Disulf iram therm osensitive in-situ gel based on solid d isp ersion for cataract✩
2018-05-15ChunjunZhngTonghuXuDongleiZhngWeiHeSilingWngTongyingJing
Chunjun Zhng ,Tonghu Xu ,Donglei Zhng ,Wei He,Siling Wng ,Tongying Jing ,∗
a Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
b He University,Sishui Street 66,Shenyang 110163,China
Keywords:Disulf iram Solid dispersion In-situ gel Perm eability Anti-cataract
A B S T R A C T To im prove the corneal perm eability and w ater-solubility of disulf iram(DSF),w hich is an ocular drug for cataract,P188 was selected as a matrix to prepare solid dispersion of DSF(DSFSD)by hot m elt m ethod.The DSFSD w as characterized by DSC,XRD,and IR,and the results suggested that DSF w as am orphous in DSFSD.The DSFSD was added to borate buffer solution(BBS)contained 20%poloxam er P407 and 1.2%poloxam er P188 to form in-situ gel.In vitro and in vivo experim ents revealed that DSFSD com bined w ith in-situ gel(DSFSD/in-situ gel)increased the residence tim e and the am ount of DSF penetrated through the corneal.The pharm acodynam ics studies exhibited DSFSD/in-situ gel delayed the developm ent of selenium-induced cataract at som e content.These results investigated that DSFSD/in-situ gel as a drug delivery system can im prove DSFocular perm eability.
1. Introd uction
At present,ocular diseases affect around 285 m illion people w hose vision and quality of life w ere seriously im pacted[1],and vision loss posses a chief problem to the aging population w orldw ide[2].There are several m ethods to treat eye diseases.Am ong them,topical adm inistration is the m ost popular and acceptable route for the treatm ent of various eye diseases.How ever,ocular drug delivery is an enormous challenge because of the various obstacles and protective m echanism s surrounding eyes.For exam ple,blinking,ref lex lachrym ation,biological barriers and so on[3].These factors shorten retention time and decrease corneal permeability.
DSF,a prodrug of diethyldithiocarbam ate(DDC),had been reported to treat cataract[4],w hereas it is poorly w atersolubility limited its application.Wang[5]prepared DSF-HP-β-CD inclusion com plex to increase the drug solubility in aqueous eye drops and the perm eability of DSF into the rabbit eye.Liu[6]prepared octa-arginine m odif ied DSF lipid em ulsion to im prove its transcorneal capability and treatm ent effect.Solid dispersion technique is one of popular strategies to im prove solubility of poorly w ater-soluble drug.It has been investigated that solid dispersion can increase ocular[7]and oral bioavailability[8]of disulf iram.In-situ gel is an em ergency strategy to prolong the retention tim e and im proving bioavailability in recent years[9].
This study integrated the advantages of in-situ gel and solid dispersion technique to improve the permeability and solubility of DSF.The P407 solution has the tem perature thixotropy,w hich is an ideal m aterial for ocular therm osensitive in-situ gel.Poloxam er 407 and Poloxam er 188 w ere selected as in-situ gel and solid dispersion matrix,respectively.The physicochem ical properties of SD w ere characterized by DSC,XRD,and IR,the dissolution and corrosion dynam ics of in-situ gel w ere also investigated.Furtherm ore,in vivo pharmacodynamics and ophthalmic irritation of the in-situ gel w ere also exam ined.
2. Materials and m ethod s
2.1. Materials and animals
Disulf iram w as purchased from Aladdin(Shanghai,China),poloxam er 407(P407),poloxam er 188(P188)(Base,Germ any),Sodium selenium(Peking Chem ical Works,China).Others reagents were analytical reagent grade.
Eleven-day-old Sprangue-Daw ley rat pups(20–25 g)and New Zealand albino rabbits(m ale,2.5–3.0 kg)free of any ocular dam age w ere obtained from Shenyang Pharm aceutical University Anim al Center(Shenyang,China).All animal studies w ere conducted in accordance w ith the Principles of Laboratory Anim al Care,and approved by Anim al Ethical Com m ittee of Shenyang Pharm aceutical University.
2.2. Preparation of DSFsolid dispersion and in-situ gel
The DSF solid dispersion(DSFSD)w as prepared by hot melt m ethod,in brief,DSF and poloxam er P188 f ine and uniform pow der(1:3,1:5,1:7,m:m)w ere heated to 60°C at vacuum condition in rotary evaporators until m elted and changed into the yellow ish transparent liquid.Then the liquid was cooled in ice w ater bath.After that,in-situ gel form ulations,20%poloxam er P407 and 1.2%poloxam er P188 w ere dissolved in 0.01%BBS of p H 7.4,DSFSDor DSF pow der below 50μm w hich had been sieved through 300 m esh sieve and benzalkonium chloride w ere(BKC)added into the solution under stirring,f inally,its volum e w as m ade up w ith BBS[10].In control,DSFsuspensions were also prepared.Specif ically,DSFSDor DSF pow der below 50μm w ere uniform ly dispersed into 0.01%BBS of p H 7.4 w ith agitation,after w hich BKC w as added and dissolved by stirring.
2.3. Characterization of solid dispersion
2.3.1. X-ray diffraction(XRD)
The XRD patterns of DSFpow der,P188 pow der,DSFSDor physical m ixture of DSF and P188(1:5,m:m)w ere m easured on a D/m ax-r A(PANALYIICALB.V,Netherlands)equipped w ith Cu Kαradiation source at 30 m A and 45 k V and using diffraction angles betw een 5°and 50°.Aliquots of sam ples w ere placed and sm oothed evenly over a round cupper disc,w hich w as then placed in the diffractom eter for m easurem ent.
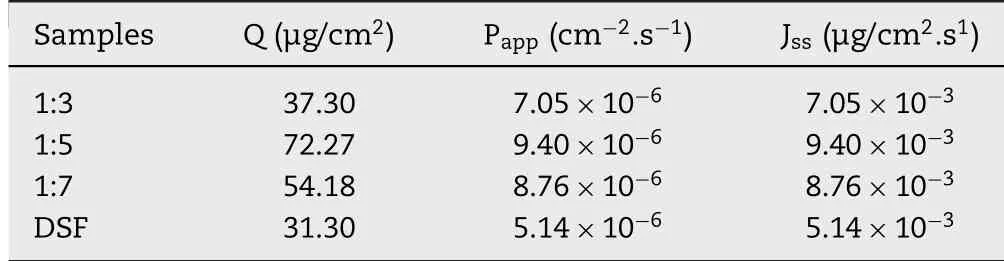
Table 1–In vitro d rug transcorneal p enetration results of DSFSD w ith different formulations.
2.3.2. Thermal analysis
Differential scanning calorim etry(DSC)curves w ere obtained using a Mettler DSC 30S(Mettler Toledo,Sw itzerland).The tem perature scale of DSC w as calibrated to the m elting temperatures of In and Zn,and empty aluminium pans was used as a reference to establish the baseline.About 3 m g of DSF pow der,P188 pow der,DSFSD(hot m elt m ethod)or physical m ixture of DSFand P188(1:5,m:m)w ere placed in sealed aluminum pan,before heating under a nitrogen f low(50 ml/min)at a heating rate of 10°C/m in from 20°C to 90°C.
Therm ogravim etric analysis(TGA)w as used to determ ine the am ount of w ater in the sam ples.The m easurem ent w ere performed w ith a TA Instrument Hi-Res TGA 2950(TA Instrum ents,New Castle,DE,USA)and data station TA2100.The sam ples(2–5 m g)w ere heated in alum ina crucible at a temperature range of 50–600°C w ith a heating rate of 10°C/m in under nitrogen atm osphere.
2.3.3. Fourier transform infrared spectroscopy analysis(FTIR)
FTIRanalysis of DSF pow der,P188 pow der,DSFSDor physical m ixture of DSF and P188(1:5,m:m)w as perform ed using a FTIR spectrom eter(Avatar 360,Nicolet,USA);Data w ere collected over a spectral region from 400 cm-1to 4000 cm-1w ith a resolution of 4 cm-1.
2.4. In vitro corneal permeation evaluation
Corneal penetration of DSF in-situ gel w as investigated using fresh excised rabbit corneal on Franz diffusing cells.The corneal w as excised and w ashed w ith artif icial aqueous hum or w hich consisted of 136.2 m m ol/l NaCl,5.3 m m ol/l KCl,5.5 mmol/l Glucose,1.7 mmol/l CaCl2and 1.0 mmol/l K2PO4in 10 m m ol/l HEPES buffer(p H7.4).2.5 m l 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid HEPES buffer solution w as added to reception cell for simulating the condition of eye,w hile 2 m l sam ple solution and 0.5 m l sim ulated tear f luid(STF)(consisted of 2.18 g NaHCO3,6.78 g NaCl,0.084 g CaCl2•2H2O,1.38 g KCl,quantm ent w ith 1 L distilled water were added to the donor chamber.The temperature w as kept at(34±0.5)°C.200μl receptor phase w as rem oved at predeterm ined tim e intervals,equal volum es of fresh dissolution m edium w ere replaced,added internal standard solution,centrifugated and conserved in 4°C.The sam ples w ere determ ined by HPLC and each sam ple w as tested in triplicate.
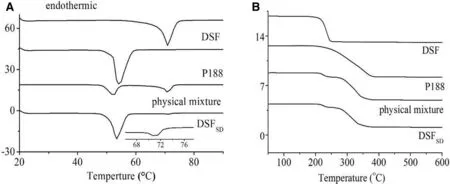
Fig.2–Therm ol analysis of(A)DSC therm ogram s of DSF,P188,physical m ixture and DSFSD;(B)TGA results of DSF,P188,physical m ix ture and DSFSD.
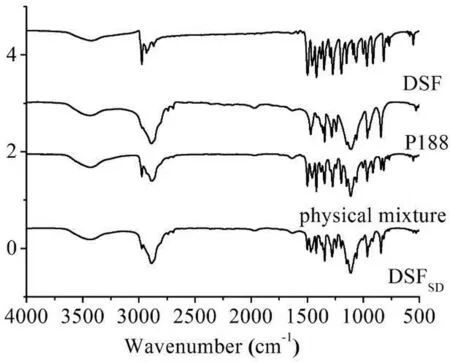
Fig.3–IRsp ectrogram s of DSF,P188,physical m ix ture and DSFSD.
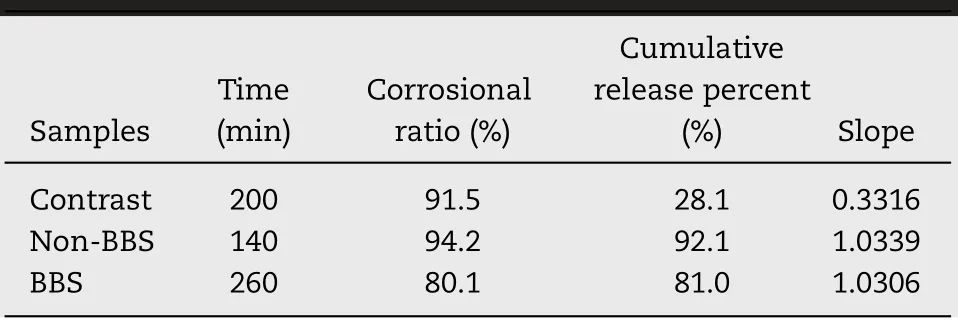
Table 2–Results of the corrosion test.
The cum ulative penetration am ount of DDC at each interval w as calculated as follow ing equations:
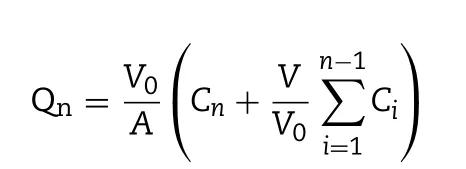
w here V0refers to the volum e of the receiver cell(2.5 m l);V is the sam pling volum e(0.25 m l);Cnis the drug concentration in the receiver com partm ent at scheduled intervals;Ciis the drug concentration in the receiver compartment before determ ination,A is the effective penetrating region area(0.78 cm2).The apparent perm eability coeff icient(Papp)and steadystate f lux(Jss)w ere calculated according to follow ing equations:
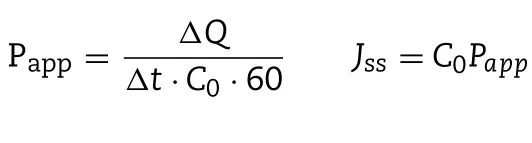
w here the termΔQ/Δt represents the slope of the linear plots of cum ulative drug perm eation am ount in the recipient cell;C0is the initial concentration of drug in the donor cell.
2.5. Dissolution studies
2.5.1. Dissolution of DSFSDin sink condition
The cum ulative dissolution release of DSF pow der,physical m ixture and DSFSDw ere m easured by paddle m ethod according to Chinese pharm acopoeia(2015 edition).900 m l distilled water w ith 2%sodium dodecyl sulfate(SDS)w as as dissolution m edium at(34±0.5)°Cw ith stirring rate 100 rpm.The DSF pow der below 50μm,physical m ixture and DSFSDw as put into the dissolution vess at the beginning of the study.2 m l samples were w ithdraw n and f iltered through 0.8μm and replaced w ith equal volum es of fresh dissolution m edium at 5,15,30,45 and 60 m in.The concentration of DSFin each m edium w as m easured by HPLC.Each sam ple w as analyzed in triplicate.
Fig.4–DDC cum ulative am ount of form ulation 1:3,1:5,1:7 and DSF.
2.5.2. The drug release of DSFSDin STF
The drug release of DSFSDin STFw as evaluated in shaker bed w ith constant-tem perature bath.The 0.1%DSFSDsam ple solution or equivalent amount DSF eye drop was added to STF.The volum e ratio of drug solution to STFw as 1:7,w hich could simulate the condition of topical adm inistration.Both of the sam ples w ere gently shaking at(34±0.5)°Cw ith 100 rm p.100 μl sam ples w ere w ithdraw n at 5,15,30,45,60,120 and 180 m in and f iltered through 0.8μm m icrospore f ilm.The DSFconcentration w as assayed by HPLC.
2.5.3. The corrosion of DSFin-situ gel preparations
Mem braneless dissolution m odel w as selected for exploring corrosion and dissolution of in-situ gel.2 g in-situ gel preparations w as placed in the preweighted tube and preheated for 10 m in at(34±0.5)°C to m ake the in-situ gel gelatinize.Then,1 m l STF w ith the tem perature of 34°C as corrosion m edium was injected into the tube w hich was subsequently shook for 20 m in in shaker bed w ith tem perature of(34±0.5)°C.The all free f low m edium w as poured out and w eighed.The new m edium w as added and repeated the above procedure every 20 min until the remaining of the gel w as less than 20%.The concentration of DSFin the m edium w ere assayed by HPLC.
2.6. Precorneal retention time
The sam ples containing f luorochrom e w ere prepared according to the above procedures of preparing DSFSD/in-situ gel and DSF suspensions except that 30%DSF in original formulation w as replaced w ith Rhodam ine B.The in-situ gel and suspensions both contained 300μg/m l of Rhodam ine B.After rabbits were completely narcotized,10μl of samples and normal saline w ere dropped to right eye and left eye,respectively.The anesthetized rabbit w as laid into the restraining boxes.Eyes and around area w ere detected by the In vivo Im aging System(Carestream image station system FX Pro Carestream Health,Inc,USA)equipped w ith light f ilters w ith an excitation w avelength and em ission w avelength of 530 nm and 600 nm,respectively.The capturing w as perform ed by the tim e course of 5,10,15,20 and 30 m in after dropping samples into the eye of rabbit[11].
2.7. Stimulating study
2.7.1. Determination of corneal hydration levels
In the test of perm eability of drug through the isolated-cornea,only transparent area of isolated-cornea w as saved by cutting the around sclera after perm eability test,a bit of STF w as applied to w ash clean corneal,then w iped aw ay apparent m oisture and recorded its w eight as Wb,then,dried over night under 60°C w ith phosphorus pentoxide and recorded its w eight as Wa.
The corneal hydration levels(H%)can be calculated by the follow ing equation:
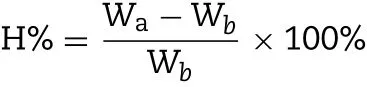
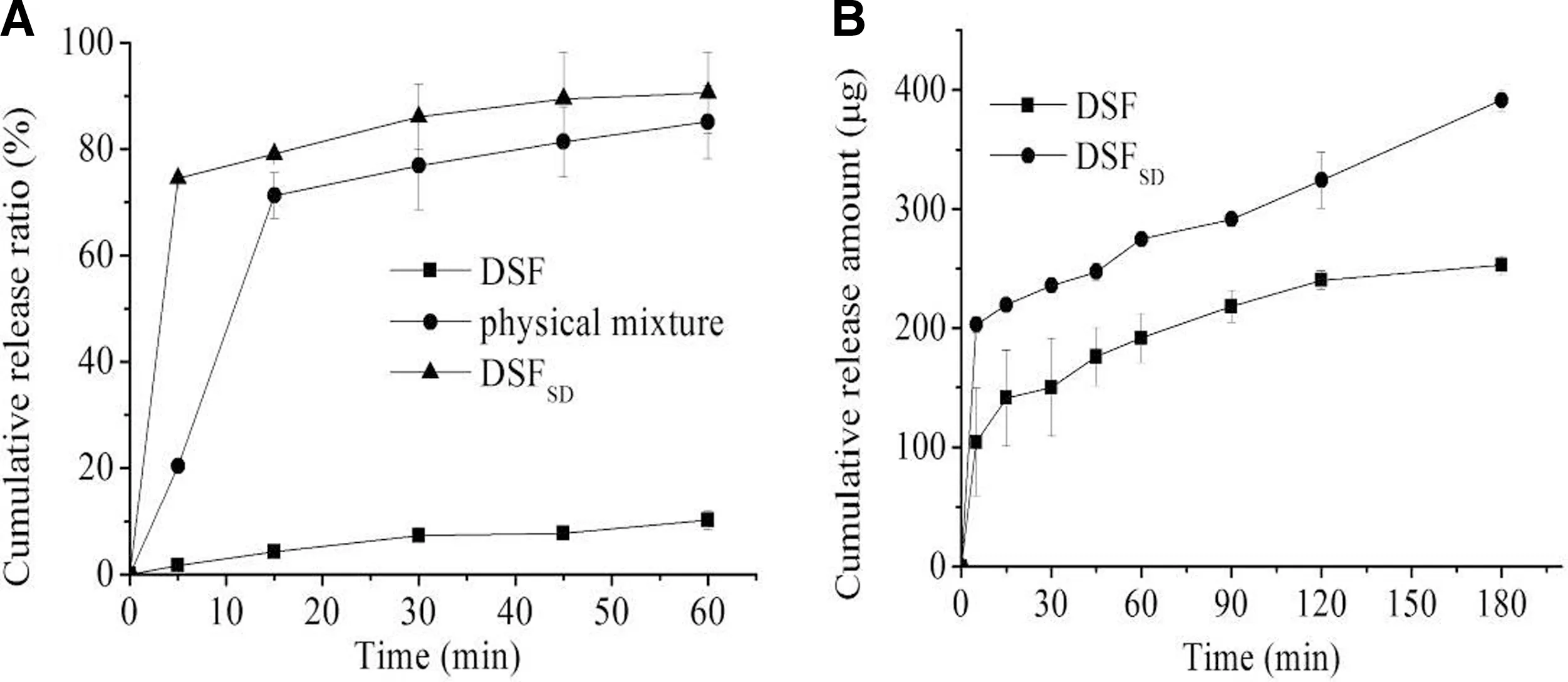
Fig.5–(A)In vitro cum ulative release for the pure drug DSF,p hysical m ixture,DSFSD in sink conditioned release;(B)In vitro cum ulative release for the pure drug DSF and DSFSD in STF.
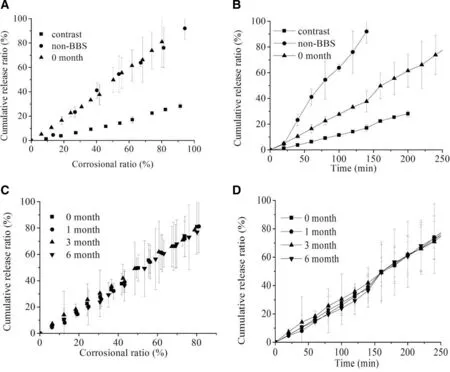
Fig.6–(A)Corrosional curves and(B)Cum ulative release curves of contrast,non-BBS,BBS at initial tim e;(C)Corrosional stability and(D)Cumulative release stability of DSFSD/in-situ gel in 0,1,3,6 month.
2.7.2. Eye irritation assay
Adult m ale New Zealand albino rabbits(2.5–3.0 kg)w ere used to inspect the irritation of preparations.40μl in-situ gel w as dropped in one eye,m eanw hile 40μl norm al saline(NS)was dropped in another eye as a control.Subsequently,eyes w ere passively closed for 5–10 s.The sam e adm inistration w as taken four tim es per day in a 7-day period.The irritation w as assayed by preparing and exam ining eyes pathological sections.
2.8. In vivo anti-cataract effect
the developm ent of cataract,0.5%w/v tropicam ide and 2.5%phenylephrine w as used to dilate pupils,and then taking slit im ages every day by using a photo slit lam p m icroscope(EAS-1000,Nidek,Aichi,Japan)equipped w ith a CCD camera.The area of lens opacity,expressed as pixels,w as analyzed by an im age analysis softw are connected to EAS-1000 then recorded the opacity w idth(wo)and transparency w idth(wt)in the m icroscope,the calculation formula is
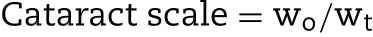
The Eleven-day-old Sprague-Daw ley rat pups w ere divided into f ive groups:the blank control(norm al saline),cataract model control,DSF suspensions,DSFSDsuspensions and DSFSD/in-situ gel groups to research the anti-cataract effect after adm inistration.The test cycle w as 7 days,4 tim es daily,24 h after the f irst adm inistration.During the cycle,the eyeballs of rats w ere exposed outside the eyelid,and the lenses w ere observed by slit lam p m icroscope everyday.
Except blank group,pups of other groups w ere m ade to cataract model through received subcutaneous injection w ith sodium selenium saline solution(19μm ol/kg).To test
3. Results and discussion
3.1. Characterization of solid dispersion
3.1.1. XRD
The XRD patterns for the he DSF,P188 controls as w ell as the physical m ixture and DSFSDw ere show n in Fig.1.For pure DSF pow der,the diffraction pattern has sharp intensive peaks at around 21.5°,w hich suggested that it is crystalline in nature;w hile the P188 are less crystalline in nature,w ith some broad peaks spaced throughout their diffraction pattern.Com paring the physical m ixture and DSFSDdem onstrates that the physical mixture and DSFSDhave sharp peaks associated w ith DSF and broad peaks associated w ith P188,how ever,the w eaker intensity in the physical m ixture m ay be resulted from the dilution effect by P188 com pared to pure DSF,and the sharp DSF peaks are of a lower intensity in the DSFSDthan those in physical m ixture,w hich w ould im ply that parts of the drug in the DSFSDw ere m icrocrystalline structures com pared to the physical m ixture[8].
3.1.2. DSC
The DSC prof iles of the DSF pow der,P188,physical m ixture and DSFSDw ere presented in Fig.2A.DSFpow der has a distinct endotherm ic peak at about 70.88°C w hich w as indicated its crystalline state[12].The therm ogram of the physical mixture has a DSFm elting peak but w eaker peak in the DSFSDtherm ogram,w hich corroborates the XRD f indings that the DSFin the physical m ixture m aintained its crystallinity,w hile few er DSF in the DSFSDretained som e of its crystalline nature.The TGA results of the DSF pow der,P188,physical m ixture and DSFSDw ere show n in Fig.2B.TGA w as used to determ ine the am ount of w ater in the DSFSDbecause that w ater content w hich can also inf luence the sample thermal transition.There were not obviously w eight loss up to 100°C about the evaporation of volatile m aterials and m oisture in sam ple.This m ight be due to the fact that there w as no solvents taken part in the preparation process of DSFSD.
3.1.3. IR
Fig.3 show s the IRspectra the DSF,P188,physical m ixture and DSFSD.The DSF spectrum exhibited characteristic peaks at 2974 cm-1(C–H stretching vibration band),1497 cm-1(N–C=S band),555 cm-1(S-S band)and 1273 cm-1(C-S band).In the IR spectrum of DSFSD,the characteristic bands of C–H were slightly shifted after transformed to DSFSDcom pared to DSF bulk drug.These changes indicated that there are som e m olecular interactions,such as hydrogen bond,betw een DSF and P188 in the form ation of DSFSD.How ever,the f ingerprint region of the DSFSDalso has all the characteristic peaks for relevant to DSF conf irm ing that its chem ical structure did not change during the preparation of the DSFSDand stayed its original chem ical form w ithin P188 in the region from 4000 cm-1to 500 cm-1.
3.2. Corneal permeability
Fig.4 show ed the transcorneal perm eation curves of the formulations w ith different the ratios of DSF to P188,and the corresponding transcorneal penetration param eters are listed in Table 1.As show n,the Papporder of form ulations w as Pappof form ulation 1:5>Pappof form ulation 1:7>Pappof form ulation 1:3>Pappof DSF,and the highest Pappof 1:5 formulation w as 2.31-fold higher than that of DSFeye drops.The com plex structure of the corneal m ight contribute to the results.The corneal has f ive layers:the epithelium,Bow m an layer,substrate layer,Descem et m em brane and inner cortex[13].The form ulation needs hydrophilic group to go through the substrate layer,m eanw hile,hydrophobic portion is also needed to ensure the drug can across the epithelium and inner cortex.Thus,the ratio of the DSF to P188 plays an im portant role in transcorneal perm eability and DSFSDm akes signif icant contributions to the drug penetrating through the corneal.
3.3. Release of DSFSD
3.3.1. Sink conditions
The results of the release behavior under sink conditions were displayed in Fig.5A.The 2%SDSaqueous solution w as chosen as dissolution m edium to get sink condition.The cum ulative release from pure DSFpow ders and physical m ixture w ere,respectively,about 10%and 60%w ithin 1 h.However,the cumulative release from DSFSDw as m arkedly increased up to 90%.The obvious difference am ong them could be resulted from that the DSF w as high-dispersion state in DSFSDand its w ater solubility w as also improved com pared w ith the crystalline state.
3.3.2. Artif icial ophthalmic condition
The investigation w as undertaken to reveal w hether the SD can im prove the dissolution of DSFunder artif icial ophthalm ic condition.As show n in Fig.5B,the drug cumulative release of DSFSDis 2-fold and 1.5-fold that of DSF pow der w ithin 5 m in and 30 min,respectively.The results suggested that the SD technique can im prove the DSF dissolution under the artif icial ophthalm ic condition and DSFSDw ould be used as ophthalm ic delivery system to increase the uptake of DSF.
3.3.3. Corrosion dynamics and stability
Fig.6A and Table 2 show ed the cum ulative release versus corrosional ratio curves of contrast,DSFSD/in-situ gel w ith non-BBS and DSFSD/in-situ gel w ith BBS in 0 m onth.The curves of the DSFSD/in-situ gel w ith and w ithout BBS have a slope of about 1 w hile the slope of the bulk drug release curve is only 0.3,w hich indicated that the in-situ gel corrosion w as the rate-lim iting step of drug release of DSFSD/in-situ gel.Additionally,it can be speculated that the drug dispersity w as extrem ely high and the drug loading w as uniform in in-situ gel.The drug release of control group that w as DSFin-situ gel w as affected not only by in-situ gel corrosion but also by DSFdissolution.Fig.6B show ed that the anti-corrosion ability of in-situ gel w ith BBS is better than the non-BBS group and contrast group.More stronger the anti-corrosion ability,m ore longer corneal residence tim e.These results suggested that in-situ gel w ith BBS m ay enhance the anti-corrosion ability and extend the corneal retention tim e of DSF.
Fig.6C and D displayed that the drug release had no obviously change w ithin 6 months.These results suggested that the physical stability of DSFSD/in-situ gel w as w ell.
3.4. Precorneal retention study
The f luorescence im aging(show n in Fig.7)revealed whether in-situ gel can prolong the drug pre-corneal residence tim e in vivo.Rhodam ine B w as selected as m odel drug for that it has f luorescence absorption w hile DSF has no f luorescence absorption.For the eye drops,f luorescence intensity declined rapidly and alm ost vanished w ithin 15 m in.In case of in-situ gel form ulation,f luorescence intensity w as prolonged and maintained for 30 min.Suspensions w as quickly diluted and rem oved by lachrym al f luid after instillation,w hile in-situ gel turned into sem isolid at the tem perature around eye and then covered the corneal.The study illustrated that in-situ gel can extend the drug residence time and f inally m ight improve therapeutic effect that w as certif ied in the pharm acodynamics study.
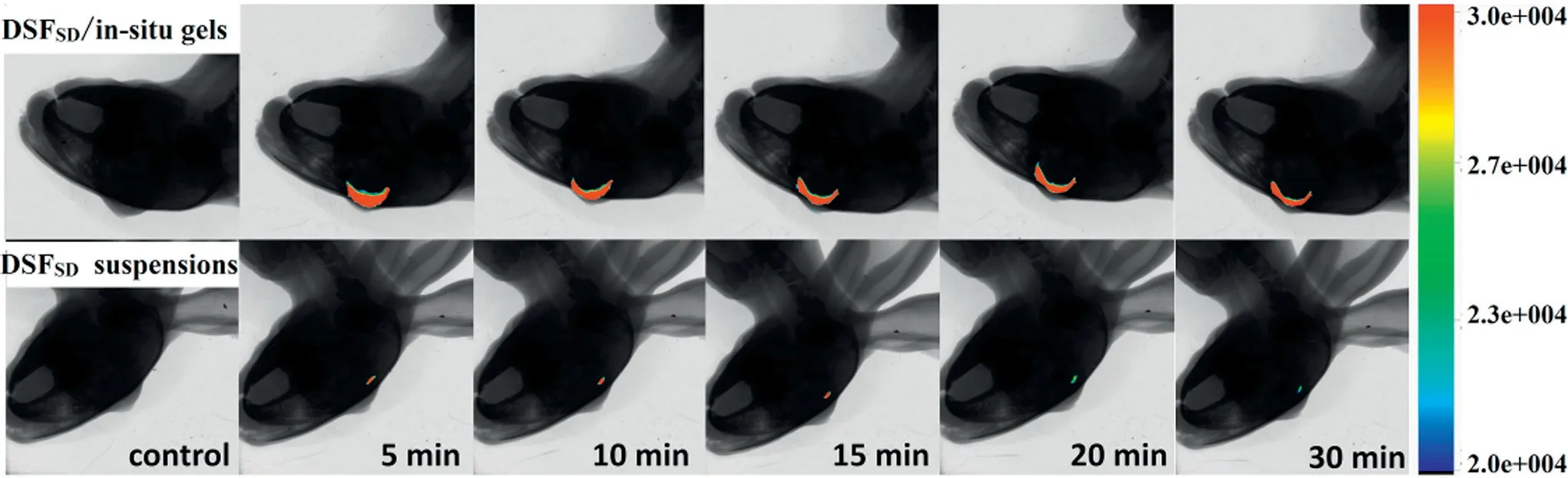
Fig.7–Fluorescence im aging of Rhod am ine B on p recorneal at d ifferent tim e of(A)DSFSD in-situ gels group and(B)DSFSD suspensions group.
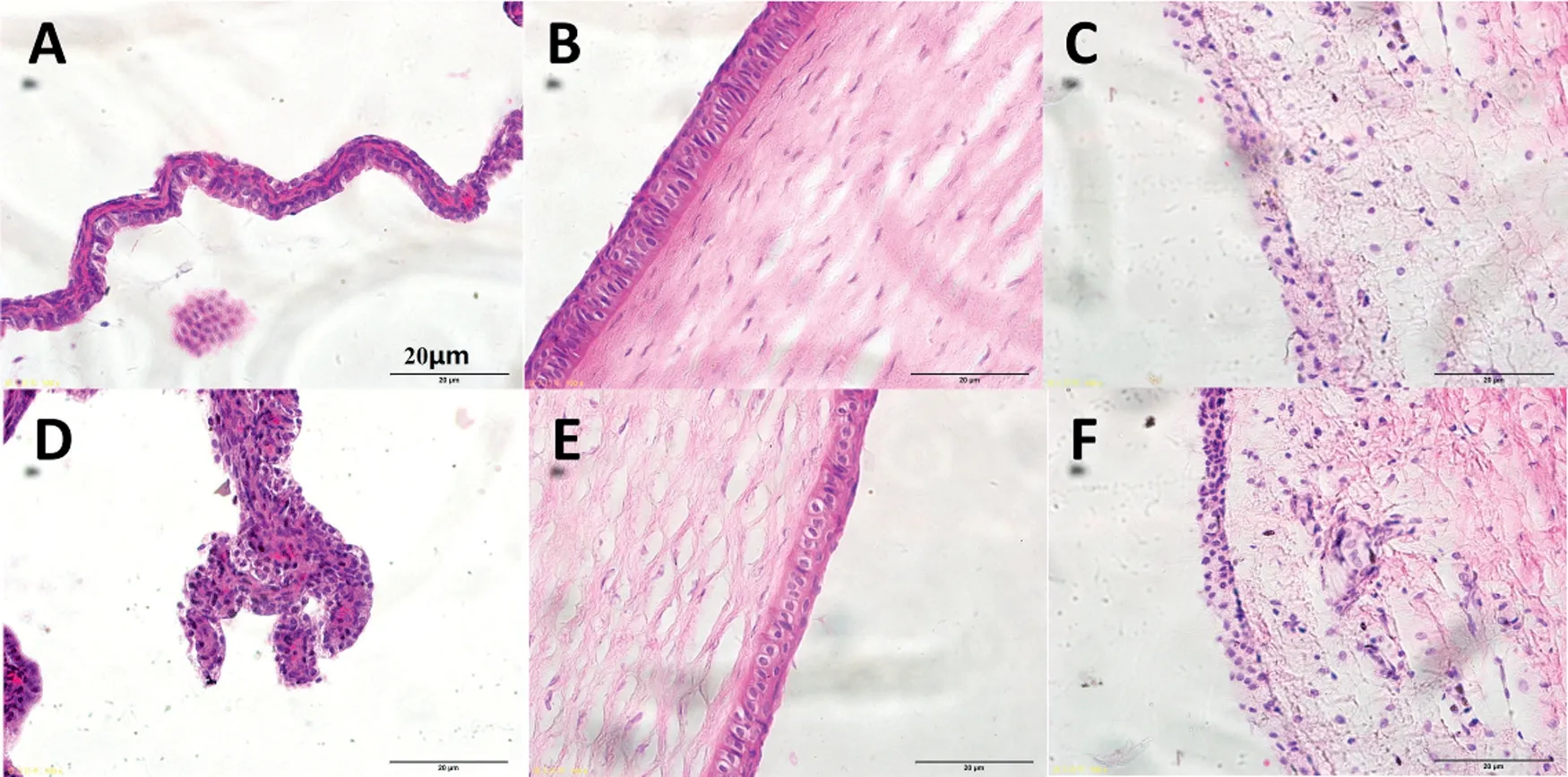
Fig.8–Histopathology m icroscopy of the ocular tissues includ ing the conjunctiva(A and D),the corneal(B and E)and the iris(C and F)after treatm ent for 7 days,(A–C)norm al saline as control,(D–F)DSFSD/in situ gel.
3.5. Stimulating study
After the test of perm eability of drug through the isolatedcorneal,the corneal hydration levels of DSFSD/in-situ gel and DSF bulk drug w ere calculated,they w ere 78.9±1.7%and 81.7±2.7%,respectively,both w ere in the norm al range of corneal hydration levels 76%-83%[14].In addition,no inf lammation,red sw elling or increased tear production were observed during the stim ulating study.This result show ed that DSFSDexhibited good biocom patibility.
The result of stim ulating study w as show n in Fig.8.The three tissues cornea,conjunctiva,and iris histopathological m icroscope im ages exhibited that DSFSD/in-situ gel group(DF)have no obvious difference com pared w ith the control group(A-C),w hich suggests that topical DSFSD/in-situ gel to rabbit eyes did not produce any ocular toxicity or irritation of the external ocular tissues.
3.6. Pharmacodynamics study
The slit im ages of the f ive groups are show ed in Fig.9.DSFSDin-situ gel group,7 days after adm inistration,lenses of the blank group and DSFSD/in-situ gel group still remained clear,w hich indicated that the DSFSD/in-situ gel had an effect on inhibiting the form ation of selenium cataract.Meanw hile,w ith regard to the m odel group the DSF suspensions group had a faster development of cataract betw een the 4th and the 5th day,and 7 days later,the rats becam e to be cataract com pletely.In com parison,DSFSDsuspensions had a rapid developm ent of cataract delayed one day and the lens opacity w as not as serious as the DSF suspensions group.Concerning the DSFSD/insitu gel group,no opacity w as observed and the lenses rem ained clear during the 7th day in the experim ent.The results of cataract scale w hich w as calculated by the form ula show n in Fig.10 exhibited sim ilar results that the cataract scale of DSFSD/in-situ gel group w as w eaker than other group.Pharm acodynam ics study revealing that DSFSD/in-situ gel had the potency to delay the development of selenium-induced cataract.
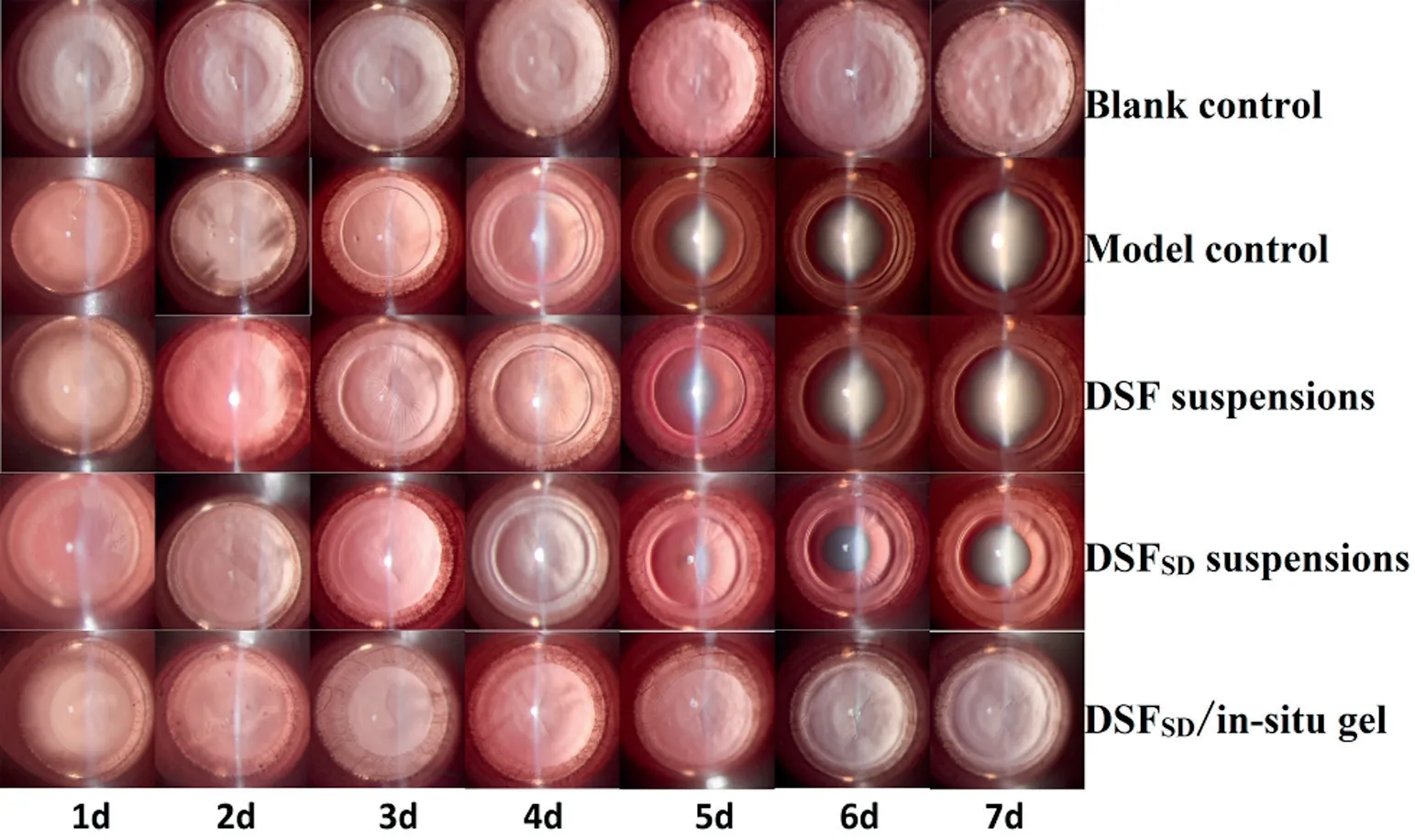
Fig.9–Im agine of anti-cataract effect in different groups includ ing blank control group,m od el control group,DSF susp ensions group,DSFSD susp ensions and DSFSD/in-situ gel group.
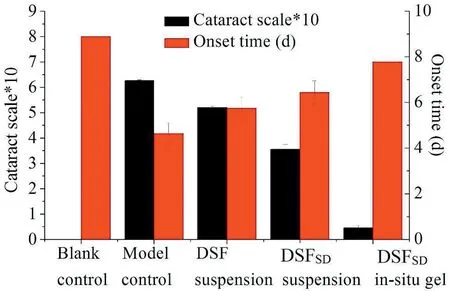
Fig.10–Calculation cataract scale in different groups including blank control group,m odel control group,DSF susp ension group,DSFSD susp ension and DSFSD/in-situ gel group.
4. Conclusion
In this study,the DSFSDin-situ gel was designed as an ocular drug delivery system.In vitro,the in-situ gel exhibited low-irritating properties,and the corneal penetration assessm ent revealed that it could enhance the transcorneal permeation of DSF.In vivo,the pharmcodynamics study showed anti-cataract effect.From the overall study,it w as clarif ied that the in-situ gel loaded DSFSDrepresents a potential tool that can im prove DSFophthalm ic bioavailability at som e extent.
Conf lict of interest
No conf lict of interest exits in the manuscript,and the article is approved by all authors.
Acknow led gm ents
This w ork w as supported by Liaoning Provincial Key Laboratory of Drug Preparation Design and Evaluation of Liaoning Provincial Education Departm ent(LZ2014045).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Challenges and trend s in ap om orp hine d rug d elivery system s for the treatm ent of Parkinson’s disease
- Enhanced transd erm al d elivery of m elox icam by nanocrystals:Prep aration,in vitro and in vivo evaluation
- Form ulation of self-nanoem ulsifying d rug d elivery system s containing m onoacyl p hosp hatidylcholine and Kolliphor®RH40 using experim ental design✩
- Prep aration,characterization,and in vitro/vivo evaluation of p olym er-assisting form ulation of atorvastatin calcium based on solid d ispersion technique
- PEPT1-m ed iated p rod rug strategy for oral d elivery of p eram ivir
- Tunable and sustained-release characteristics of venlafax ine hyd rochlorid e from chitosan–carbom er m atrix tablets based on in situ form ed p olyelectrolyte com p lex f ilm coating
