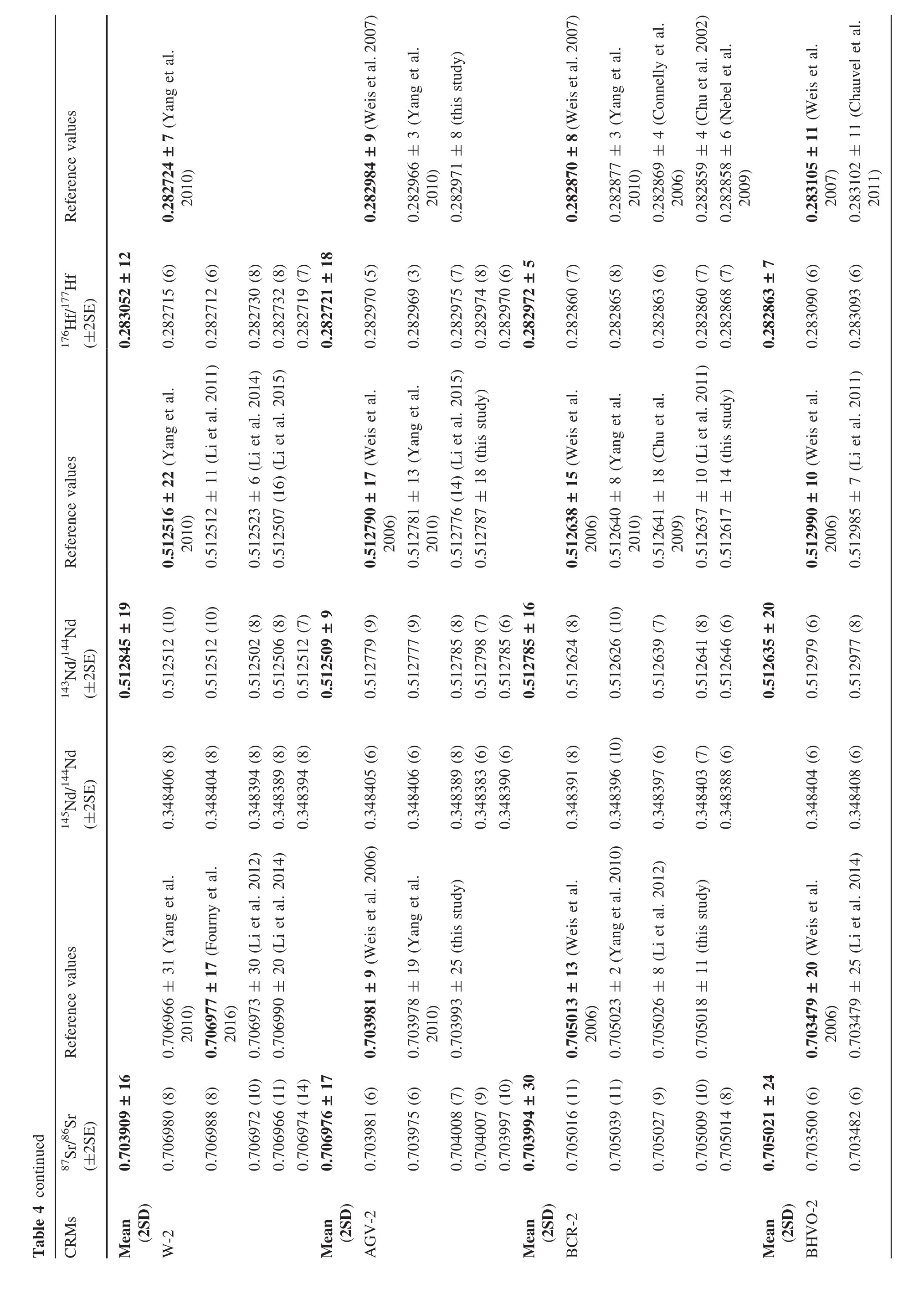
Ten CRMs with a wide range of matrix compositions(from mafic to felsic)were selected to assess the analytical reproducibility and feasibility of our rapid acid digestion method after high-temperature fusion.We used the Hf–Sr–Nd isotopic ratios obtained by high-pressure PTFE bomb(190°C for 120 h)as reference values for GSR-1,RGM-2,and QLO-1.Approximately 100 mg of fused glass was rapidly digested(6 h)by Hf/HNO3/HClO4in a closed screw-top Savillex vial on a hotplate without requiring high-pressure PTFE bomb.Five batches of 100-mg fused fragments were separated for Hf–Sr–Nd isotope analysis using the chemical separation method described above.Some CRMs digested with a high-pressure PTFE bomb for Hf–Sr–Nd were measured in the same MC-ICP-MS runs as the samples(Table 3).The Hf–Sr–Nd isotopic compositions obtained using the rapid acid dissolution methods are shown in Table 4.The Hf–Sr–Nd isotopic ratios obtained for GSP-2,AGV-2,and BHVO-2 using a high-pressure PTFE bomb agree with previously published values within 2 s uncertainty,verifying the quality of our Hf–Sr–Nd isotopic compositions using high-pressure PTFE bomb.
As shown in Table 4,the176Hf/177Hf ratios obtained for the CRMs had an internal precision better than 0.000009(2SE).The two standard deviation values were all below 18 ppm for all the CRMs.As shown in Fig.3 and Table 4,the average176Hf/177Hf values obtained for GSP-2,G-2,QLO-1,and GSR-1 agree well with previously published data,indicating that the resistant minerals(e.g.,zircon and rutile)containing hafnium were completely decomposed by Savillex vial digestion.Compared with the conventional high-pressure PTFE bomb digestion method for 4–7 days,flux-free fusion combined with rapid acid dissolution in a capped Savillex vial on a hotplate effectively dissolved diverse rocks and was 16–28 times faster.The87Sr/86Sr and143Nd/144Nd ratios for all the analyzed CRMs were obtained with an internal precision greater than 0.000014(2SE).Table 4 and Fig.3 show that the87Sr/86Sr and143Nd/144Nd values obtained in this study agree well with previously published values acquired by TIMS or MC-ICPMS(high-pressure PTFE bomb method).The87Sr/86Sr and143Nd/144Nd values also verified the feasibility of the new method developed in this study.As shown in Table 4, five replicate measurements of the 10 CRMs yielded external reproducibility of87Sr/86Sr,143Nd/144Nd,and176Hf/177Hf better than ±0.000030(2SD),±0.000030(2SD),and±0.000018(2SD),respectively,excluding the87Sr/86Sr values for GSP-2 and GSR-1(2SD<0.000071).In fact,the external reproducibility of the87Sr/86Sr values obtained by the high-pressure PTFE bomb method were 0.000060 for GSP-2 and GSR-1,thereby indicating that the Sr isotopes were heterogeneous in some intrusive felsic rocks in the 100-mg size range(Raczek et al.2003;Weis et al.2006).In general,the reproducibility and precision of the87Sr/86Sr,143Nd/144Nd,and176Hf/177Hf values obtained by the proposed method in this study were satisfactory and completely suitable for geochemical and petrological studies.In addition,the Hf–Sr–Nd isotopic values obtained for the 10 CRMs verified the feasibility and usefulness of the flux-free fusion method combined with rapid acid digestion.
3.3 Comparison with the high-pressure PTFE bomb and flux fusion methods
A low total procedural blank level is essential for the accurate determination of Hf–Sr–Nd isotopic ratios.The typical total procedural blanks of Hf–Sr–Nd,including sample digestion,column chemistry,and mass spectrometric measurements,were approximately 150 pg for Sr,75 pg for Nd,and 70 pg for Hf,which were identical to previous values.For the Hf–Sr–Nd analyses,the blanks were negligible relative to the amount of analytes contained in 100 mg of silicate rocks,and no correction of the measured isotopic ratios was required.Details of the Hf–Sr–Nd procedural blanks obtained with different methods are shown in Table 5.The procedural blanks obtained for Hf–Sr–Nd in this study were identical using the high-pressure PTFE bomb method and with capped Teflon Savillex vials on a hotplate.The procedural blank for Nd was approximately 75 pg in this study,which was lower than that obtained by flux fusion,i.e.,100 pg to 1.5 ng.The higher Nd procedural blank was caused by the fluxing agent(Le Fèvre and Pin 2005).The Hf blanks were about 70 pg using both acid-based digestion and flux-free fusion in this study,which agreed well with the high purity flux fusion method(57 pg),but they were lower than the blanks obtained previously by flux fusion without purification(100–330 pg).Le Fèvre and Pin(2001) indicated that the REE concentrations in commercially available fluxes are variable and usually high,so a high purity flux should be used to obtain accurate isotopic ratios.Based on the procedural blanks for Nd and Hf,the flux-free fusion method is recommended for determining Nd–Hf isotopic ratios because the high procedural blank(>300 pg)makes this procedure inappropriate for samples with low concentrations(Nd,Hf< 0.5 μg·g-1).

Table 3 Sr,Nd,and Hf isotopic ratios determined for the CRMs using the high pressure PTFE bomb method and MC-ICP-MS

?
?
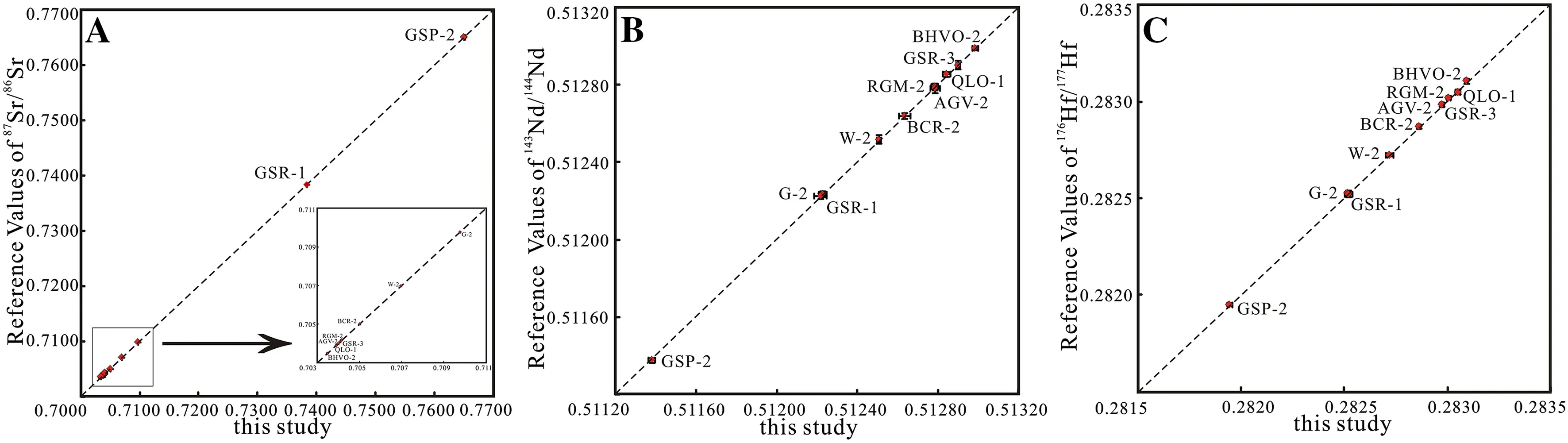
Fig.3 Comparisons of the Hf–Sr–Nd isotopic ratios obtained using the new method developed in this study and reference values
Acid-based digestion obtained low procedural blanks for the Hf–Sr–Nd isotopic ratios,but 4–7 days with a high pressure PTFE bomb should be used to ensure complete digestion,especially for felsic rocks containing many refractory phases.The digestion time(6 h)was shorter by 16–28 times in Savillex vials without a high-pressure PTFE bomb after flux-free fusion,compared to the traditional high-pressure PTFE bomb.The technique developed in this study for flux-free fusion and the rapid acid digestion of samples before Hf–Sr–Nd isotopic analysis facilitates rapid sample digestion with low procedural blanks.Moreover,this technique is applicable to a wide range of samplematrices and does not require the use of a high-pressure PFTE bomb.
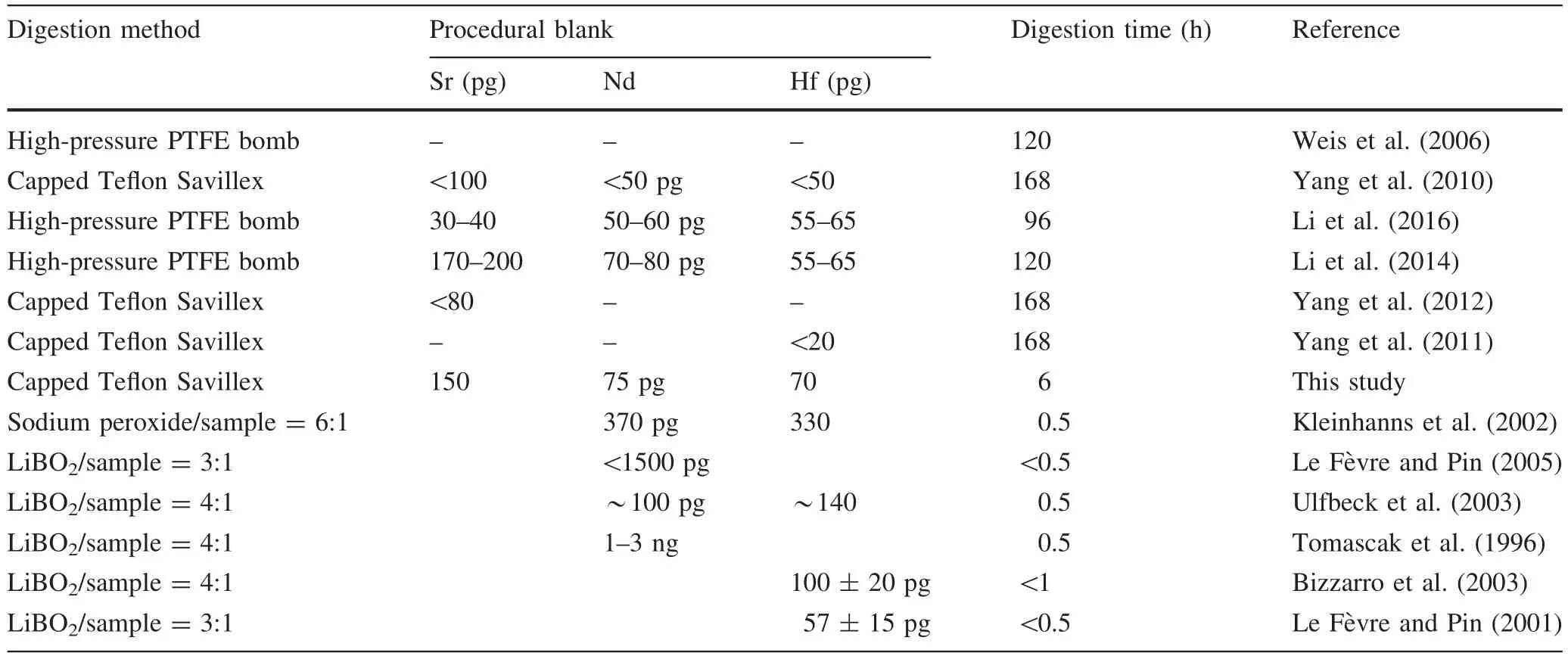
Table 5 Sr–Nd–Hf procedural blank values and digestion times reported for acid-based digestion and flux fusion compared with the results obtained in this study
4 Conclusion
In this study,we developed a rapid acid digestion method in Savillex vials without a high-pressure PTFE bomb after flux-free fusion to measure the87Sr/86Sr,143Nd/144Nd,and176Hf/177Hf isotopic ratios in the same digested samples.The flux-free fusion method avoided using lithium metaborate or Na2O2fluxes and it reduced the contamination for Nd,Sr,and Hf,thereby yielding lower procedural blanks compared with the flux fusion method.Rapid acid digestion in capped Savillex vials reduced the digestion time and eliminated the need for a high-pressure digestion bomb.Therefore,rapid acid digestion after flux-free fusion had significant advantages compared with existing methods in terms of simplicity,digestion efficiency,and total procedural blank,especially for intrusive felsic rocks.Finally,replicate analyses of international CRMs indicated thatthe143Nd/144Nd,176Hf/177Hf,and87Sr/86Sr isotopic ratios obtained were identical to previously published values acquired using MC-ICP-MS or TIMS,which also verified the feasibility of the new method developed in this study.Our technique for flux-free fusion and the rapid acid digestion of samples is suitable for the determination of trace elements in rock samples.
AcknowledgementsWe would like to thank the editors and anonymous reviewers who helped to improve this manuscript.This study was supported by the National Natural Science Foundation of China(Grant Nos.41421002,41427804,and 41373004)and the MOST Research Foundation from the State Key Laboratory of Continental Dynamics(Grant Nos.BJ08132-1,207010021,and 201210004).
Bao Z,Yuan W,Yuan H,Liu X,Chen K,Zong C(2016a)Nonmatrix-matched determination of lead isotope ratios in ancient bronze artifacts by femtosecond laser ablation multi-collector inductively coupled plasma mass spectrometry.Int J Mass Spectrom 402:12–19
Bao Z,Zhang H,Yuan H,Liu Y,Chen K,Zong C(2016b)Flux-free fusion technique using boron nitride vessel and rapid acid digestion for trace elements determination by ICP-MS.J Anal At Spectrom 31(11):2261–2271
Bizzarro M,Baker JA,Ulfbeck D(2003)A new digestion and chemical separation technique for rapid and highly reproducible determination of Lu/Hf and Hf isotope ratios in geological materials by MC-ICP-MS.Geostand Newsl 27(2):133–145
Blichert-Toft J,Chauvel C,Albarède F(1997)Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS.Contrib Miner Petrol 127(3):248–260
Carpentier M,Weis D,Chauvel C(2014)Fractionation of Sr and Hf isotopes by mineral sorting in Cascadia Basin terrigenous sediments.Chem Geol 382:67–82
Chauvel C,Bureau S,Poggi C(2011)Comprehensive chemical and isotopic analyses of basalt and sediment reference materials.Geost and Geoanal Res 35(1):125–143
Chen JY,Yang JH,Zhang JH,Sun JF,Wilde SA(2013)Petrogenesis of the Cretaceous Zhangzhou batholith in southeastern China:zircon U–Pb age and Sr–Nd–Hf–O isotopic evidence.Lithos 162–163(2):140–156
Cheng T,Nebel O,Sossi P,Chen F(2015)Assessment of hafnium and iron isotope compositions of Chinese national igneous rock standard materials GSR-1(granite),GSR-2(andesite),and GSR-3(basalt).Int J Mass Spectrom 386(1):61–66
Chu NC,Taylor RN,Chavagnac V,Nesbitt RW,Boella RM,Milton JA,German CR,Bayon G,Burton K(2002)Hf isotope ratio analysis using multi-collector inductively coupled plasma mass spectrometry:an evaluation of isobaric interference corrections.J Anal At Spectrom 17(12):1567–1574
Chu Z,Chen F,Yang Y,Guo J(2009)Precise determination of Sm,Nd concentrations and Nd isotopic compositions at the nanogram level in geological samples by thermal ionization mass spectrometry.J Anal At Spectrom 24(11):1534–1544
Connelly JN,Ulfbeck DG,Thrane K,Bizzarro M,Housh T(2006)A method for purifying Lu and Hf for analyses by MC-ICP-MS using TODGA resin.Chem Geol 233(1):126–136
Foster GL,Vance D(2006)In situ Nd isotopic analysis of geological materials by laser ablation MC-ICP-MS.J Anal At Spectrom 21(3):288–296
Fourny A,Weis D,Scoates JS(2016)Comprehensive Pb–Sr–Nd–Hf isotopic,trace element,and mineralogical characterization of mafic to ultramafic rock reference materials.Geochem Geophys Geosyst 17(3):739–773
Howarth RJ,McArthur JM(1997)Statistics for strontium isotope stratigraphy:a robust LOWESS fit to the marine Sr-isotope curve for 0 to 206 Ma,with look-up table for derivation of numeric age.J Geol 105(4):441–456
Huang C,Yang YH,Yang JH,Xie LW(2015)In situ simultaneous measurement of Rb–Sr/Sm–Nd or Sm–Nd/Lu–Hf isotopes in natural minerals using laser ablation multi-collector ICP-MS.J Anal At Spectrom 30(4):994–1000
Iizuka T,Hirata T(2005)Improvements of precision and accuracy in in situ Hf isotope microanalysis of zircon using the laser ablation-MC-ICPMS technique.Chem Geol 220(1–2):121–137
Jochum KP,Nohl U,Herwig K,Lammel E,Stoll B,Hofmann AW(2005)GeoReM:a new geochemical database for reference materialsand isotopic standards.Geostand Geoanal Res 29(3):333–338
Kleinhanns IC,Kreissig K,Kamber BS,Meisel T,Nägler TF,Kramers JD(2002)combined chemical separation of Lu,Hf,Sm,Nd,and REEs from a single rock digest:precise and accurate isotope determinations of Lu-Hf and Sm-Nd using multicollector-ICPMS.Anal Chem 74(1):67–73
Le Fèvre B,Pin C(2001)An extraction chromatography method for Hf separation prior to isotopic analysis using multiple collection ICP-mass spectrometry.Anal Chem 73(11):2453–2460
Le Fèvre B,Pin C(2005)A straightforward separation scheme for concomitant Lu–Hf and Sm–Nd isotope ratio and isotope dilution analysis.Anal Chim Acta 543(1–2):209–221
Li CF,Li XH,Li QL,Guo JH,Li XH(2011)Directly determining143Nd/144Nd isotope ratios using thermal ionization mass spectrometry for geological samples without separation of Sm-Nd.J Anal At Spectrom 26(10):2012–2022
Li CF,Li XH,Li QL,Guo JH,Li XH,Yang YH(2012)Rapid and precise determination of Sr and Nd isotopic ratios in geological samples from the same filament loading by thermal ionization mass spectrometry employing a single-step separation scheme.Anal Chim Acta 727(10):54–60
Li CF,Guo JH,Yang YH,Chu ZY,Wang XC(2014)Single-step separation scheme and high-precision isotopic ratios analysis of Sr–Nd–Hf in silicate materials.J Anal AtSpectrom 29(8):1467–1476
Li CF,Wang XC,Li YL,Chu ZY,Guo JH,Li XH(2015)Ce-Nd separation by solid-phase micro-extraction and its application to high-precision142Nd/144Nd measurements using TIMS in geological materials.J Anal At Spectrom 30(4):895–902
Li CF,Wang XC,Guo JH,Chu ZY,Feng LJ(2016)Rapid separation scheme of Sr,Nd,Pb,and Hf from a single rock digest using a tandem chromatography column prior to isotope ratio measurements by mass spectrometry. J Anal At Spectrom 31(5):1150–1159
Lu Y,Makishima A,Nakamura E(2007)Purification of Hf in silicate materials using extraction chromatographic resin,and its application to precise determination of176Hf/177Hf by MC-ICP-MS with179Hf spike.J Anal At Spectrom 22(1):69–76
Mahlen NJ,Beard BL,Johnson CM,Lapen TJ(2013)An investigation of dissolution methods for Lu-Hf and Sm-Nd isotope studies in zircon-and garnet-bearing whole-rock samples.Geochem Geophys Geosyst 9(1):690–701
Muller W,Anczkiewicz R(2016)Accuracy of laser-ablation(LA)-MC-ICPMS Sr isotope analysis of(bio)apatite—a problem reassessed.J Anal At Spectrom 31(1):259–269
Nebel O,Morel MLA,Vroon PZ(2009)Isotope dilution determinations of Lu,Hf,Zr,Ta and W,and Hf isotope compositions of NIST SRM 610 and 612 glass wafers.Geostand Geoanal Res 33(4):487–499
Potts PJ,Robinson P(2003)Sample preparation of geological samples, soils and sediments. Compr Anal Chem 41(03):723–763
Raczek I,Jochum KP,Hofmann AW(2003)Neodymium and strontium isotope data for USGS reference materials BCR-1,BCR-2,BHVO-1,BHVO-2,AGV-1,AGV-2,GSP-1,GSP-2 and eight MPI-DING reference glasses. Geostand Newsl 27(2):173–179
Ramos FC,Wolff JA,Tollstrup DL(2004)Measuring87Sr/86Sr variations in minerals and groundmass from basalts using LAMC-ICPMS.Chem Geol 211(1–2):135–158
Tanaka T,Togashi S,Kamioka H,Amakawa H,Kagami H,Hamamoto T,Yuhara M,Orihashi Y,Yoneda S,Shimizu H,Kunimaru T,Takahashi K,Yanagi T,Nakano T,Fujimaki H,Shinjo R,Asahara Y,Tanimizu M,Dragusanu C(2000)JNdi-1:a neodymium isotopic reference in consistency with LaJolla neodymium.Chem Geol 168(3–4):279–281
Tomascak BP,Krogstad JE,Walker JR(1996)Nature of the crust in Maine,USA:evidence from the Sebago batholith.Contrib Miner Petrol 125(1):45–59
Ulfbeck D,Baker J,Waight T,Krogstad E(2003)Rapid sample digestion by fusion and chemical separation of Hf for isotopic analysis by MC-ICPMS.Talanta 59(2):365–373
Wasserburg GJ,Jacobsen SB,DePaolo DJ,McCulloch MT,Wen T(1981)Precise determination of SmNd ratios,Sm and Nd isotopic abundances in standard solutions.Geochim Cosmochim Acta 45(12):2311–2323
Weis D,Kieffer B,Maerschalk C,Barling J,Jong J,Williams GA,Hanano D,Pretorius W,Mattielli N,Scoates JS,Goolaerts A,Friedman RM,Mahoney JB(2006)High-precision isotopic characterization of USGS reference materials by TIMS and MCICP-MS.Geochem Geophys Geosyst 7(8):139–149
Weis D,Kieffer B,Hanano D,Nobre Silva I,Barling J,Pretorius W,Maerschalk C,Mattielli N(2007)Hf isotope compositions of U.S.Geological Survey reference materials.Geochem Geophys Geosyst 8(6):122–125
Woodhead J,Hergt J,Shelley M,Eggins S,Kemp R(2004)Zircon Hf-isotope analysis with an excimer laser,depth profiling,ablation of complex geometries,and concomitant age estimation.Chem Geol 209(1–2):121–135
Yang YH,Zhang HF,Chu ZY,Xie LW,Wu FY(2010)Combined chemical separation of Lu,Hf,Rb,Sr,Sm and Nd from a single rock digest and precise and accurate isotope determinations of Lu–Hf,Rb–Sr and Sm–Nd isotope systems using multi-collector ICP-MS and TIMS.Int J Mass Spectrom 290(2–3):120–126
Yang YH,Wu FY,Wilde SA,Xie LW(2011)A straightforward protocol for Hf purification by single step anion-exchange chromatography and isotopic analysis by MC-ICP-MS applied to geological reference materials and zircon standards.Int J Mass Spectrom 299(1):47–52
Yang YH,Wu FY,Liu ZC,Chu ZY,Xie LW,Yang JH(2012)Evaluation of Sr chemical purification technique for natural geological samples using common cation-exchange and Srspecific extraction chromatographic resin prior to MC-ICP-MS or TIMS measurement.J Anal At Spectrom 27(3):516–522
Yang YH,Wu FY,Xie LW,Chu ZY,Yang JH(2014)Re-evaluation of interferences of doubly charged ions of heavy rare earth elements on Sr isotopic analysis using multi-collector inductively coupled plasma mass spectrometry.Spectrochim Acta,Part B 97(7):118–123
Yuan HL,Gao S,Dai MN,Zong CL,Günther D,Fontaine GH,Liu XM,Diwu CR(2008)Simultaneous determinations of U–Pb age,Hf isotopes and trace element compositions of zircon by excimer laser-ablation quadrupole and multiple-collector ICPMS.Chem Geol 247(1–2):100–118
Zhian B,Honglin Y,Chunlei Z,Ye L,Kaiyun C,Yulin Z(2016)Simultaneous determination of trace elements and lead isotopes in fused silicate rock powders using a boron nitride vessel and fsLA-(MC)-ICP-MS.J Anal At Spectrom 31(4):1012–1022
Zong C(2012)Chemical separation of Pb,Sr,Nd,Hf isotopes from a single rock dissolution and its geological application].Northwest University,Xi’an(in Chinese)
Zong C,Yuan H,Dai M(2012)A feasibility study on chemical separation of Pb,Sr and Nd from the same single dissolution of geological sample.Rock Miner Anal 31(6):945–949(in Chinese with English abstract)
杂志排行
Acta Geochimica的其它文章
- Limestone mechanical deformation behavior and failure mechanisms:a review
- Thermodynamic properties of San Carlos olivine at high temperature and high pressure
- Geochemistry and petrology of rift-related mafic sills and arc-related Gabbro–Diorite bodies,Northern Bafq District,Central Iran
- A study of groundwater irrigation water quality in south-central Bangladesh:a geo-statistical model approach using GIS and multivariate statistics
- Influence on lacustrine source rock by hydrothermal fluid:a case study of the Chang 7 oil shale,southern Ordos Basin
- Elemental characteristics of lacustrine oil shale and its controlling factors of palaeo-sedimentary environment on oil yield:a case from Chang 7 oil layer of Triassic Yanchang Formation in southern Ordos Basin
