Optimization of a fermented pumpkin-based beverage to improve Lactobacillus mali survival and α-glucosidase inhibitory activity:A response surface methodology approach
2018-05-24KohUUthumpornARosmAIrfnYPrk
W.Y.KohU.UthumpornA.RosmA.R.IrfnY.H.Prk
aFood Technology Division,School of Industrial Technology,Universiti Sains Malaysia,Minden 11800,Penang,Malaysia
bBioprocess Division,School of Industrial Technology,Universiti Sains Malaysia,Minden 11800,Penang,Malaysia
cDepartment of Applied Microbiology and Biotechnology,Yeungnam University,Gyeongsan,South Korea
Abstract
Keywords:Probiotics;Response surface methodology;Box-Behnken;Hyperglycaemia;Functional food
1.Introduction
Type-2 diabetes mellitus is a long term endocrine and metabolic disorder diseases featured by persistent hyperglycaemia.This might lead to multiple complications and can cause cardiovascular disease.In Type 2 diabetes,there are difficulties for insulin to stimulate glucose uptake by skeletal muscle,fat and liver cells from the blood[1].
One of the therapies towards alleviating Type 2 diabetes is promoting the efficacy of the body achieving optimal blood glucose after meal.Inhibitors of α-glucosidase,which assembles the disintegration of disaccharide into glucose,are plausible in impeding glucose absorption.Therefore,inhibition of αglucosidase is contemplated to be a potent strategy for the hyperglycemia management.The common synthetic antidiabetic drugs,such as meglitinides,biguanides,thiazolidinediones and sulfonylureas,are costly and might also have significant side effects such as gastrointestinal disturbance and weight gain.For this reason,researchers have shifted focus in search for more adequate and safer anti-hyperglycemic products from natural substances which reportedly have lesser side effects[2].
Pumpkin(Cucurbita pepo)belongs to the family ofCucurbitaceae.Pumpkin flesh contains a variety of phenolic compounds,flavonoids,vitamins,as well as minerals.It also has low calorie content(17kcal/100g flesh)[3].One of the major carotenoids in pumpkin fruit(>80%)is β-carotene,which contributes to the high nutritional value of pumpkins[4].Traditionally pumpkin is cooked in variety of dishes or used to make desserts and beverages.However,pumpkin contains a high level of insulin-dependent sugars,which is problematic for diabetic patients[5].Therefore,in this study,fermentation is utilized in the development of pumpkin based beverage in which the microorganisms could utilize the sugar during fermentation.This reduces the insulin-dependent sugars so that this beverage is more suitable for the consumption of diabetic patients.Moreover, fermentation of vegetables and fruits such as pumpkin may not only improve the food safety levels and prolong the shelf life,but may also enhance the availability of certain nutrients[6].
According to the definition of FAO/WHO in 2002[7],‘probiotics are live microorganisms that confer health benefits to host when administered in adequate amounts’.There is a growing demand for dairy alternatives by cause of lactose intolerance,milk protein and cholesterol allergic,stimulating development of non-dairy probiotic foodstuff[8].Probiotic beverages which are fruit and vegetable based are viewed positively by the public with regards to health and therefore have become one of the main fruit and vegetable intake methods[9].Several probiotic beverages based on fruits and vegetables have been studied including artichoke[10],pineapple[11],carrot[12],broccoli and cherry[13].
Probiotic microorganisms must have high survivability through the gastrointestinal tract which is harsh for most microorganisms such as to tolerate acid,bile and gastrointestinal enzymes,eventually attached to and set up colonies in the intestinal epithelium in order to improve the host’s health[14].Pumpkin flesh have the appropriate matrix with pH value more than five and also adequate level of oxygen for the probiotic bacteria to survive long enough over gastric passage,where the matrix’s buffering capability increases the gastric tract’s pH and further stabilize as well as provide protection to the probiotics[15].From this point of view,pumpkin could serve as a delivery carrier for sufficient viable probiotics to assure active healthy benefits to the host.
In previous studies(Koh,W.Y.,Uthumporn,U.,and Rosma,A.,unpublished work)we have demonstrated thatLactobacillus maliK8 isolated from kefir grains exhibited high α-glucosidase inhibition activity.In the presentresearch,L.maliK8 was used as an inoculum for the fermentation of a pumpkin-based beverage in order to further explore the concept of pumpkin fruits could serve as a delivery vehicle for probiotics.The primary objectives of this study were(1)to optimize the variables i.e. pumpkin puree concentration,fermentation temperature and inoculum concentration levels for the development of a fermented pumpkin-based beverage that is stable for 4 weeks of cold storage,(2)to establish the relationship among the aforementioned variables with fermentation time,the survival of probiotics in fermented pumpkin-based beverage after treated with simulated gastrointestinal fluids,α-glucosidase inhibitory activity and sensory overall acceptability after 4 weeks of cold storage.Response surface methodology was employed to accomplish these objectives so that the anti-hyperglycemic functional beverage product can provide convenient probiotics and nutrients intake to the general public,which may better manage hyperglycemic condition in Type-2 diabetes patients.
2.Materials and methods
2.1.Preparation of inoculum
Lactobacillus maliK8(isolated from ke fir grains)was used for the fermentation of pumpkin-based beverage.The cultures were first grown in sterileLactobacillusde Man Rogosa Sharpe broth,incubated(48h,37°C)and then centrifuged(14,310×g,20min).Subsequently,the pellets were suspended in sterilized saline solutions and re-centrifuged with the aforementioned settings.The washed cells were then adjusted in sterilized saline to three different concentrations which are 6 Log colony forming units per mL(CFU/mL),7 Log CFU/mL,and 8 Log CFU/mL,respectively.Each of these suspension concentrations were employed at 1mL/100mL across the different fermentation series.
2.2.Preparation of pumpkin based beverage
Fig.1 outlined the preparation for the pumpkin based beverage.Freshly collected pumpkins were washed thoroughly with chlorinated water(50ppm sodium hypochlorite)and the fruits were diced into cubes.The pumpkin cubes was steamed at 100°C for 20min in a laboratory autoclave to soften the flesh so that they can be easily homogenized into puree.The pumpkin puree and distilled water were measured according to the formulations generated by response surface methodology coupled with Box-Behnken Design in Design Expert®(Table 1)and mixed in 250ml Erlenmeyer flask.The pumpkin juice was autoclaved(121°C,20min)and cooled down beforeL.maliK8 inoculum was added at 1mL/100mL and fermented according to the independent parameters as in Table 1.The pH value of the pumpkin based beverage was monitored every hour using a pH meter(Mettler-Toledo,Switzerland)with a glass electrode at room temperatures throughout the fermentation.The time required to decrease to pH4.5 was recorded as the fermentation time(TpH4.5)based on pH 4.5 being set as the fermentation endpoint in probiotic beverage production[16].Finally,the end beverage products were kept at 4°C prior to use.

Fig.1.Flow chart of preparation of pumpkin based beverage.

Table 1 Box-Behnken design of the observed responses(fermentation time(TpH4.5),L.mali survival in gastrointestinal condition(SGI),α-glucosidase inhibitory activity percentage(α-G)and sensorial acceptability(SOA)score of the fermented pumpkin-based beverage)under different experimental conditions.
2.3.Survival of L.mali K8 in fermented pumpkin based beverage after incubated in simulated gastrointestinal fluids
The fermented products were stored at 4°C for 4 weeks andL.maliK8 survival rates at every week of storage after simulated gastrointestinal juices exposure were determined.Simulated gastric juice at pH 2 was imitated by preparing hydrochloric acid buffer consisting of 8g/L sodium chloride,0.2g/L potassium chloride,8.25g/L sodium phosphate dibasic dehydrate,14.35g/L sodium phosphate monobasic,0.1g/L calcium chloride,0.18g/L magnesium chloride hexahydrate and 3g/L pepsin.The pumpkin based beverage samples(10mL)was inoc-ulated into 90mL of simulated gastric juice and was subjected to incubation(37°C,3h)in a rotary shaker.
After incubation,the solution were centrifuged and washed with 0.85%(w/v)pre-reduced saline solution and added into 9mL of simulated intestinal juice(pH 7.4)consisting of 6.50g/L sodium chloride,0.84g/L potassium chloride,0.22g/L calcium chloride,1.39g/L sodium bicarbonate,0.50g/LL-cysteine hydrochloride,3g/L bile salt and 1g/L pancreatin(Sigma-Aldrich).Consequently, the solution was incubated with consistent shaking(37°C,3h).Subsequently,1mL of samples was inoculated to 9mL of pre-reduced phosphate buffer(0.1M,pH7.4) and was subjected to stomacher homogenization(5min)and then centrifugation(10,000g,10min,4°C).Serial dilution of pellet with saline solution was made and spread-plated on MRS agar added with 0.05%(w/v)L-cysteine hydrochloride and was subjected to incubation(37°C,48h).Viable cells were enumerated and conveyed as Log CFU/mL after incubation.The survival rates(%)was determined as(log CFU N1/log CFU N0)×100% where N1represented the total viable count of the microbial strain in pumpkin beverage after treatment whereas the total viable count of the microbial strain in pumpkin beverage before treatment was represented by N0.All experiments were performed in triplicate[17].
2.4.α-Glucosidase enzyme inhibitory activity
Rat intestinal acetone powder(Sigma-Aldrich,I1630)was used as an enzyme source.Ultra-pure water was used to dissolve the acetone powder at 10mg/mL and the mixture was subjected to centrifugation(16,000×g,30min,4°C),and the supernatant was used as the α-glucosidase solution in this assay.Beverage sample was centrifuged at 5939gfor 10min at 4°C.The pH of supernatant was adjusted to pH 7.4 and centrifuged again at 13,362gfor 10min at 4°C.The supernatant was then filtered through a 0.22μm membrane filter before conducting enzyme inhibition assay[18].
A total of 100μL beverage samples(at each storage time,week 0,1,2,3 and 4)or acarbose(Cayman Chemical)(10mg/mL)were mixed with 100μL α-glucosidase enzyme solution and 100μL of phosphate buffer(0.1mol/L,pH 6.9)and then was subjected to pre-incubation(25°C,5min).Consequently,100μL of 5mmol/Lp-nitrophenyl α-D-glucopyranoside solution was added as substrate after pre-incubation and was subjected to incubation(25°C,10min).After the incubation,α-glucosidase enzyme inhibition was measured by taking the absorbance at 405nm and the data was calculated using equation below[19].

where x0,y0,x and y are the absorbance with enzyme and without the sample,absorbance without enzyme or the sample,absorbance with enzyme and the sample,and absorbance with the sample but without enzyme,respectively.
2.5.Sensory evaluation
Sensory properties of pumpkin based beverage were evaluated by an untrained sensory panel(n=50),which was performed immediately after beverages removal from storage conditions.Beverages were served in food serving trays with codes.Panelists were asked to evaluate appearance(colour),smell,taste,texture(consistency)and overall acceptability of the pumpkin based beverages at week 0,1,2,3,and 4 of storage.A seven-point hedonic scale was used by the panelists to evaluate sensory attributes of experimental pumpkin based beverage at each storage time.The limit of acceptance was rated at 2.5 which means that any attributes evaluated below that score was considered to represent the end of shelf-life[20].
2.6.Experimental design
Response Surface Methodology coupled with Box-Behnken design was utilized to optimize the levels of fermentation temperature and ingredient concentration.
The range for independent variables i.e.fermentation temperature(X1),inoculum concentration(X2)and pumpkin puree concentration(X3)were derived from preliminary experiments results(Table 1).The twelve factorial points and centre points were repeated for five times.Fermentation time at the fermentation endpoint pH 4.5(TpH4.5),α-glucosidase inhibitory activity(α-G),survival ofL.maliK8 in pumpkin based beverage treated with simulated gastrointestinal fluids(SGI)and sensory overall acceptability(SOA)after 4 weeks of cold storage were selected as combination responses presented in Table 1.Each experimentation was replicated three times and observed responses were calculated through their mean values.These experiments were undertaken in a random way to minimize perceived unexpected variability in the responses.
The actual values of independent variables’as shown in Table 1 were coded using the equation below:

Where x,xi,x0andΔx are the coded value,corresponding actual value,actual value in the centre of the domain and increment ofxicorresponding to a variation of one unit of x,respectively.
The mathematical model corresponding to the composite design is:

Where,y,β0,βi,βii,βijare the predicted response(TpH4.5,α-G,SGI and SOA),constant coefficient,linear coefficient,quadratic coefficient and cross product coefficient,respectively and Xiand Xjare assigned as independent variable while ε represented as error or noise[21].
2.7.Statistical analysis
For the storage quality experiments,all samples were replicated three times and the data were depicted as mean±standard deviations.Differences between statistical significance were evaluated using one-way analysis of variance.
Design Expert®10.0.1 were used for data analysis of response surface methodology experimental design and predicted or estimated responses calculations.The statistical experiment’s validity was confirmed through additional experiments.
3.Results and discussion
3.1.Model fitting
Response surface methodology optimization is more favourable than the single parameter optimization in terms of lower cost,shorter time,smaller space and quantity of raw material required for experimentation.The adequacy of second order quadratic polynomial models describing the effects of three independent variables:pumpkin puree concentration(X1),inoculum concentration(X2)and fermentation temperature(X3)on responses(TpH4.5,α-G,SGI and SOA after 4 weeks refrigerated storage).A model is considered as well fitted with the experimental data when significant regression model(Pm<0.05)and non-significant(PIof>0.05)lack of fit is obtained[22].As demonstrated in Table 2,all proposed mathematical models could be utilized sufficiently to estimate the dependent variables as a function of the independent variables.At all cases,the statistical models consisted of significant determination coefficeints(R2>0.98),non-significant P&HIPHEN;value for lack of fit relevant to pure error(p>0.05)and low correlation variation %(C.V.%).The adequate precision level of all models were well above the threshold value of 4 indicating adequacy of the signals and model to navigate the design[23].
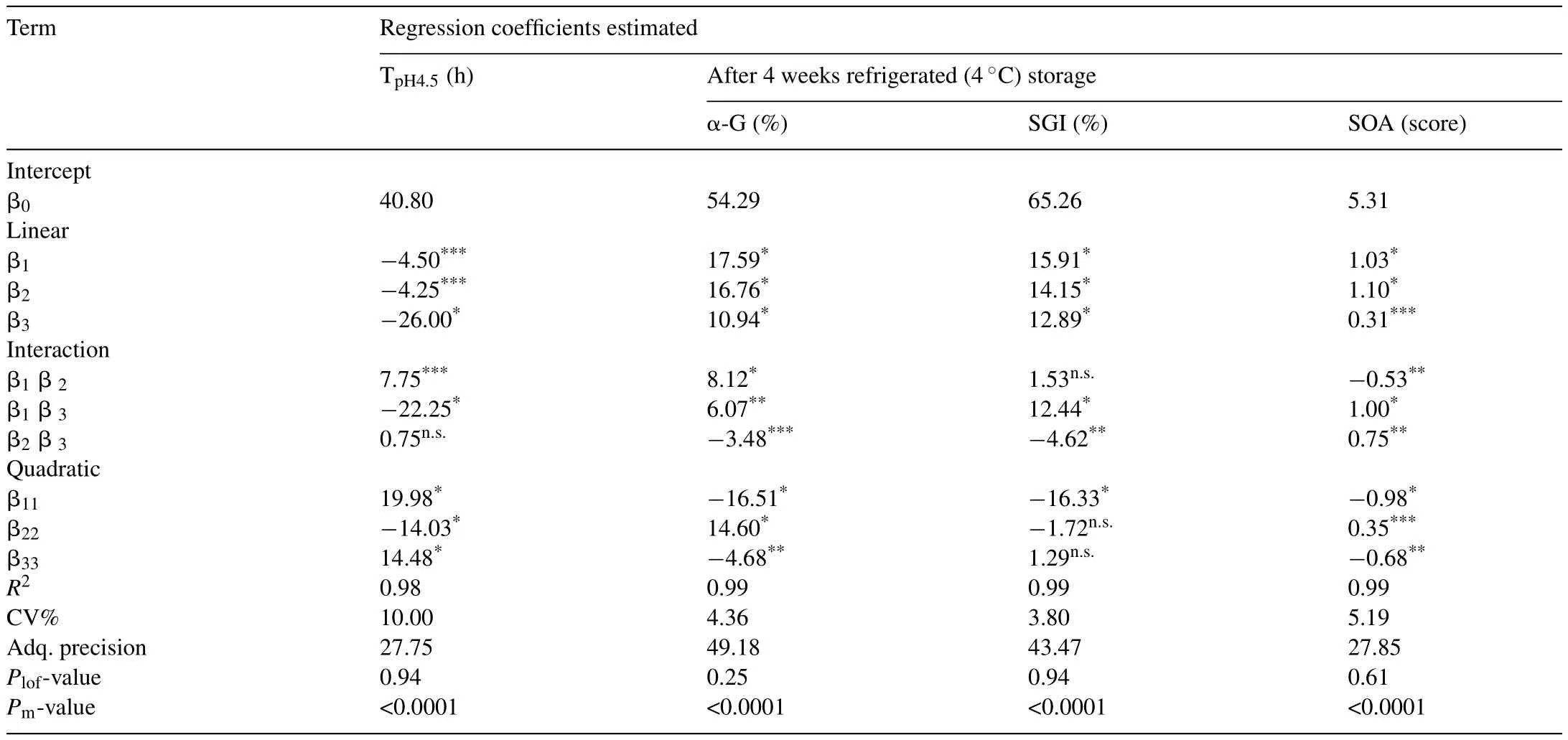
Table 2Estimated coefficients of the fitted second-order polynomial models for the responses(fermentation time(TpH4.5),L.mali survival in gastrointestinal condition(SGI),α-glucosidase inhibitory activity percentage(α-G)and sensorial acceptability score(SOA)of the fermented pumpkin-based beverage).
The four responses of interest were fermentation time at the fermentation endpoint pH4.5(TpH4.5),α-glucosidase inhibitory activity(α-G),survival ofL.maliK8 in the beverage after incubation in simulated gastric and intestinal fluids(SGI),and sensory overall acceptability(SOA)following 4 weeks storage under refrigeration(4°C)condition.The outcomes of the 17 experiment runs using the Box-Behnken designs were presented in Table 2.The values for TpH4.5ranged from 12 to 120h,and the maximum point was found using formulation of X1=40%,X2=7 Log CFU/mL,X3=20°C.The minimum TpH4.5was determined in the existence state of experimental conditions of X1=30%(v/v),X2=8 Log CFU/mL and X3=40°C.A broad range of values for SGI after 4 weeks refrigerated storage was also found(survival rate of 18.04−90.86%).The maximum survival was found under the conditions of X1=40%(v/v),X2=7 Log CFU/mL and X3=40°C.On the other hand,α-G values after 4 weeks storage ranged from 10.12 to 95.97% inhibition,and the greatest value was set up in conditions of X1=40%,X2=8 Log CFU/mL,X3=30°C.The values for SOA after 4 weeks storage was ranged widely from 1.89 to 6.99.The maximum point was found using experimental conditions of X1=30%,X2=8 Log CFU/mL,X3=40°C.These conditions differ according to the responses,therefore in order to acquire short TpH4.5coupled with high values of α-G,SGI and SOA,an optimum process was examined.
As demonstrated in Table 2,the outcome of analysis of variance postulated the quadratic model was significant at each of the responses investigated.Based on the data provided in Table 2,second order polynomial equations excluding non-significant(p>0.05)factors were provided showing the relationships between the independent variables and responses,as shown in Eqs.(3)–(6),respectively.

3.1.1.Fermentation time
The pH value 4.5 was selected as the fermentation endpoint because it’s safer for the food products to be stored at this pH value and any food products with pH value above 4.5 can be conducive for food pathogen growth[11].As deduced from Table 2,the linear term of fermentation temperature(X3)had the largest effect(Fvalue=26.00)on the TpH4.5followed by the interaction term of pumpkin puree concentration and temperature(X1X3)and quadratic term of pumpkin puree concentrationBesides that,the linear term of pumpkin puree concentration(X1)and inoculum concentration(X2),interaction term of pumpkin puree and inoculum concentration(X1X2),and the quadratic terms for inoculum concentration and temperature (,respectively)demonstrated significant values(p<0.05)(Table 2).Nevertheless,the interactions between inoculum concentration and temperature(X2X3)does not contribute significantly to the values of TpH4.5.The statistical model demonstrated significant coefficient(r2=0.9845),and non-significantp-value for lack of fit relevant to pure error(Plof=0.9481;p>0.05).The polynomial Eq.(3) fitted the response TpH4.5adequately.
3.1.2.α-Glucosidase inhibitory activity
All the estimated coefficients of the fitted second-order polynomial models were significant in α-glucosidase inhibitory activity after 4 weeks refrigeration storage response.The linear term of pumpkin puree concentration(X1)demonstrated the greatest effect(Fvalue=17.59)(Table 2),trailed along by the linear term of inoculum concentration(X2),and the quadratic term of pumpkin puree concentration. The predicted model in α-glucosidase inhibitory activity response possessed high coefficient of determination(r2=0.9985)and insignificant lack of fit(PIof=0.2523),indicates well fit to the mathematical Eq.(4).
3.1.3.Survival of L.mali K8 in beverage after incubation in simulated gastric and intestinal fluids
The quadratic term corresponding to pumpkin puree concentration,with the highestFvalue(16.33),illustrated the greatest contribution(p<0.05)to the SGI model,trailed along by the linear terms of pumpkin puree(X1)and inoculum concentration(X2)(Table 2).Additional terms i.e.linear term of fermentation temperature(X3),interaction terms corresponding to pumpkin puree concentration and temperature(X1X3),and inoculum concentration and temperature(X2X3)also demonstrated significant values(p<0.05)(Table 2).While,the term corresponding to the interactions pumpkin puree and inoculum concentrations(X1X2),the quadratic terms of inoculum concentrationand fermentation temperatureillustrated a negligible effect.The predicted model demonstrated coefficient of determination(r2)was 0.9951,whereas lack of fitp-value was 0.9375 which implies a good fitting to Eq.(5).
3.1.4.Sensory overall acceptability
Based on the ANOVA test as shown in Table 2,all independent variables affecting the SOA response significantly(p<0.05).The inoculum concentration in its linear term(X2)had the most significant effect on SOA,accompanied by linear term of pumpkin puree concentration(X1)and interactions between pumpkin puree concentration and temperature(X1X3).The predicted model showed coefficient of determination,r2=0.9875,with the lack of fitp-value=0.6052,which indicates an adequate fitting to the mathematical model(Eq.(6)).
3.2.Response surface model and contour plot interpretation
The regression equations were presented in threedimensional surface plots and two-dimensional contour plots.This aid in visualizing the relationship between responses and each variable’s experimental levels and two test variables’interactions.Significance of the variables can be noted through the elliptical or circular form of the contour plots.A saddle or elliptical contour plot of response surfaces denotes that there were significant interactions between the correlated variables,while a circular contour denotes otherwise[24].
The response surfaces and contour plots produced by the model for TpH4.5,α-G,SGI and SOA after 4 weeks of storage(Figs.2–5,respectively)demonstrated the relationship between independent and dependent variables,two variables were portrayed in one three-dimensional surface plot while the other variable were retained at zero.All responses(TpH4.5,α-G,SGI and SOA values)were sensitive to slight modifications of the independent variables(pumpkin puree concentration,inoculum concentration and fermentation temperature).
3.2.1.Fermentation time
The effect of inoculum and pumpkin puree concentration was displayed in a two-dimensional contour and three-dimensional response surface plot(Fig.2a),which showed the factors’linear and quadratic effects respectively. The increment in pumpkin puree concentration associated with the decrease in the response of TpH4.5,from 82 to 18h.However,a continued increase in the pumpkin puree concentration(~35% to 40%)produces an increase in TpH4.5response.On the other hand,the increment of inoculum concentration decreases the TpH4.5reaching its maximum value at~6.5 Log CFU/mL,followed by a constant value and then slow decrease.
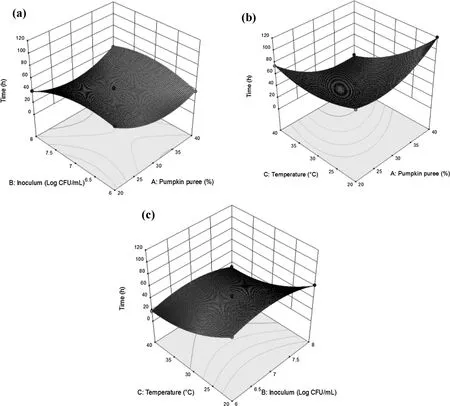
Fig.2.Response surface plots illustrating the interactive effects of(a)Pumpkin puree concentration vs Inoculum concentration,(b)Pumpkin puree concentration vs Fermentation temperature,(c)Inoculum concentration vs Fermentation temperature on fermentation time(pH 4.50 set as the fermentation endpoint)of the fermented pumpkin-based beverage.
Fig.2b illustrated the quadratic and linear effects of fermentation temperature and pumpkin puree concentration variables.The increase in fermentation temperature stimulated the decrease TpH4.5response,but continued increase in the fermentation temperature(~35 to 40°C)induced increase in TpH4.5response.As can be observed in Fig.2c,there is negligible interaction between fermentation temperature and inoculum concentration(p>0.05).Furthermore, there is observable linear effects of fermentation temperature and inoculum concentration.Fig.2c demonstrated that an increase of fermentation temperature reduces TpH4.5values,with the same effect being observed in inoculum and pumpkin puree concentration.These findings propose that shorter TpH4.5could be achieved by increasing temperature,inoculum and pumpkin puree concentration.
3.2.2.α-Glucosidase inhibitory activity
Fig.3a illustrated inoculum and pumpkin puree concentration’s quadratic effects on α-G values after 4 weeks of storage.As inoculum concentration increases up to approximately 7.0 Log CFU/mL,α-G increases,but further increase in this variable(~7.0 to 7.5 Log CFU/mL)α-G response became plateau and eventually increase again.Fig.3b shows the quadratic and linear effects of fermentation temperature and pumpkin puree concentration variables.An increase in pumpkin puree concentration stimulated an increase in the response of α-G reaching its maximum point at~35%,and then slightly decrease.While fermentation temperature and inoculum concentration’s linear effects can be observed Fig.3c,where the increase of fermentation temperature increases the α-G values.The same effect can be observed for inoculum and pumpkin puree concentration as well.The positive impact of these parameters propose that higher α-G is achievable by increasing temperature,inoculum and pumpkin puree concentration.
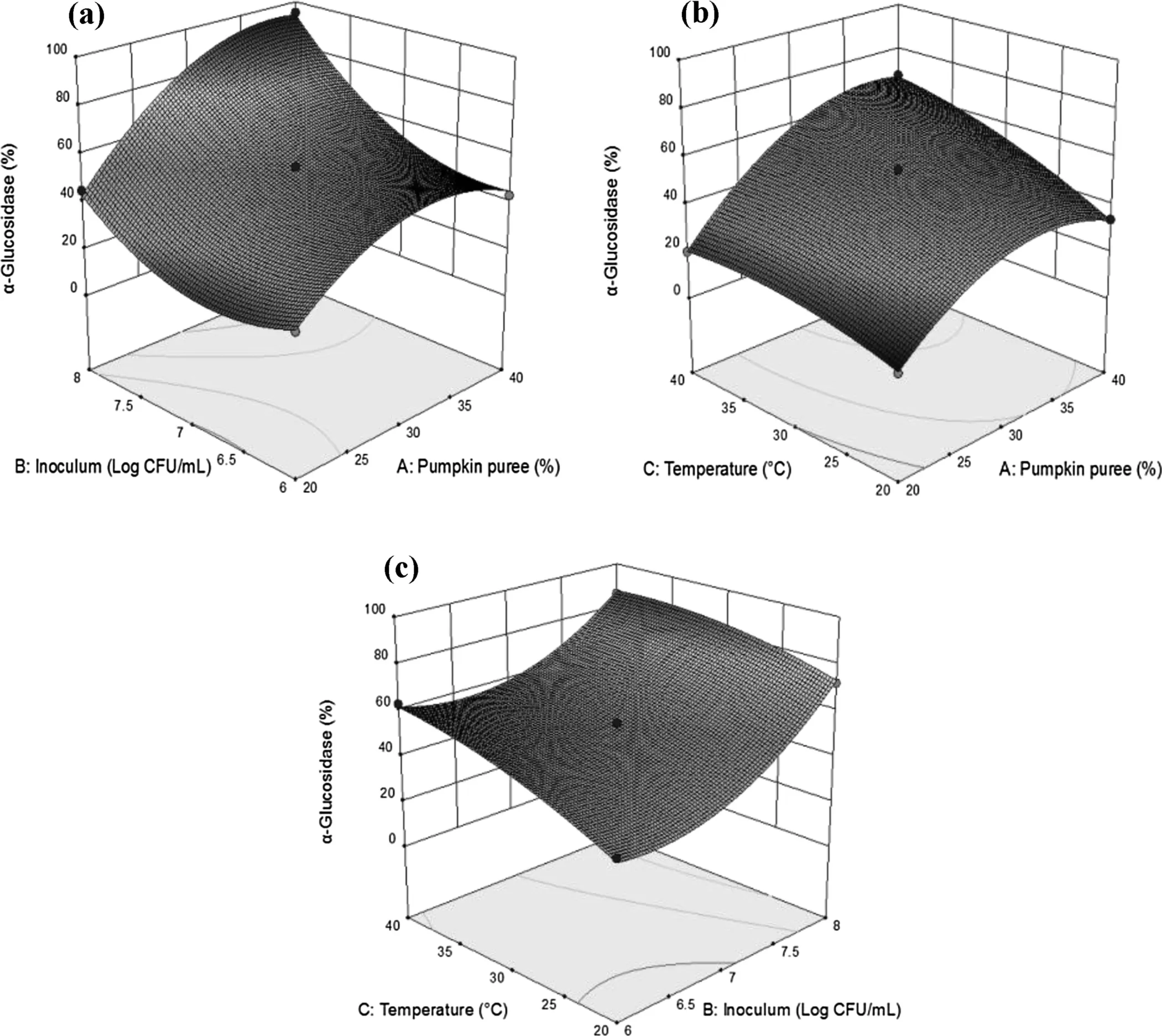
Fig.3.Response surface plots illustrating the interactive effects of(a)Pumpkin puree concentration vs Inoculum concentration,(b)Pumpkin puree concentration vs Fermentation temperature,(c)Inoculum concentration vs Fermentation temperature on α-glucosidase inhibitory activity percentage of the fermented pumpkin-based beverage after 4 weeks refrigerated(4°C)storage.
3.2.3.Survival of L.mali K8 in beverage after incubation in simulated gastric and intestinal fluids
The contour plot in Fig.4a presented a circular shape,indicating insignificant interaction between inoculum and pumpkin puree concentrations(p>0.05).Quadratic and linear effects of temperature and pumpkin puree concentration variables can be observed in the interaction plot(Fig.4b).In this case,there is significant interaction between the two variables(p<0.05).While the rise in temperature increases the SGI value after 4 weeks storage,the increment of pumpkin puree concentration up to~30% resulted in the increment in the SGI response whereas more elevation in pumpkin puree concentration causes a decrease in SGI values.There is also significant interaction between both temperature and inoculum concentration(p<0.05)as observed in Fig.4c.The plot shows that a continuing increment of both temperature and inoculum concentration advocates the increase in SGI values.These findings postulated that higher SGI could be achieved by increasing temperature,inoculum and pumpkin puree concentration.

Fig.4.Response surface plots illustrating the interactive effects of(a)Pumpkin puree concentration vs Inoculum concentration,(b)Pumpkin puree concentration vs Fermentation temperature,(c)Inoculum concentration vs Fermentation temperature on L.mali survival in the gastrointestinal condition of the fermented pumpkinbased beverage after 4 weeks refrigerated(4°C)storage.
3.2.4.Sensory overall acceptability
Fig.5a–c illustrated the contour and response surface plots for SOA after 4 weeks storage.The quadratic and linear effects of inoculum and pumpkin puree concentration on SOA response can be clearly observed in Fig.5a.Therefore,an elevation in inoculum concentration produces an elevation in SOA and the same effect can be observed for pumpkin puree concentration.As illustrated in Fig.5b,while the increase in temperature increases the SOA values,the increase of pumpkin puree con-centration from 20% to 30% resulted in a preliminary increase in SOA trailed around with a rapid decrease.The Fig.5c illustrated the linear and quadratic effects of both temperature and inoculum concentration.The SOA score increased as a result of the elevation of temperature reaching its maximum value at~30°C,followed by a constant value and then slow decreased.

Fig.5.Response surface plots illustrating the interactive effects of(a)Pumpkin puree concentration vs Inoculum concentration,(b)Pumpkin puree concentration vs Fermentation temperature,(c)Inoculum concentration vs Fermentation temperature on sensory overall acceptability score of the fermented pumpkin-based beverage after 4 weeks refrigerated(4°C)storage.
3.3.Predictive models verification
An optimization investigation was performed based on these findings to find the optimal ingredient concentrations as well as optimal fermentation conditions for individual and all combined response with the aim to obtain fermented pumpkin-based beverage product which exhibit short TpH4.5and high values of α-G,SGI and SOA properties and stable for at least 4 weeks refrigerated storage.
The optimal conditions for each separate response with the experimental and predicted values were showed in Table 3.Optimal formulation was obtained at an approximation of 40% pumpkin puree concentration,8 Log CFU/mL inoculum and at 35°C.The optimal fermentation temperature values for short TpH4.5,high α-G,SGI and SOA were within the range of 20–40°C and is consistent with de Castro et al.in 2009[25],pointing out that lactic beverage fermented at 40°C achieved maximum viability of lactic acid bacteria.Besides that,Filannino et al.in 2015[13]demonstrated thatLactobacillusspp.fermented in 10%(v/v)broccoli puree presented a discerning cells viability.On the other hand,Speranza et al.in 2012[26]applied 8 Log CFU/mL of probiotic inoculum level for anchovies fermentation and the fermented product maintained high survival of the tested probiotic strains without affecting the sensory acceptance.
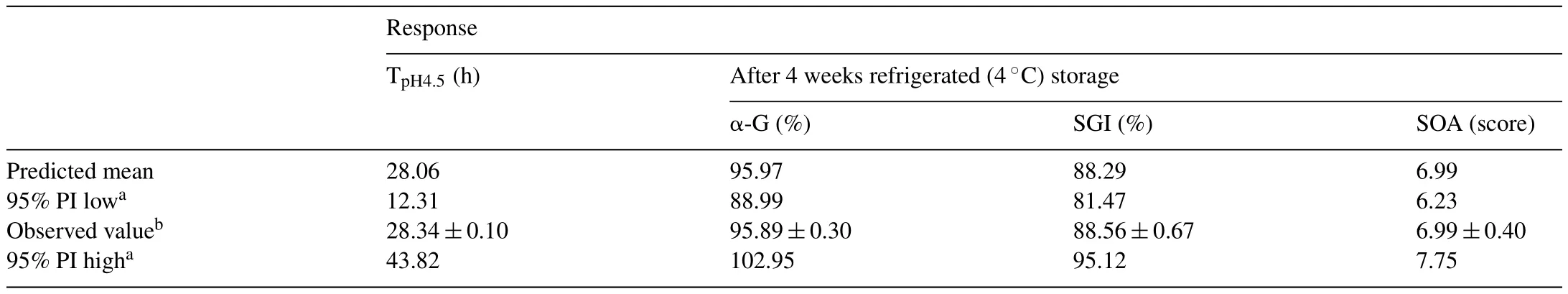
Table 3Estimated predicted and observed values for the responses(fermentation time(TpH4.5),L.mali survival in gastrointestinal condition(SGI),α-glucosidase inhibitory activity percentage(α-G)and sensorial acceptability score(SOA)of the fermented pumpkin-based beverage)within their intervals.
As can be seen in Table 3, the optimal conditions gave TpH4.5,α-G,SGI and SOA values of 28.34h;95.89%;88.56%;and6.99 score,respectively.Results showed that there is insignificance difference in the predicted and experimental values.Hence,the models acquired in this research could be employed to optimize the fermentation temperature and ingredient concentrations of pumpkin based beverage to obtain short TpH4.5and prominent values for useful properties such as α-G and SGI with satisfied SOA after 4 weeks refrigerated storage.
3.4.Storage quality
Acarbose is a commercially standard drug available to delay starch and oligosaccharides degradation,decreasing the absorption rate of glucose and inhibits the increase in postprandial blood glucose.However abdominal distention,flatulence and possibly diarrhea are reported as some of its side effects.Searching for safe and effective inhibitors from natural sources are of emerging interest[19].
The inhibitory effects of the inoculated optimized beverage,non-inoculated pumpkin juice and acarbose on α-glucosidase are shown in Fig.6.α-Glucosidase inhibiting activities of the samples were in the following order:Acarbose>inoculated optimized beverage>non-inoculated pumpkin juice.High levels of α-glucosidase inhibition(92.03%–95.89%)activity for as long as 4 weeks of cold storage observed inL.maliK8 inoculated optimized beverage is comparable to that in acarbose(98.93%).α-Glucosidase inhibition of the inoculated optimized beverage product gradually increases throughout the storage study,suggested that K8 strain continued to secrete bioactive components which are potent α-glucosidase inhibitors during storage.Our findings showed that the inoculation ofL.maliK8 strain did not only enhance but may also increase the pumpkin juice αglucosidase inhibitory capacity with storage(Fig.6).

Fig.6.Changes in α-glucosidase inhibition(%)in fermented pumpkin-based beverages(obtained from optimized fermentation condition)during refrigerated(4 °C)storage in comparison with acarbose(positive control)and non-inoculated pumpkin puree(negative control).Each value in the table represents the mean±SD(n=3).Error bars represent the standard deviation of all trials.Different superscripts have mean ranks that are significantly different from each other.Mean values with different uppercase alphabets(A-G)indicate significantly difference(p<0.05)among storage period(weeks);mean values with different lowercase alphabets(a–g)indicate significant difference(p<0.05)among samples.
Jin et al.in 2013[27]have reported pumpkin-rich diets prevent excessive rise of the blood glucose level after a mixed carbohydrate diet,which might be due to the potential αglucosidase inhibition activity of pumpkin polysaccharides in its fruits.Adams et al.in 2011[28]concluded that pumpkin fruit have active hypoglycemic properties and antidiabetic effects due to its abundance of pectin(dietary fibre)and non-pectin polysaccharies which when consumed is purported to improved glucose tolerance and control glycaemia by lessening the blood glucose elevation.Therefore,the fermentation of pumpkin juice by theL.maliK8 bacteria could enhance the α-glucosidase inhibition of pumpkin juice tremendously due to the generation of oligosaccharides hydrolyzed by the bacteria from polysaccharides in pumpkin juice[29].Moreover,naturally occurring plant’s polyphenols in pumpkin juice could bind to the reactive sites of enzymes and altering its catalytic activity,thus are considered potent and safe inhibitors of carbohydrate hydrolysing enzymes[30].
Strong inhibition of intestinal α-glucosidase could be a potent strategy for the management of Type-2 diabetes[31].Therefore,we suggest that inhibition activity against α-glucosidase could be part of the possible mechanisms of the optimized beverage in dietary management of diabetes,by retardation of starch hydrolysis in the gastrointestinal tract.Additionally,no sugar was added in the production of fermented pumpkin based beverage and the non-insulin dependent sugars in pumpkin puree can be fermented byL.maliK8 to produce metabolites that might improve the functional properties of the beverage and is suitable for diabetic folklore consumption.
As illustrated in Fig.7,the survival rates ofL.maliK8 in optimized beverage after incubation in simulated gastric and intestinal fluids showed variation of less than 2% throughout the storage period.The viability ofL.maliin optimized beverage during refrigerated storage period of 4 weeks and after treated in simulated gastrointestinal fluids was consistently over the minimum recommended level of 6 Log CFU/g implied positive health potency in the intestines[32].
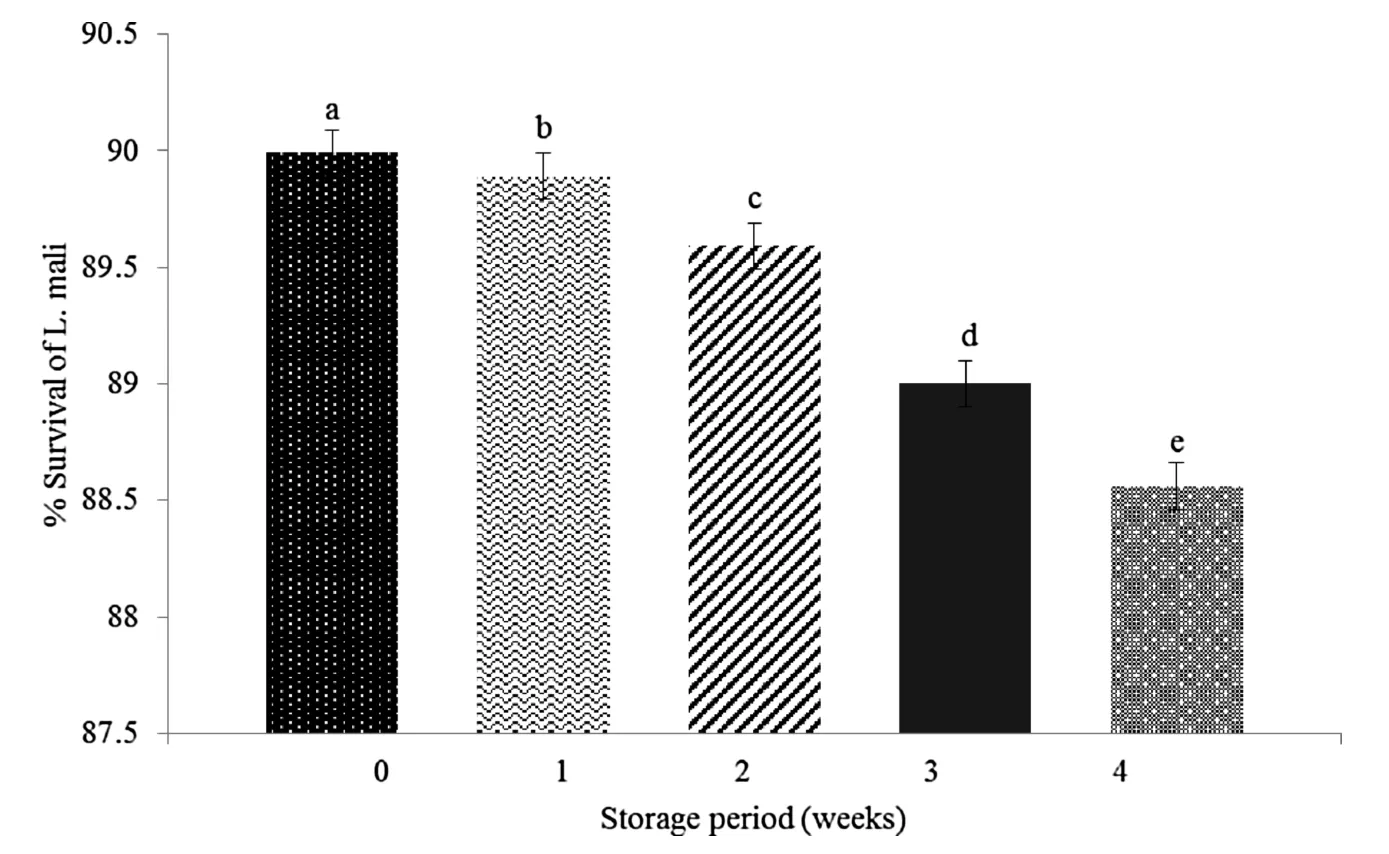
Fig.7.Survival rate(%)of L.mali in the fermented pumpkin-based beverage(obtained from optimized fermentation condition)during refrigerated(4°C)storage.Error bars represent the standard deviation of all trials.Different superscripts have mean ranks that are significantly different from each other.Mean values with different lowercase alphabets(a–g)indicate significant difference(p<0.05)between storage period(weeks).
Du et al.in 2011[33]reported that there is high resistance of the oligosaccharides from pumpkin pulp towards hydrolysis in artificial human gastric juice and can stimulate theLactobacilligrowth.In addition,Lactobacillistrains are able to metabolize and adhere to pumpkin’s polysaccharides[15].Therefore,we proposed that the polysaccharides and high fiber content in the fermented pumpkin based beverage may acts as a buffer against the acidic condition in the stomach by binding to excess acids produced by the digestive system protecting the probi-otic viability during refrigerated storage[34].This increases the survival rate of the probiotic microorganism in the product during the refrigerated storage.Through this it is postulated that the pumpkin juice could serve as a suitable food matrix with polysaccharides promoting microbial survival rates.
On the other hand,refrigeration storage was favorable to the preservation of probiotic populations in fermented pumpkin based beverage.Similar to what was detected in this research study for fermented pumpkin based beverage,Shukla et al.in 2012[35]disclosed that probioticL.acidophilusin whey and pineapple beverage maintained at 107CFU/mL throughout 4 weeks cold storage(4°C);whereas for the probiotic pineapple and whey beverage stored at 30°C,the total viable count ofL.acidophilusin declined gradually and after 5days no viability of probiotic cells was observed.
Optimized fermented pumpkin based beverage was able to maintain its sensory attributes for at least 4 weeks under cold storage as shown in Fig.8 as the difference observed between the storage period were insignificant(p>0.05).The sensory acceptance for the optimized fermented beverage can be accred-ited to compounds such as free amino acids, exopolysaccharides and interesting volatile compounds(esters,aldehydes,phenyl,ketones,acids,alcohols,furan and benzothiazole),which were the byproduct of the fermentation enriching the pumpkin juice[5].

Fig.8.Seven-point hedonic sensory evaluation of the fermented pumpkin-based beverage(obtained from optimized fermentation condition)stored at 4°C for 4 weeks.
4.Conclusions
Survival rate and α-glucosidase inhibitory activity ofLactobacillus maliK8 in fermented pumpkin-based beverage were maximize.Optimized fermented pumpkin-based beverage product achieved high sensory acceptance and 95% α-glucosidase inhibitory activity.After treatment with simulated gastrointestinal fluids and 4 weeks cold storage,L.maliK8 maintained up to 88% survival rate.In conclusion,pumpkin-based beverage has the potential to be used as a probiotic carrier and to cultivateL.malifor the development of a functional anti-hyperglycemic drink.
Acknowledgments
The authors would like to appreciate and acknowledge ASEAN University Network (AUN)and Korea Association of the Southeast Asian Studies for providing financial support under ASEAN-ROK Academic Exchange Programme 2016/2017;and Universiti Sains Malaysia(USM)Vice Chancellor Award Scholarship and grant(RUI,1001/PTEKIND/811339).
杂志排行
食品科学与人类健康(英文)的其它文章
- Nutritional and medicinal characteristics of Chinese giant salamander(Andrias davidianus)for applications in healthcare industry by artificial cultivation:A review
- A critical review on phytochemical profile and health promoting effects of mung bean(Vigna radiata)
- Trichosanthes dioica Roxb.:A vegetable with diverse pharmacological properties
- Alterations of attention and impulsivity in the rat following a transgenerational decrease in dietary omega-3 fatty acids
- Fluorescence sensor based on glutathione capped CdTe QDs for detection of Cr3+ions in vitamins
- Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed
