Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1
2018-05-24SyedElToumyJoslineSliWlElKshkChristelMrtyGillesBedouxNthlieBourgougnon
Syed A.El-ToumyJosline Y.SliWl A.El-KshkChristel MrtyGilles BedouxNthlie Bourgougnon
aChemistry of Tannins Department,National Research Centre,Dokki,12622 Cairo,Egypt
bChemistry of Natural Compounds Department,National Research Centre,Dokki,12622 Cairo,Egypt,
cCentre de Recherché et d’enseignement Yves Coppens,Campus de Tohannic,BP 573,56017 Vannes,France
Abstract
Keywords:Egyptian plants;Anti-HSV-1;Polyphenols;Vero cell
1.Introduction
Infection with theHerpes SimplexVirus(HSV) can be due to eitherHerpes SimplexVirus type 1(HSV-1)orHerpes SimplexVirus type 2(HSV-2).HSV-1 is a highly contagious infection,which is common and endemic throughout the world[1,2].HSV-1 is usually transmitted with infected oral secretions and it can cause more serious aggravation such as encephalitis,keratitis or gingivostomatitis.HSV-related opportunistic infections cause a variety of malignancies.Recently,some synthetic nucleoside analogues[3]were applied in the primary steps of infection as anti-herpetic agents but were found to developed drug-resistant species mostly in patients who are immune-compromised[4–6].Therefore,there is an increasing need to find new alternative antiviral compounds where pure compounds of plant origin have shown to possess antiviral activity against HSV types.It was revealed that their mechanism of function is either through inhibiting viral replication or viral genome synthesis(Fig.1).HSV-1 and also a multitude of viruses employ glycosaminoglycan(GAG)as initial attachment receptors during infection of their host cell.polyphenols are observed to target HSV-1 glycoprotein’s that interact with GAGs and,in turn,prevent their association with cell surface GAGs as well as subsequent binding receptors.This inhibitory effect is shown(1)against cell-free virus,(2)during the viral attachment and fusion stages,and(3)in the intercellular junction spread of HSV-1,which is mediated by its glycoprotein’s[7–9].The Phytochemicals possessing antiviral activity against HSV types are flavonoids[10],alkaloids[11],saponins[12],terpenes[13],quinones[14],lignans[15],polysaccharides[13],tannins[16],thiosul finates[17],steroidal glycoside[18]and proanthocyanidin[19].

Fig.1.Proposed HSV-DNA replication by natural polyphenolic compounds.
Twenty five species of Egyptian plants belonging to 13 families were selected for this study as many Egyptian medicinal species have significance medical treatments and have been used to treat many diseasese.g.stroke,diabetes,Alzheimer’s disease,atherosclerosis,liver functions,respiratory,blood and nervous systems as well as they possess anti-inflammatory and antioxidant activities,from which the previously mentioned medicinally active components[10–19]are obtained.
In the present study,the HSV-1/Vero cell line model is designed for assessing the ability of viral productivity of a number of Egyptian plants extracts and their flavonoid fractions.The chemical composition of the active fractions of these two plants was investigated to give seven pure compounds with potent anti-herpetic activity.
2.Materials and methods
2.1.Materials and Chemicals
1H(500MHz)and13C(125MHz)NMR:Joel spectrometer(Kyoto,Japan)in DMSO-d6;UV:Shimadzu spectrophotometer model UV-240(Kyoto,Japan);CC:Sephadex LH-20(Fluka,UK);PC was carried out on Whatman No.1 and 3MM paper(Sigma Aldrich,USA)using the following solvent systems:(1)BAW(nBuOH/AcOH/H2O,4:1:5(upper layer));(2)H2O;and(3)AcOH/H2O(15:85).The sodium salt Zovirax IV,25mg/mL was purchased from the Wellcome Foundation Ltd(UK).
2.2.Plant material
The different plants with their different parts were collected from different regions in Egypt(Table 1).Identification of the plant was confirmed by both the Department of flora,Agricultural Museum,Ministry of Agriculture and Herbarium of the Department of Botany,Faculty of Science,Cairo University.Voucher specimens were kept in the National Research Centre Herbarium,Cairo,Egypt.

Table 1List of selected Egyptian plants.
2.3.Preparation of extracts
The plant materials collected were air dried and crushed into powder.Most of the collected plants were extracted with 75% aqueous methanol except forEuphorbia cooperiflowers was extracted by chloroform:methylene chloride(1:1),Psidium guayavaseeds with acetone andRetama raetamseeds with ethyl acetate.The extraction was done thrice at 40°C.Then,the resulting liquid was collected,filtered and reduced through evaporation under vacuum by a rotary evaporator(Heidolph,Germany)at 45°C and dried by use of a freeze dryer.
2.4.Isolation of the compounds
Compounds 1–23 were isolated by conventional chromatographic methods.The chloroform:methylene chloride(1:1)extract of the flowers ofEuphorbia cooperiwas fractionated on a polyamide 6S column chromatography,usingn-Butanol-water saturated as solvent system for elution,led to the desorption of three fractions.Compound 1 was isolated from fraction I by preparative thin layer chromatography followed by crystallization in methanol and identified as 7-galloyl catechin(1).Compounds 2 and 3 were isolated from fraction II by applying on a Sephadex column chromatography and crystallized in a mixture of chloroform/methanol and identified as gallic acid(2)and kaempferol 3-O-β-(6''-O-galloyl)-glucopyranoside(3).Application of fraction III to Sephadex LH-20 column elution with EtOH led to the desorption of two successive bands.These bands were concentrated to afford two pure samples of ellagic acid(4)and kaempferol 3-O-β-glucopyranoside(5).
The aqueous methanolic extract prepared with the leaves ofMorus Albawas subjected to the top of a column containing 500g of polyamide 6S.Gradient elution started with water followed by H2O/EtOH mixtures to give eight fractions.Elution with 50% EtOH of fraction IV led to desorption of the major band,crystallization of the received band afforded quercetin 3-O-β-glucopyranoside(6).Compounds 7–9 were obtained from the fifth fraction by Sephadex LH-20 column chromatography using ethanol for elution.They were elucidated as quercetin 3-O-β-(6''-O-galloyl)-glucopyranoside(7),quercetin(8)and kaempferol(9)by using chromatographic and spectroscopic techniques as well as comparing with its authentic sample.
Fraction III resulted from the polyamide 6S column of the 70% methanolic extract ofAcaciaalbdaileaves,was therefore re-fractionated on Sephadex LH-20 Column chromatography using 40% EtOH (4:6;EtOH:H2O)solvent system for elution,yielded the individual pure samples of quercetin-3-O-α-L-rhamnopyranosyl(1→6) β-D-glucopyranoside(10)and kaempferol-3-O-α-L-rhamnopyranosyl(1→6)β-D-glucopyranoside(11).Application of the obtained material of fraction IV on Sephadex LH-20 column and eluted with 60% EtOH,has led to the separation of pure samples of Kaempferol-3-O-α-L-rhamnopyranoside(12)and Quercetin-3-O-α-L-rhamnopyranoside(13).
A polyamide column 6S usingn-Butanol-water saturated as the mobile phase for theAcacia nilotica( flowers)led to the separation of four distinct fractions.The first fraction afforded a pure sample of Taxifolin(14).Application of the obtained material of the second fraction on Sephadex LH-20 column and eluted with 30% EtOH,has led to the separation of pure samples of catechin(15).Finally,the pure compound quercetin-3-O-β-D-glucopyranosyl(1→2)β-D-glucopyranoside(16)was obtained in a pure form from a cellulose column chromatography of fraction V using ethanol as eluent.
Curcumin(17)was isolated as major compound fromCurcuma longamethanolic extract.The active concentrated ethyl acetate extract ofRetama raetam(seeds)was then subjected to Sephadex LH-20 column chromatography and eluted with water followed by different ratios of water/ethanol to give rise to five fractions.Genistein-8-C-glucoside(18)was separated from fraction I by fractionation over Sephadex LH-20 column using MeOH/H2O.Apigenin-6-C-glucoside(19)and Apigenin-8-C-glucoside(20)were isolated as pure compounds from fraction II by using Sephadex LH-20 column andn-BuOH saturated with H2O as developing system.Applying the third fraction on Sephadex LH-20 column and eluted by ethanol to obtain the pure natural apigenin(21)
From the fourth fraction of the methanolic extract of theCentaurea glomerata(aerial parts),6-O-methoxy quercetin-7-O-β-D-glucopyranoside(22)and4’,6-O-dimethoxy quercetin-7-O-β-D-glucopyranoside(23)were separated in a pure form by applying on the Sephadex LH-20 column and eluted by 40% EtOH.The structure of the isolated compounds was elucidated on the basis of spectral analysis(Table 4).
Only seven compounds of all the compounds isolated(1–3,7–9&17)showed anti-herpetic activity(Fig.2).

Fig.2.Structures of anti-herpetic compounds isolated.
2.5.Cells
African green monkey kidney cell line(Vero cell line no ATCC CCL-81)were grown in Eagle’s minimum essential medium(MEM,Laboratory Euro-bio)supplemented with 8%(v/v)Fetal Calf serum(FCS,Euro-bio,France),to which 1% of antibiotics PCS(Penicillin 10,000IU/mL,Colimycine 25,000IU/mL,Streptomycine 10mg/mL,Sigma)was added.All Cells were cultured at 37°C,in a 95% air,5% CO2(v,v)atmosphere and routinely examined every 3 days.
2.6.Viruses
A virus stock ofHerpes simplexVirus type 1,wild 17 strain ACVSand PFASwas obtained from Pr.Agut,(EA 2387,Génétique et Pouvoir Pathogène des Virus,Hopital Pitié Salpétrière,Paris,France).Virus stock were prepared by incubating Vero monolayers(75cm2culture flasks seeded with 350,000 cells/mL)at low multiplicity and incubating at 37°C,in a 95% air,5% CO2(v,v)atmosphere.Two or three days after infection,the suspension were frozen and thawed twice,before clearing the preparation by centrifugation at low speed to remove cell debris.The resulting supernatant aliquot was stored at−70°C until used. Virus titrations were performed by the Reed and Muench dilution method[20],using 10 wells on 96-wells micro titter plates per dilution.The virus titter was estimated from cytopathogenicity and expressed as 50% infectious doses per millilitre(2×107.4ID50/mL).
2.7.Cytotoxicity and Antiviral activity of drugs by neutral red dye method
Using the Vero cell/HSV-1model,cytotoxicity was evaluated by incubating cellular suspensions(3.5×105Vero cells/mL)with various dilutions(concentration of 200,50,10,5 and 1μg/mL,4 wells per concentration)of drugs in 96-well plates(72h,37°C in 5% CO2)in Eagle’s MEM containing 8% FCS.Cytotoxicity by cell viability was tested using the neutral dye method from Le Contel[21].
100μL of cellular suspension(3.5×105Vero cells/mL)in Eagle’s MEM containing 8% FCS was incubated with 50 μL of a dilution of the drugs in 96-well plates(72h,37°C in 5% CO2)using a multichannel Titertek®pipette.Three replicates were infected using 50μL of medium and a virus suspension at multiplicity of infection(MOI)of 0.001 ID50/cells were then transferred into each well containing the dilution compound and were incubated for 3days without change of the medium,at 37°C in 5% CO2.Cells and virus controls were run simultaneously.After microscopic examination to check the growth of virus,50μL of neutral red dye(0.15% in saline,pH 5.5)was added to each well and the cultures were incubated for 45min at 37°C[22].Excess dye was removed by rinsing with phosphate buffered saline(PBS,pH7.2;Biomérieux)and the neutral red incorporated by the viable cells was eluted into 100μL/well of citrate ethanol buffer.The anti-herpetic compound Zovirax(acylcovir(9-(2-hydroxy ethoxy methyl)guanine))was used as a reference drug with concentration ranging from 0.5 to 5μg/mL.After shaking the tray for 20min,whereby the cell culture monolayer was completely destroyed,the absorbance(OD)of the wells was read in a multichannel spectrophotometer(Packard Spectra CountTM)at 540nm.The OD was directly related to the percentages of viable cells that were inversely proportional to cytopathic effect(CPE)ratio.The straight line of the regression was determined for each assay and for each plate on the basis of cell controls(0% CPE)and virus controls(100% CPE)[23].The 50% cytotoxic concentration(CC50)of the compound or extracts was defined as the concentration that reduced the OD of the treated cells to 50% of that of controls.The 50% antiviral effective concentration(EC50)was expressed as the concentration that achieved 50% protection of virus-infected cells from the HSV-induced destruction.The percent protection(% P)was calculated by the following formula:

where(ODt)HSV is the absorbance of the test sample,(ODc)HSV is the absorbance of the virus-infected control(no compound),and(ODc)MOCK is the absorbance’s of the mockinfected control.The ratio of(ODc)HSV to(ODc)MOCK is expressed as“% of control”.
2.8.Spectral analysis of some compounds
2.8.1.7-O-Galloyl-Catechin,(1)
Colourless needles,Rf-values(×100):53(HOAc-15),67(BAW);UV λmaxnm(MeOH):230 sh,278;1H NMR and13C NMR data are shown in Table 4.
2.8.2.Kaempferol-3-O-(6''-O-galloyl-β-D-glucopyranoside),(3)
Amorphous,yellow powder,Rf-values(×100):30(HOAc-15),52(BAW);UV λmaxnm:(MeOH)230,244,252,315;1H NMR and13C NMR data are shown in Table 4.
2.8.3.Quercetin-3-O-(6''-O-galloyl-β-D-glucopyranoside),(7)
Yellow powder,Rf-values(×100):08(H2O),45(HOAc-15),60(BAW);UV λmaxnm:MeOH:253,263sh,294 sh,351,+NaOMe:271,328sh,410;1H NMR and13C NMR data are shown in Table 4.
2.9.Statistical analysis
Results are expressed as mean±standard deviation(SD).The statistical analyses were carried out with the software Minitab®17 using one-way analysis of variance(ANOVA)according to the Fisher’s Least Significant Difference(LSD)test at 5% level to evaluate the differences between extracts or extraction parameters.
3.Result and discussion
3.1.Cytotoxicity Antiviral evaluation
Since HSV-1 drugs cause more risk than benefit due to their low efficiency and viral resistance,the need to find herbal remedies and/or natural products is becoming a must[24].On the other hand,many plants extracts as well as their bioactive constituents showed antiviral therapy without the extensive severe toxic effects of the synthetic drugs[25,26].Table 2,presents a first screening of antiviral activities of the 25 Egyptian plant extracts obtained by cell viabilityin vitro.
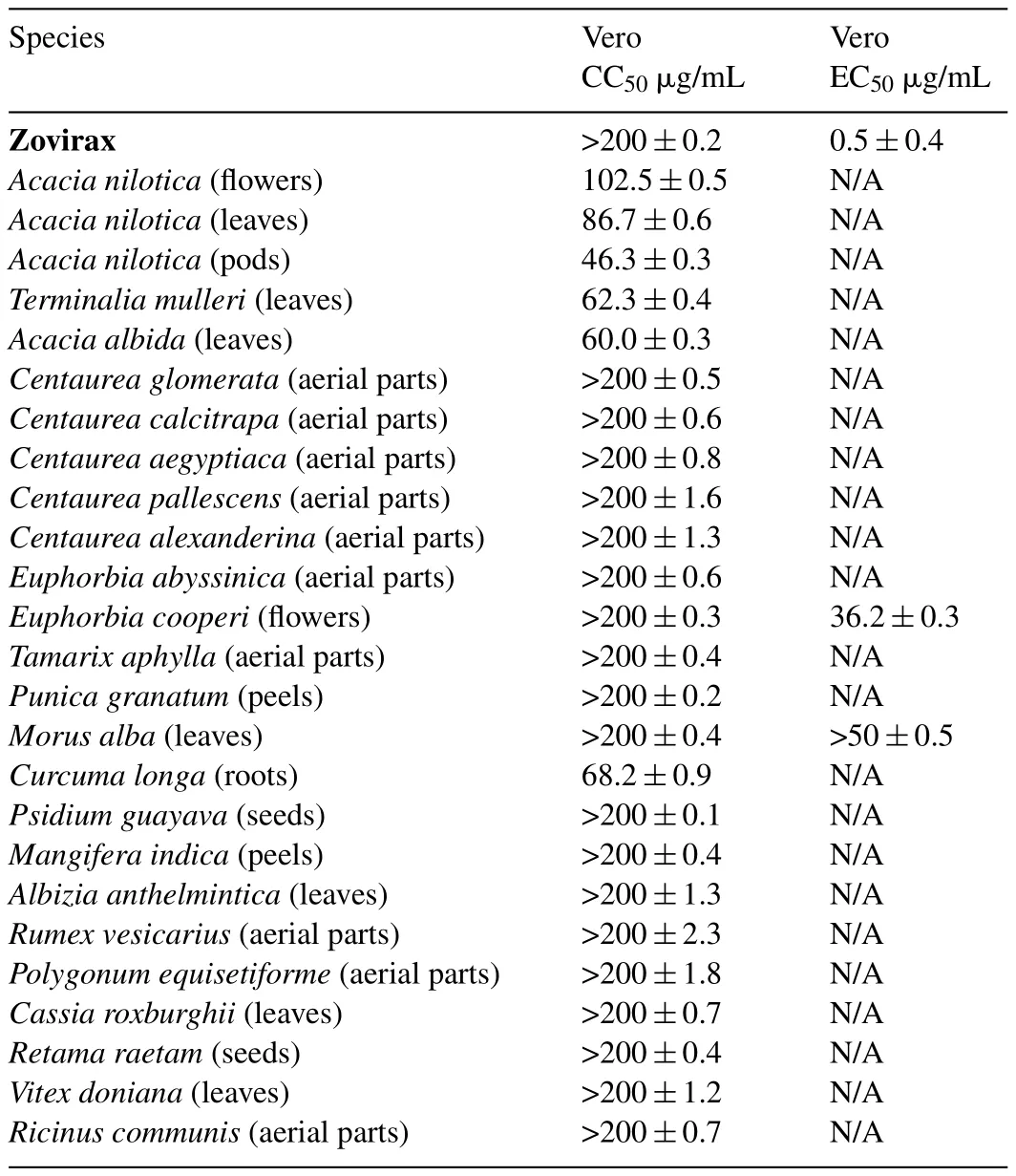
Table 2Anti-herpetic(HSV-1)activity of some Egyptian plant extracts.
After 3days of treatment,microscopically visible alteration of normal cell morphology was observed and viability assay showed destruction of cell layer.No cytotoxic effect of the compounds on the Vero cells was observed in the range of the concentrations assayed for all compounds exceptAcacia,Terminalia,Curcumaextracts.Medium percentages of cell destruction of four extracts of the Acacia speciesAcaciaspecies,Curcum alonga andTerminalia mulleriwere observed on Vero cell line as they possessed moderate antiviral inhibition with the 50% cytotoxic concentration CC50=102.5,86.7,46.3,60,68.2 and 62.3μg/mL,respectively.
OnlyEuphorbiaflowers andMorusleaves extracts present antiherpetic activity with EC50:36and>50μg/mL,respectively for MOI of 0.001 ID50/cells.Euphorbia coopireextract showed 70% cellular protections at 200μg/mL concentration at a multiplicity of infection(MOI)of 0.001 ID50/cells.While,theMorus albashowed inhibition rate of 50% for the same concentration.
Zovirax–at 1μg/mL conferred total protection of 100% against HSV-1 yet these results are considered promising and need further investigations to be done to improve the results.
Furthermore,fractionation of these extracts obtained flavonoids that showed anti-herpes activity against HSV-1 as well.This was in accordance with the increase of published papers about the antiviral activity of flavonoids in the last two decades[27–29].Some of the isolated and identified compounds such as quercetin and kaempferol were previously mentioned in literature that they possess antiviral properties[30,31].Other flavonoids for example;galangin,quercetin,procyanidin and pelargonidin as well as procyanidin C-1 are found to be virucidal against HSV[32,33].
Thus,the antiviral activity of the pure compounds isolated was further examined by different concentrations and was reported in(Table 3),whereby the gallic acid and the 7-galloy catechin showed potent activity with CC50=49.8 and 43.2μg/mL,respectively.While the other isolated compounds;kaempferol 3-O-β-(6''-O-galloyl)-glucopyranoside,quercetin 3-O-β-(6''-O-galloyl)-glucopyranoside,quercetin and kaempferol showed partial anti-herpetic activity with CC50=124.1,175.6,78.1,and 76.1μg/mL,respectively.Another compound curcumin isolated fromCurcuma longa also possessed significant anti-herpetic activity with CC50=49.8μg/mL.In comparison to the Zorivax(with total protection>100%)the results maybe low but due to their different viral inhibition mechanisms and low toxicity they could be used as complement in the HSV-1 infections treatment.

Table 3 Anti-herpetic(HSV-1)activity of some phenolic compounds.
3.2.Phenolic composition
The structures of the isolated secondary metabolites were elucidated on the basis of spectroscopic analysis[34,35].Among the most active compounds(Fig.1)obtained from the extracts ofEuphorbia coopireflowers andMorus alba leaves;compounds 1,3 and 7 were described below.
Compound 1 was strongly positive(a dark blue color)to the ferric chloride reagent and exhibitedRf-values and UV spectral data similar to those of Catechin derivatives.The1H NMR spectrum of 1 was similar to that of Catechin except for the additional signal atδ7.27(2H,s),due to the presence of a galloyl group. The proton resonances of H-6 and H-8 appeared atδ6.38 and 6.28ppm more down field than observed in Catechin[36],besides the13C NMR spectrum ensured esterification at posi-tion C-7 followed from the upfield shift of C-7 toδ150.83,while the recognizable down field shift at C-6 and C-8 resonances to 101.47 and 101.19,respectively.Compound 1 was identified as 7-O-Galloyl-Catechin[37].
Compound 3 was obtained as yellow amorphous powder withRfvalues and chromatographic properties(a purple colour under UV lamp turning to bright yellow when fumed with ammonia vapours and gives yellow colour when sprayed with Naturstoff reagent)similar to those of flavonol glycosides.Complete acid hydrolysis yielded kaempferol as the aglycone moiety together with gallic acid while the sugar moiety gave glucose,in which all of them were co-chromatographed with authentic samples.1H NMR spectrum of 3 showed a pair of doublet signals atδ7.93 and 6.75(J=8.9Hz)assignable to H-2',6'and H-3',5',respectively characteristic for ring B,and a two metacoupled signals appeared atδ6.38and6.18(J=2Hz)assignable to H-8 and H-6,respectively characteristic for ring A,which together characteristic for kaempferol moiety[38].A doublet signal of the anomeric proton appeared atδ5.44(J=7.4Hz)for the sugar moiety whose β–con figuration was estimated from its value[34].The downfield shift of the anomeric proton suggested its estrification[39]which was con firmed by the presence of the singlet signal atδ6.91 assignable to the two equivalent protons of the gallic acid and by the down field shift for H-6''a&b atδ4.26 and 4.16,respectively[40].The13C NMR spectrum of 3 showed twenty five signals,six of them attributed to the sugar moiety,thirteen to the kaempferol moiety and the remaining six signals for galloyl moiety.The C-2 down field shift at 157.10 con firmed the glycosylation at C-3 position[41].While,the down field shift of the C-6''atδ63.16 was evidence that the hydroxyl group at this positions was galloylated.Therefore,compound 3 was identified as kaempferol-3-O-β-D-(6''-galloyl)glucopyranoside.
Rfvalues,colour reactions and UV spectral data(λmaxMeOH:258 and 352nm)of compound 7 suggested it is a quercetin-3-O-substituted molecule[35].Complete acid hydrolysis yielded quercetin and gallic acid in the organic layer,glucose moiety in the aqueous layer that was Co-PC.1H NMR spectrum of this compound was similar to those belonging to quercetin moiety which gave an ABX system with the signals atδ7.68(d,J=2.1Hz,H-2'),7.70(dd,J=8.5&2.1Hz,H-6'),6.83(d,J=8.5Hz,H-5'),for ring B and at a meta-coupled signals atδ6.47(d,J=2.1Hz,H-8),and 6.28(d,J=2.1Hz,H-6)for ring A.The presence of an anomeric proton with β con figuration was proved from the doublet signal atδ5.55(J=7.4)[34].A singlet signal appeared atδ7.01 belonging to gallic acid which was determined to be attached to the 6-position of the sugar moiety due to the down field shift of H-6''atδ4.35 and 4.30 as well as the down field shift of the anomeric proton itself.13C NMR spectrum was in agreement with the above data.Moreover,the HMBC spectrum of 7 ensured the correlation between the quercetin,glucopyranose,and gallic acid moieties according to the cross peaks;one due to the coupling between H-1''and C-3 and the other due to the coupling between H-6''a and C-7'''.Comparison of the spectral data of 7 with those of published in literature revealed the structure of 7 to be quercetin 3-O-β-(6''-O-galloyl)-glucopyranoside[40].
3.3.Conclusion
The present study concludes that eight plant extracts and seven pure compounds of the 25 different Egyptian plant extracts exhibited potent anti-HSV-1 activities.Among them,the phenolic compounds,namely gallic acid,7-O-Galloyl-Catechin and curcumin were found to possess the strongest antiviral activity.Due to the lack of approved drugs in treating adenoviral infection,gallic acid,7-O-Galloyl-Catechin and curcumin might be a potential therapeutic agent for treating this disease.

Table 41H and13C NMR data for the isolated compounds.
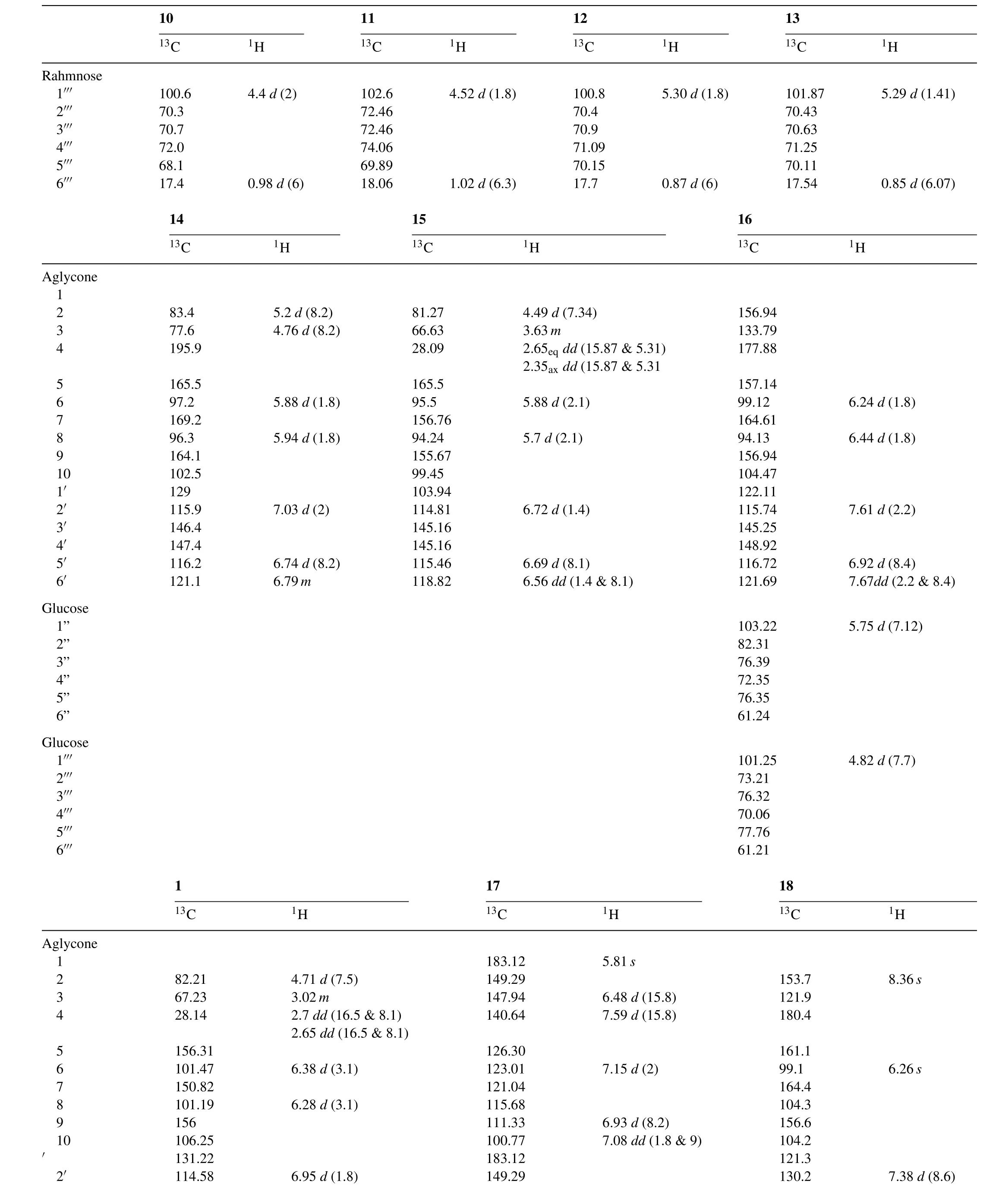
Table 4(Continued)

Table 4(Continued)
Acknowledgments
The authors thank the Academy of Scientific Research&Technology(ASRT,Egypt)and Centre de Recherché et d’enseignement Yves Coppens,Campus de Tohannic,BP 573,56017 Vannes(France)for supporting this study within the IMHOTEP program(Project No.EGY/FR 8-013).
杂志排行
食品科学与人类健康(英文)的其它文章
- Nutritional and medicinal characteristics of Chinese giant salamander(Andrias davidianus)for applications in healthcare industry by artificial cultivation:A review
- A critical review on phytochemical profile and health promoting effects of mung bean(Vigna radiata)
- Trichosanthes dioica Roxb.:A vegetable with diverse pharmacological properties
- Alterations of attention and impulsivity in the rat following a transgenerational decrease in dietary omega-3 fatty acids
- Optimization of a fermented pumpkin-based beverage to improve Lactobacillus mali survival and α-glucosidase inhibitory activity:A response surface methodology approach
- Fluorescence sensor based on glutathione capped CdTe QDs for detection of Cr3+ions in vitamins
