Fluorescence sensor based on glutathione capped CdTe QDs for detection of Cr3+ions in vitamins
2018-05-24ChiFngPengYingYingZhngZhiJunQinZhengJunXie
Chi-Fng PengYing-Ying ZhngZhi-Jun QinZheng-Jun Xie
aState Key Lab of Food Science and Technology,Jiangnan University,Wuxi 214122,PR China
bSchool of Food Science and Technology,Jiangnan University,Wuxi 214122,PR China
cNational Engineering Research Center for Functional Food,Jiangnan University,Wuxi 214122,PR China
dYangzhou Entry-Exit Inspection and Quarantine Bureau,Yangzhou 225000,PR China
Abstract
Keywords:CdTe QDs;Fluorescence;Chromium ions;Vitamin supplements
1.Introduction
Metal ions are widely used in various industrial processes and routinely released into environment,eventually leading to harmful effects of human and animal health.For example,salts of chromium(Cr)have been widely used in leather tanning,electroplating and paint pigment industries.Under overloading condition,Cr3+exhibits high toxicity and can bind DNA,affecting cellular structures and components[1].However,the ingestion of Cr(III)vitamin supplements,such as chromium(III)picolinate,has become common since Cr3+is a micronutrient essential of human and animal for the proper functioning of living organisms[2,3].The deficiency of Cr3+in the human body can increase the risk of diabetes and cardiovascular disease[4].
Many analytical methods have been developed for the detection of Cr3+ions,such as atomic absorption spectrometry[5],chromatography[6],inductively coupled plasma mass spectrometry[7,8],atomic fluorescence spectrometry[9,10],flow injection chemiluminescence[11,12],and electrochemical sensors[13],etc.However,most of these methods are time-consuming or highly-trained operators practical applications.Thus,it is important to develop sensitive and simple methods for detection of Cr3+ions.
Semiconductor quantum dots(QDs)based optical sensors have attracted great attention due to broad excitation spectra,component-tunable emission,excellent photostability,and flexibility in tuning the surface functionality[14–19].For metal ions,a large number of QD-based probes were reported based on direct and nonspecific interactions between QDs and metal ions.For example,calf-thymus DNA modified CdS,p-Sulfonatocalix[4]arene coated CdSe@ZnS,mercaptosuccinic acid-capped CdS QDs and calix[6]arene coated CdTe@SiO2were reported to be very specific and sensitive Hg2+optical sen-sors[20–27].Ding et al.utilized L-glutathione capped(GSH)ZnSe quantum dots(QDs)for ultrasensitive detection of Cu2+[28].Gan et al.developed a mercaptopropionic acid stabled CdTe quantum dots for the detection of Ag+[29].Many QDs were used to sense chromium ions.For example,Zhang et al.synthesized CdTe QDs using glutathione (GSH) as the stabilizer and the fluorescence intensity of the GSH-QDs could be selectively reduced by Cr6+[9,10].Zhao et al.developed a fluorescent method with denatured bovine serum albumin(dBSA)-capped Mn-doped ZnS QDs for Cr6+detection[30].The CdS QDs,synthesized by wet chemical method using pectin biopolymer as capping agent and taurine as an antioxidant, can be selectively quenched by Cr3+ions[31].The electrochemically synthesized TGA–CdSe quantum dots(QDs)can be quenched by Cr3+ions[32].The fluorescent carbon dots were prepared by a facile one-pot hydrothermal method using degrease cotton and can be used as an effective fluorescent probe for the detection of Cr6+ions[33].The fluorescent carbon dots-linked isophorone diisocyanate and beta-cyclodextrin(FCDs-IPDI-CD)can be used as an effective fluorescent probe for the detection of Cr6+ions[34].The graphene quantum dots(GQDs)are viable fluorescent probes for the determination of Cr6+and ascorbic acid in an on-off-on mode[35].The ammonium citrate derived carbon quantum dot(C-dots),synthesized via one-step hydrothermal approach,was developed as an effective on-off-on fluorescent probe for selective and sensitive detection of Cr6+and sulfites[36].Although these works demonstrated good analytical performance in detecting chromium ions,so far few works have been applied to Cr3+ions in vitamin supplements.
In this work,we prepared glutathione(GSH)capped CdTe QDs through a one-pot process.The GSH-QDs were found to be selectively quenched by Cr3+,Cu2+and Ag+,and the GSHQDs also can be used for the detection of Cr3+or chromium(III)picolinate(Cr(pic)3)by masking Cu2+and Ag+ions.
2.Material and methods
2.1.Reagents and apparatus
CdCl2,Na2TeO3,trisodium citrate and L-glutathione(GSH)were purchased from Sigma-Aldrich(Shanghai,China).Sodium borohydride(NaBH4)was from Aladdin reagent(Shanghai,China).All the metal salts were of analytical grade and purchased from Aladdin reagent(Shanghai,China).Ultra-pure water used throughout the experiments was prepared with a Milli-Q Pure system.The vitamin supplements samples used were acquired from local the chemist’s shop.
The fluorescence intensity spectra were recorded by a FluoroMax4 fluorescence spectrometer(Horiba JY,USA).The emission spectra were recorded in the wavelength range of 500–650nm upon excitation at 430nm.The FTIR spectrum was performed with a NEXUS FTIR spectrometer(Thermo Scientific,USA).The ultraviolet-visible(UV-vis)spectra were obtained by a UV-2802pcs spectrometer(Unico,China).
2.2.Preparation of GSH-capped CdTe QDs
The GSH capped CdTe(GSH-CdTe)QDs were prepared according to the reported method with slight modifications[37].Brie fly,H2O(85mL),CdCl2(8mL,0.04M)and trisodium citrate(1mL,0.68M)were added into a two-neck flask.Afterwards,glutathione(1mL,0.33M),Na2TeO3(4mL,0.01M)and NaBH4(1mL,2.64M)were added under vigorous stirring.After stirring at room temperature for 1 h, the mixture was heated at 110°C for 1h.Then the mixture was cooled to room temperature.The obtained QDs were precipitated by addition of ethyl alcohol(150mL).The precipitates were centrifuged at 8000 rpm for 15 min and dried under vacuum.The obtained QDs were dispersed in ultrapure water(4μM,calculated by the concentration of cadmium)and preserved at 4°C for in the dark.
2.3.Sensing of Cr3+ions
The GSH-QDs were diluted to 100nM in phosphate buffer(1.0mM,pH 7.0).Then 100μL of GSH-QDs and 100μL of Cr3+standard solutions(0–5000nM)were mixed thoroughly.After incubated at room temperature for 20min,the fluorescence spectra were recorded with excitation at 430nm.
2.4.Selectivity
In order to test the selectivity of the proposed method,various metal ions(Cu2+,Ag+,Cr3+,Ca2+,Mg2+,K+,Co2+,Fe3+,Sr2+,Al3+,Pb2+,Hg2+,Mn2+,Cd2+,Ni2+,Zn2+and Ba2+)were detected using the above method.The concentrations of all the above metal ions were 1.0μM.
2.5.Analysis of real samples
The vitamin tablets samples were pulverized.The obtained powder(0.5g)were mixed with 22.5mL methanol solution(25%,v/v)and 2.5mL sodium sulfide solution(1.0mM).The mixture was extracted under ultrosonic treatment for 5min.After centrifuged at 2000rpm for 5min,the supernatant was filter through disposable filter membrane(0.45μm).The filtrate was 50-fold diluted by phosphate buffer(10mM,pH 7.0),and then detected using the above method.
3.Results and discussion
3.1.Characterization of GSH-QDs
As shown in Fig.1,the GSH-QDs have a maximum absorption peak at 532nm and the fluorescent emission peak at 565nm with excitation wavelength at 430nm.The size of GSH capped CdTe QDs is about 3.3nm,which were calculated in virtue of the following empirical equation:D=(9.8127×10−7)λ3−(1.7147×10−3)λ2+1.0064λ−194.84[38].In the above equation,D(nm)is the diameter of a given QDs,and λ(nm)is the wavelength of the first excitonic absorption peak of the UV–vis absorption spectra.

Fig.1.UV–vis and fluorescence emission spectra of GSH-QDs.
As shown in the FTIR spectra(Fig.2),the peaks at about 3500–3300nm,3300nm,2600–2500nm,1700nm and 1600nm represented the stretching vibration of NH3,OH,SH,NH and CO groups.The characteristic absorption broad bands at about 3410–3253cm−1corresponded to the stretching vibrations of O-H and N-H.The sharp peak at 1398cm−1was from the bending vibration of C-NH.The high intensity of the peak at 1587cm−1was ascribed to the asymmetric stretching vibrations of the carboxylate anions.The disappearance of 2525cm−1,vibration of S-H group likely resulted from the complex formation between thiols and the Cd atom of the surface of CdTe QDs[37].The abundant hydrophilic groups presented on the surface can significantly improve the stability of GSH-QDs[39].

Fig.2.FTIR of GSH capped CdTe QDs and GSH.
3.2.Sensing of Cr3+ions
The GSH-QDs was found that Cu2+,Ag+and Cr3+ions can quench the fluorescence,but Cr6+ions can’t.Zhang et al.reported that the fluorescence intensity of the GSH-QDs could be selectively reduced by Cr6+while slightly quenched by Cr3+[9,10].The obvious different characters from the reported the GSH-QDs were mainly due to the different preparations of GSH-QDs since the GSH-QDs were prepared through a one-pot process here.
In order to obtain sensitive respond to Cr3+ions,the incubation time between Cr3+ions and the GSH-QDs were investigated first.As shown in Fig.3,the highest fluorescence quenching by Cr3+ions reached within 20min and kept stable for about 2h(F/F0,F and F0represent the fluorescence intensity in the presence and absence of metal ions).The minimum F/F0could be obtained when 100–400nM GSH-QDs were applied for Cr3+sensing(Fig.4).In order to develop a sensitive assay,20min incubation and 100nM GSH-QDs were set for the next experiments.
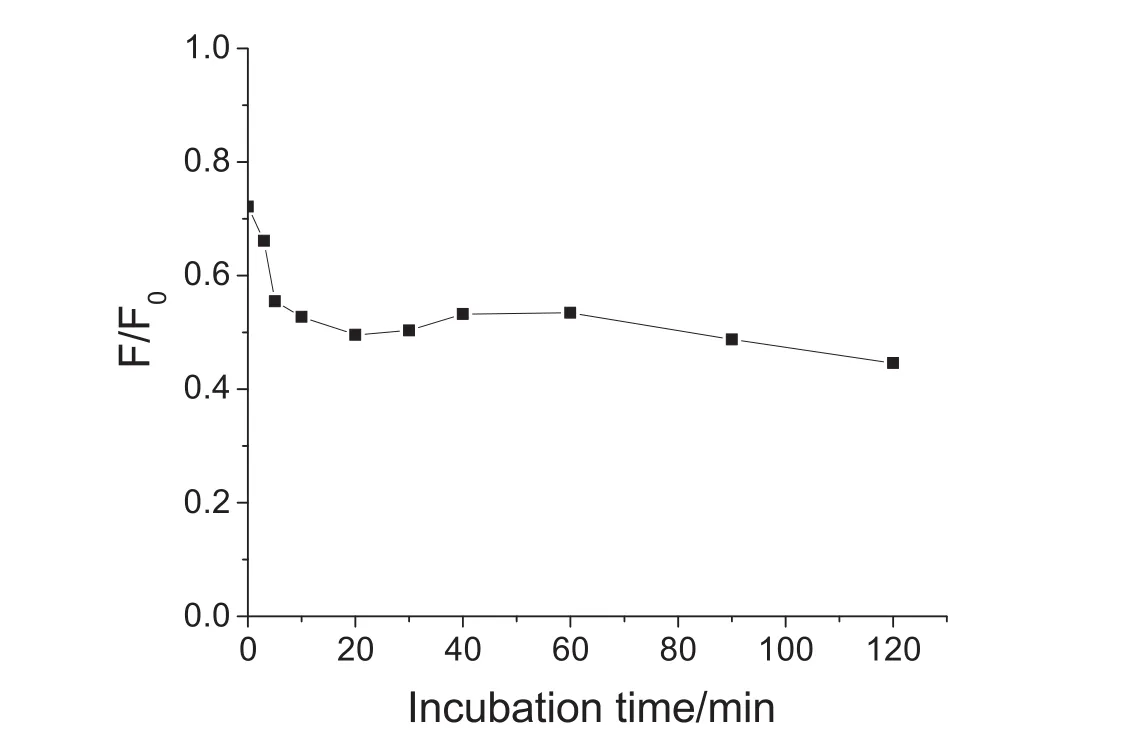
Fig.3.Effect of the incubation time of the GSH capped CdTe QDs and Cr3+ions.

Fig.4.Effect of the QDs concentration.
The The fluorescence spectra of GSH-QDs were recorded after the GSH-QDs were incubated with various concentrations of Cr3+ions.As shown in Fig.5,the fluorescence quenching by Cr3+ions could be described by a typical Stern-Volmer equation in the concentration range of 0–2000nM,respectively.The Stern-Volmer equation was as follow:F0/F=1+Ksv[C],C is the metal ion molar concentration,andKsvis Stern-Volmer constant.The deviations were observed at higher concentrations of Cr3+ions,which probably were attributed to the simultaneous existence of both static and dynamic quenching[32].The limits of detection calculated by 3σ/s(σ is the standard deviation of blank samples and s is the slope of linear curve)were 3.0nM.It should be pointed out that no obvious emission peak shifting was observed after incubated with Cr3+ions.This result suggested that the GSH layer still existed on the surface of QDs and the Cr3+-GSH complex formed[9,10].
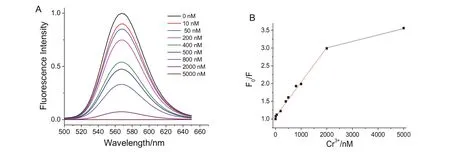
Fig.5.Fluorescence spectra of GSH-QDs in the presence of various concentrations of Cr3+(A)with respective analytical curve plotted as F0/F(B).
3.3.Selectivity
Various metal ions were tested on the fluorescence quenching of GSH-QDs.The concentrations of Cu2+,Ag+and Cr3+were 1.0μM and concentrations of Ca2+,Mg2+and K+ions were 10mM.The other metal ions were set at 10μM.As shown in Fig.6,all the other metal ions,except Cu2+,Cr3+and Ag+ions,have very weak effect on the fluorescence of GSH-QDs.The results demonstrated that the GSH-QDs could be applied for the sensing of Cu2+,Cr3+and Ag+ions.
In order to develop specific assay for Cr3+ions,Na2S(100μM)was selected to mask Cu2+and Ag+ions.As shown in Fig.7,Na2S can mask the fluorescence quenching by both Cu2+and Ag+ions(2.0μM).These results suggested that competition of thiol-containing ligands may exist among Cu2+,Ag+,Cd2+.It was reasonable since theKspvalues of Ag2S(Ksp=2×10−49)and CuS(Ksp=6×10−36)are much lower than that of CdS(Ksp=8×10−27),which lead to have stronger affinities of Cu2+and Ag+towards thiol group on the surface of GSH-QDs[40].The fluorescence quenching by Cr3+ions was mainly due to the coordination between GSH with Cr3+ions,probably leading to the inner filter effect of Cr3+ions[9,10].Interestingly,chromium(III)picolinate(Cr(pic)3)also can quench the fluorescence of GSH-QDs(data not shown),thus Cr(pic)3could be detected using this probe.
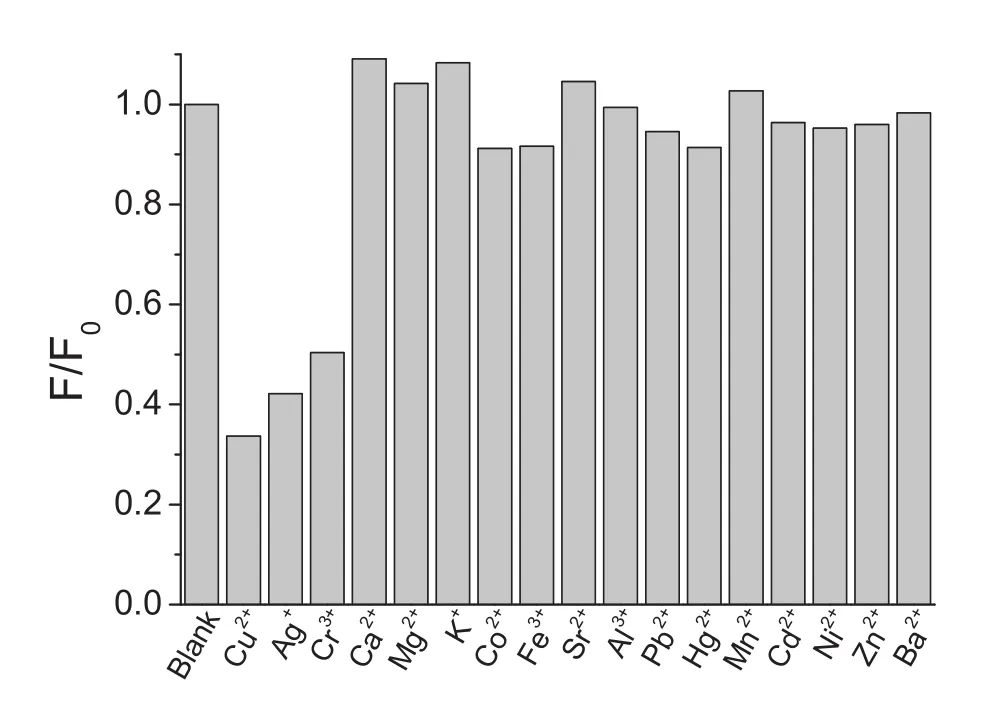
Fig.6.Selectivity of the fluorescence assay for various metal ions.

Fig.7.Effect of sulfide on the fluorescence quenching by metal ions.The metal ions(a–d)are Cu2+,Cr3+,Ag+and their mixture,respectively.All the concentrations of Cu2+,Cr3+and Ag+were 2.0μM.
This method was comparable with the most sensitive method and showed much wide linear range[30].The comparison with other fluorescence probes for Cr3+was listed in Table 1.The GSH-QDs can be quenched by Cr3+,Cu2+or Ag+,which also suggested that these QDs have the potential to be used to detect the three kinds of metal ions by carefully selecting masking regents[43,40].Meanwhile,it should be pointed out that the one-pot process preparation of the GSH-QDs was much more facile than the two-step process,which was commonly used in fluorescent CdTe QDs preparation.

Table 1Comparison of several fluorescence methods with this work.
3.4.Sample analysis
To demonstrate the practical application of the GSH-QDs,vitamin tables were spiked with 20,100 and 500μg/g Cr3+,respectively.After pretreated and diluted,the samples were detected using the above method.As shown in Table 2,the recoveries from 92.5% to 106% were obtained.The GSH-QDs were also applied in the determination of 3 tablets containing Cr(pic)3.The comparison the data of the proposed method to other data obtained by flame absorption atomic spectroscopy(FAAS)(Table 3)showed that all the differences were within5%.This result con firmed the high application potential of this fluorescent method in practice.
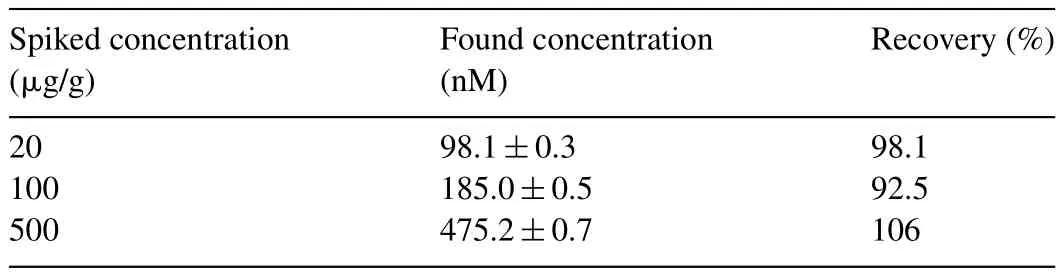
Table 2Recoveries of Cr3+in vitamin tablets.(n=3).
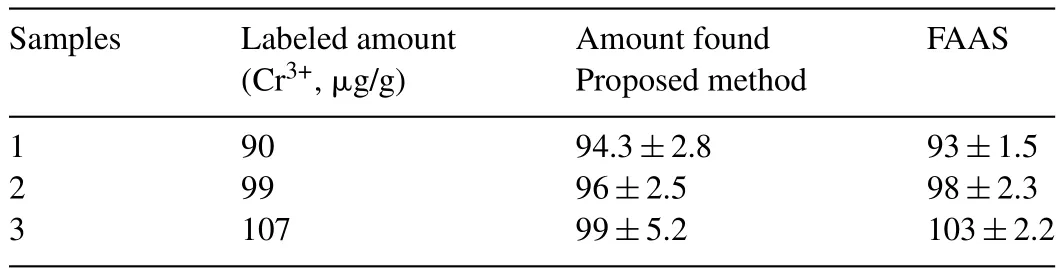
Table 3Comparison of the determination results between proposed and reference method.(n=3).
4.Conclusion
The present study demonstrated the potential application of GSH-QDs,synthesized through one-pot method, as a useful fluorescent probe for Cr3+ions.A sensitive assay for Cr3+ions was developed based on fluorescence quenching of QDs by the metal ions.The QDs were also a successful alternative for quantitative determination of chromium complex in vitamin supplements by masking Cu2+and Ag+ions.The proposed method showed to be easy-to-use,highly sensitive and selective.
Conflict of interest
All authors declare that they have no Conflict of interest.
Ethical approval
Informed consent:Not applicable.
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
The work was supported by the National Natural Science Foundation of China(31371767),the National S&T support program of China(2015BAD17B02),Jiangsu Entry-Exit Inspection and Quarantine Bureau China(2017KJ19),the National Engineering Research Center for Functional Food,Jiangnan University and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province,Jiangnan University.
杂志排行
食品科学与人类健康(英文)的其它文章
- Nutritional and medicinal characteristics of Chinese giant salamander(Andrias davidianus)for applications in healthcare industry by artificial cultivation:A review
- A critical review on phytochemical profile and health promoting effects of mung bean(Vigna radiata)
- Trichosanthes dioica Roxb.:A vegetable with diverse pharmacological properties
- Alterations of attention and impulsivity in the rat following a transgenerational decrease in dietary omega-3 fatty acids
- Optimization of a fermented pumpkin-based beverage to improve Lactobacillus mali survival and α-glucosidase inhibitory activity:A response surface methodology approach
- Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed
