Enhanced transd erm al d elivery of m elox icam by nanocrystals:Prep aration,in vitro and in vivo evaluation
2018-05-15QinYuXiyingWuQungngZhuWeiWuZhongjinChenYeLiYiLu
Qin Yu,Xiying Wu,Qungng Zhu,Wei Wu,b,Zhongjin Chen,∗,Ye Li,Yi Lu,b,∗∗∗
a Shanghai Dermatology Hospital,Shanghai200443,China
b Department of Pharmaceutics,School of Pharmacy,Fudan University,Shanghai 201203,China
c Shaanxi Academy of Traditional Chinese Medicine,Xi’an 710003,China
Keywords:Meloxicam Nanocrystals Transderm al delivery Nanoprecipitation Acid-base neutralization
A B S T R A C T
1. Introd uction
Meloxicam (MLX),a highly potent non-steroidal antiinf lam m atory drug(NSAID),can inhibit cyclooxygenase activity and block the synthesis of prostaglandins.In clinic,MLX is eff icient in relieving pain and inf lammatory sym ptom s in joint diseases such as rheum atoid arthritis and osteoarthritis[1].Due to the low toxicity,MLX is a good alternative for patients that cannot tolerate other NSAIDs.Nonetheless,the poor solubility and gastrointestinal side effects,including bellyache,dyspepsia,ulceration and bleeding,greatly lim it the clinical application of MLX[2].Transderm al delivery provides an optimal route for MLX to bypass the gastrointestinal adverse effects.In addition,MLX displays distinctive characteristics suitable for transderm al delivery,such as low dose,relatively low m olecular w eight(354.1),moderate lipophilicity(logP=1.91 at p H 5.0),and excellent derm al tolerability[3].How ever,the poor w ater-solubility of MLX presents as a hurdle to eff icient transderm al delivery.Various approaches em ploying penetration enhancers[4],lipid vesicles[5]and microemulsions[6]have been adopted to im prove the transderm al delivery of MLX.
Nanocrystals are drug crystals w ith a size generally less than 1μm[7].Size reduction to nanom eter range signif icantly increases the apparent solubility and dissolution rates due to the enorm ously increased specif ic surface.Therefore,nanocrystals w ere initially invented to im prove the oral bioavailability of poorly soluble drugs[8–10].Recently,nanocrystals found new applications in transdermal drug delivery[11–13].The f irst successful m arketing of a rutin nanocrystal product(Juvedical®)in cosm etic m arket greatly encourages research and developm ent of pharm aceutical transderm al products[10].The proposed m echanism s for enhanced transderm al perm eation by nanocrystals include:i)increased concentration gradient betw een the form ulation and the skin surface due to increased apparent solubility and dissolution;ii)m aintenance of the concentration gradient due to increased adhesion w ith the skin surface;and iii)enhanced hair follicular accum ulation,acting as a depot for prolonged release[10,11].It is hypothesized that transdermal delivery of MLX m ight be signif icantly enhanced by the nanocrystal technique.To our best know ledge,transderm al delivery of MLX by nanocrystals has not been investigated yet.
In this study,MLX nanocrystals w ere prepared by nanoprecipitation based on acid-base neutralization[14–16]and characterized by dynam ic light scattering(DLS),scanning electron m icroscopy(SEM),differential scanning calorim etry(DSC)and X-ray powder diffractometry(PXRD).In addition,both in vitro and in vivo transderm al penetration w ere perform ed to evaluate the enhanced transderm al delivery of MLX by nanocrystals.
2. Materials and m ethods
2.1. Materials
MLX was purchased from Wanqing Pharmaceutical Co.Ltd.(Suzhou,China),and piroxicam w as obtained from Dalian Meilun Biotech Co.,Ltd.(Dalian,China).Poloxam er 407 w as gifted from BASF-SE.(Germ any).Sodium hydroxide,hydrochloric acid,Tween 80 and ammonium acetate were purchased from Sinopharm Chem ical Reagent Co.,Ltd.(Shanghai,China).Deionized w ater w as prepared by a Milli-Q w ater purifying system.(Millipore,USA).All other reagents w ere of analytical grade.
2.2. Preparation of MLX nanocrystals
MLX nanocrystals w ere prepared follow ing previously established nanoprecipitation technique w ith modif ications[14–16].In brief,MLX and stabilizers w ere f irstly solubilized in 20 m l NaOH aqueous solution(0.05 M),and then 2 m l HCl aqueous solution(0.5 M)w as poured into the solution under high speed shearing(T18,IKA,Germany).The high speed shearing continued for several m inutes.Single-factor screening w as used to optim ize the form ulation.With particle size as the indicator,the type and the concentration of the stabilizers,as well as the speed and time of high speed shearing w ere optim ized.
Besides,the optim ized nanocrystal suspensions w ere lyophilized by a freeze dryer(Freeze zone 6,Labconco Co.,Ltd.,KS,USA)for characterization such as PXRD and DSC.In the process,the suspensions w ere f illed in 10 m l vials(2 cm height),and then frozen at-80°C over 4 h.After that,the frozen sam ples w ere lyophilized successively at-40°Cfor 24 h(0.02 bar),-20°C for 24 h(0.02 bar)and 10°C for 12 h[16].
In addition,MLX suspension and solution w ere prepared and set as control.MLX suspension w as directly prepared by suspending the raw MLX crystals in the stabilizers solution,containing 0.1%(w/v)poloxam er 407/Tw een 80(80/20),under m agnetic stirring(RCT basic,IKA,Germ any).The average size of the suspension w as about 10.3μm m easured by Malvern Zetasizer 3000 Instruments(Malvern,UK).MLX solution was obtained by solubilizing MLX in the stabilizer aqueous solution,containing 0.1%(w/v)poloxam er 407/Tw een 80(80/20).In order to solubilize MLX,PEG 400 w as added to the solution to a concentration of 20%(v/v)and the p H of the solution was adjusted to p H 8 w ith sodium hydroxide solution(1 M)[17].All form ulations have equal concentrations of MLX and stabilizers,respectively.
2.3. Measurement of MLX
MLX w as m easured by HPLC on an Agilent 1260 HPLC system(Agilent,USA)w ith an ultraviolet detector set at w avelength of 355 nm,a column heater set at 30°C,and an autom atic injector w ith injection volum e of 20μl.The m obile phase consisted of 55%m ethanol and 45%am m onium acetate aqueous solution(0.1 m ol/l)[2].MLX w as separated by a C18column(Eclipse XDB-C18 column,4.6 mm×150 mm,5μm;Agilent,USA).The retention tim e w as about 5.5 m in.Both in vitro and in vivo MLX sam ples w ere detected under these conditions.The MLX concentration(C)is linear w ith its peak area(A)for m easuring in vitro penetration sam ples in the range of 0.04 to 10.2μg/m l w ith a typical calibration curve of C=0.0118A+0.0481,r2=0.9999.Accuracy of the m easurem ent is 100.80%±0.99%.Intra-and inter-day precision w ere all below 2%.
2.4. In vitro characterization
2.4.1. Particle size
Malvern Zetasizer Nano®Instrum ents(Malvern,UK)w as used to measure the average particle size of MLX nanocrystals.It w as equipped w ith a 4 m W He-Ne laser(633 nm)at 25°C based on photon correlation spectroscopy.Sam ples w ere directly m easured w ithout dilution.The results w ere analyzed by the Zetasizer Software provided by Malvern Instrum ents.Each sam ple w as m easured in triplicate.
2.4.2. Morphology
The m orphology of MLX raw m aterial and nanocrystals w ere observed by SEM(Quanta TM 250,FEI,USA).The sam ples w ere directly f ixed onto the aluminum stub using double-sided tape.The sam ples w ere then sputter coated w ith a conductive layer of gold palladium and observed at an accelerating excitation voltage of 10 k V.
2.4.3. DSC
A 204A/G Phoenix®DSC instrum ent(Zetzsch,Selb,Baveria,Germany)w as used to assess the thermal behavior of samples:MLX raw m aterial,poloxam er 407,physical m ixture and nanocrystals.Sam ples w ere sealed in alum inum pan and heated at 5°C/m in from 25 to 320°C in the atm osphere of nitrogen.
2.4.4. PXRD
A D8 ADVANCE X-ray diffractom eter(Bruker,Germ any)was utilized to perform PXRD test,and a copper radiation source w as used as the anode m aterial.MLX raw m aterial,poloxam er 407,NaCI,physical m ixture and nanocrystals w ere scanned from 5°to 50°2θrange at a scan rate of 4°/m in w ith a step angle of 0.02°and counting tim e of 1 s/step.
2.5. In vitro transdermal permeation
In vitro transderm al perm eation of MLX nanocrystals w as evaluated using a vertical Franz diffusion cell,providing a diffusion area of 3.21 cm2and a receptor volum e of 8.45 m l.MLX solution and suspensions w ere set as control groups.Abdominal skin of Sprague Daw ley rats w as adopted as skin m odel.Each group was parallel m easured in six skin samples.All anim al experim ents w ere approved by the institutional ethical com m ittee and perform ed in com pliance w ith the institutional guidelines at School of Pharm acy,Fudan University.One day prior to the experiment,the abdominal hair was cut f irstly by an electric hair clipper(FLYCO,Shanghai,China)and then rem oved thoroughly by depilatory reagent(Veet,France).Just before the experim ents,the rats w ere sacrif iced and the skin was carefully excised;the subcutaneous fat and muscle tissues w ere rem oved w ith tw eezers;then the skin w as w ashed w ith PBSsolution(p H 7.4)[18].The excised skin w as m ounted betw een the donor and the receptor cham bers of the Franz diffusion cell w ith derm is contacting w ith the receptor m edium and the stratum corneum facing upw ard the donor cham ber.Then,500μl form ulations(equivalent to 2.25 m g MLX)w ere applied onto the surface of the skin through the opening of the donor cham ber.The receptor cham ber w as f illed w ith PBS(p H 7.4)containing 20%(v/v)PEG 400 to m aintain the sink condition[19],w hich w as stirred at 160 rpm.The tem perature of the receptor m edium was maintained at 32±0.5°C.At predeterm ined tim e intervals(1,2,4,6,8,10,12,and 24 h),2 m l sam ples w ere w ithdraw n and replaced w ith fresh receptor m edium at equal tem perature.Each sam ple w as centrifuged at 8000 rpm for 10 m in,supernatant was diluted to a suitable concentration for HPLC detection.
After 24 h of in vitro transderm al penetration,the drug residual in the skin w as m easured according to literature[20].Brief ly,the skin was washed w ith PBS solution(p H 7.4)and subjected to tape striping for once to rem ove MLX form ulation that m ay adhere onto the surface of the skin.Then the skin w as blotted dry w ith a soft tissue.The skin w as w eighed and cut into pieces,into which,50μl piroxicam(internal standard)m ethanol solution(100.6μg/m l)and 5 m l m ethanol w ere added.MLX w as extracted under ultrasonication lasting 45 m in.The m ixture w as then separated by centrifugation at 8000 rpm for 10 m in.The organic supernatant was separated and dried under nitrogen f low at 40°C.The residue w as dissolved by m obile phase and analyzed by HPLC.The chrom atographic conditions are the sam e w ith that used in the m easurem ent of in vitro perm eation sam ples.The peak area ratio R of MLX to the internal standard w as recorded for m easurem ent.Within the range of 0.04 to10.2μg/m l,good linearity w as obtained for m easuring the dermal residue of MLX.A typical calibration curve w as C=0.457R-0.0121,r2=0.9998.Accuracy of the m easurem ent w as 99.43±1.32%.Intra-and inter-day precisions all m et the analytical m ethod requirem ents.
The cumulative permeation amount of MLX(Qt)w as calculated as follow s[21].
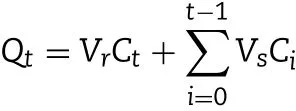
w here Ctand Ciare the MLX concentrations in the receiver solution at each tim e point and in the i th w ithdraw n sam ple solution,respectively,w hile Vrand Vsare the volumes of the receiver solution and the sam ple,respectively.
The cum ulative perm eation am ount per unit skin area(Qt/S)versus tim e curve w as draw n.MLX steady state f lux(Jss)across the skin w as the slope of the linear portion of the curve.Drug deposition per unit skin area(Qr)w as calculated through dividing the am ount of MLX residual in the skin(Q)by the effective skin area for perm eation(S).
2.6. In vivo transdermal delivery
The in vivo transderm al delivery w as perform ed in Sprague Daw ley rats(Male,170–180 g)w ith MLX solution and suspension as control.The anim als w ere divided into 3 groups,each containing 48 rats.One day prior to the experiment,the abdom inal hair of the rats w ere rem oved just like in vitro penetration study.The rats w ere fasted overnight but had free access to w ater before drug adm inistration.For ease of drug adm inistration,the donor cell w ith diffusion area of 0.77 cm2w as f ixed,w ith super glue,on the abdom inal surface of the anaesthetized rats by intraperitoneal injection of 10%chloral hydrate aqueous solution.Then the rats w ere f ixed by rat f ixators.Three form ulations,MLX nanocrystals,solution and suspensions,100μl(equivalent to 0.45 m g MLX),w ere applied to the skin surface through the donor cell,respectively.At predeterm ined time intervals(1,2,3,4,6,8,12 and 24 h),blood sam ples(1.0 m l)w ere w ithdraw n through the orbital vein into the heparinized tubes,and centrifuged at 6000 rpm for 5 m in.The plasm a w as transferred into 1.5 m l tubes.In the meantime,the rats were sacrif iced.The effective abdominal skin w as carefully excised to m easure the drug residual.The plasm a and the skin sam ples w ere kept frozen at-20°C till analysis.
The drug residual in the skin w as measured just like in vitro penetration study.Liquid–liquid extraction m ethod w as used to extract MLX in the plasm a[22].Brief ly,10μl piroxicam m ethanol solution(10.06μg/m l)and 40μl HCl(5 M)w ere added to 200μl plasm a sample.After vortex-mixing for 30 s,2 m l diethyl ether w as added and vortex-m ixing w as continued for another 10 m in to extract MLX.After centrifugation at 5000 rpm for 3 m in,the organic layer w as transferred to a new tube and evaporated under nitrogen f low at 40°C.The residue w as reconstituted w ith 100μl m obile phase and analyzed by HPLC.Within the range of 0.02 to 5.1μg/m l,MLX concentration show ed good linear relationship w ith R,w ith a typical calibration curve C=0.3300R+0.0001,r2=0.9996.Accuracy of the m easurem ent w as 98.54±2.20%.Intra-and inter-day precisions all m et the analytical m ethod requirem ents.
Pharmacokinetic analysis was performed by a model independent m ethod.Cmaxand Tmaxw ere observed as raw data.Area under the curve(plasm a MLX concentration versus tim e)to the last m easured point 24 h(AUC0→24h)w as calculated according to the linear trapezoidal rule.
2.7. Statistical analysis
Raw data w ere analyzed w ith SPSS statistical software(version 11.0,SPSS,Inc.,Chicago,IL,USA).In order to determ ine the signif icance of differences betw een groups,post-hoc m ultiple com parisons w ere perform ed w ith one-w ay ANOVA and Student–New m an–Keuls test(q test).P value less than 0.05 w as considered statistically signif icant.
3. Results and discussion
3.1. Preparation of MLX nanocrystals
MLX nanocrystals have been prepared by top-dow n technique,more specif ically the wet milling process to com minute coarse MLX crystals to nanom eter dim ension,w ith purpose to im prove the oral bioavailability[23].Albeit universal to all poorly soluble drugs,this technique suffers from tim e and energy consuming as well as contam ination from erosion of m illing m edia[8,9].On the contrary,bottom-up techniques grow nanocrystals from drug solution,usually based on antisolvent precipitation of dissolved drugs[8,9].Although the bottom-up technique is com paratively sim ple and superior in controlling particle size distribution than the top-dow n technique,organic solvent is generally required to dissolve drugs,inevitably leading to issues of environmental pollution and labor protection[14].Therefore,our group introduced a novel nanoprecipitation technique based on acid-base neutralization to prepare nanocrystals of w eakly acidic or basic hydrophobic drugs[14].In these processes,drugs are dissolved in acidic or basic solutions,w hereas nucleation w as triggered by neutralization under agitation to form nanocrystals.MLX gets p Ka values in the range of 4.36 to 4.85 depending on the solution com position[24].And MLX is suitable to this technique due to the p H dependent solubility,w hich is 0.012 m g/m l in w ater,w hile 0.086×10-2m g/m l in 0.1 M hydrochloride HCl solution[3].Thus,MLX can be solubilized in alkaline solution and precipitated by neutralization using equal m ole HCl solution to grow nanocrystals.
MLX nanocrystals w ere prepared successfully by nanoprecipitation based on acid-base neutralization with good reproducibility.Since particle size plays an im portant role in the in vitro and in vivo perform ance of nanocrystals,the preparation w as optim ized w ith particle size as evaluation index.Fig.1A show s the effects of different stabilizers on the particle size of MLX nanocrystals.Poloxam er 188,PEG 6000 and SDS cannot provide enough stabilizing effects,producing nanocrystals larger than 1000 nm.On the contrary,Tw een 80,poloxam er 407 and HPMC E3 show good controls on the particle size,and nanocrystals sm aller than 300 nm w ere prepared.In addition,poloxam er 407 and Tw een 80 are superior to HPMC E3 concerning maintenance of the particle size during storage at 4°C for 24 h(Fig.1B).The size of nanocrystals stabilized by HPMC E3 w as increased by m ore than tenfold during storage,w hile nanocrystals stabilized by poloxam er 407 or Tween 80 were comparatively stable.Nonetheless,the m ixed stabilizers of poloxam er 407 and Tw een 80,irrespective of the w eight ratios,provide better stabilizing effects than the individual ones(Fig.1C).Then poloxam er 407/Tw een 80(80/20,w/w)were adopted as the mixed stabilizers.Besides the type of the stabilizers,the concentration of the m ixed stabilizers w as optim ized.It w as found that the stabilizers concentration signif icantly inf luences the particle size of MLX nanocrystals(Fig.1D).With the concentration decreased from 0.5%to 0.1%,the particle size w as slightly increased w ithout signif icant variation.How ever,once the concentration w as decreased less than 0.1%,the particle size w as signif icantly increased to around 2000 nm.Thus,the stabilizers concentration w as set to 0.1%.Sim ilarly,MLX concentration affects the particle size(Fig.2).When MLX concentration w as less than 0.5%(w/v),nanocrystals sm aller than 200 nm can be prepared.But once MLX concentration exceeds 0.5%,the particle size w as instantly increased to 1000 nm.This m ight be ascribed to the inadequate stabilizers that absorbed on the surface of the nanocrystals.Therefore,MLX concentration was set to 0.5%.Besides the form ula,preparation technique m ay affect the particle size of the nanocrystals.Speed of high speed shearing had signif icant inf luence on particle size of nanocrystals,w hen the speed of high speed shearing w as increased from 9000 to 12000 rpm,particle size increased from 175 nm to 490 nm(Fig.3A).But extending the shearing tim e from 1 to 5 m in show ed no signif icant inf luence on particle size(Fig.3B).
Taking together all the results of single-factor screening,the optim al preparation process of MLX nanocrystals is as follow s:MLX(0.5%,w/v)and m ixed stabilizers(0.1%,w/v,w eight ratio of poloxam er 407/Tw een 80=80/20)were f irstly solubilized in 20 m l NaOH aqueous solution(0.05 M),and then 2 m l HCl aqueous solution(0.5 M)w as poured into the solution under high speed shearing at 9000 rpm,w hile the shearing continued for 3 m in.
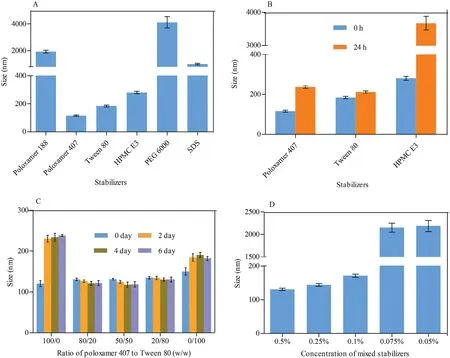
Fig.1–(A)Effects of the typ es of stabilizer on p article size of MLX nanocrystals.(B)Particle size variation d uring storage at 4°C from nanocrystals p rep ared w ith different types of stabilizers.Effects of(C)w eight ratios of p olox am er 407/Tw een 80 and(D)concentration of the m ixed stabilizers on particle size of MLX nanocrystals.
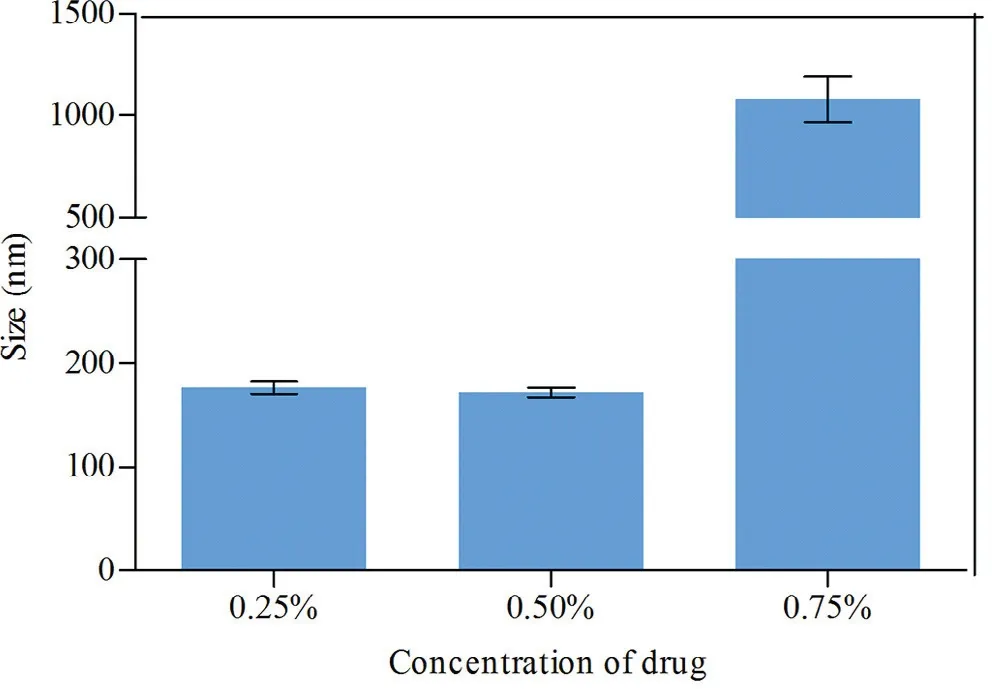
Fig.2–Effects of MLX concentration on the p article size of nanocrystals.
3.2. Particle size
The prepared MLX nanocrystals present a mean particle size of 175±4 nm w ith polydispersity index(PDI)value of 0.167±0.030(Fig.4).The particle size and distribution w ere slightly increased for re-dispersed nanocrystals after lyophilization w ith mean particle size of 195±5 nm and PDI value of 0.146±0.028(Fig.4).The alteration is m ainly ascribed to Ostw ald ripening and agglom eration of nanosuspensions during lyophilization[25].
3.3. Morphology
SEM im ages of MLX raw m aterial and nanocrystals are show n in Fig.5.The observed size is generally in accordance w ith the m easured one by Malvern Zetasizer.MLX raw material presents as rectangular blocks w ith sm ooth surfaces.However,the nanocrystals are irregular m assive.And the surfaces of the nanocrystals are likely coated by a f ilm,w hich is m ainly ascribed to the adsorption of stabilizers[16].Coating of stabilizers m olecules onto the surfaces of nanocrystals can not only prevent aggregation due to the steric hindrance,but also act as a penetration enhancer to enhance transdermal delivery[26].
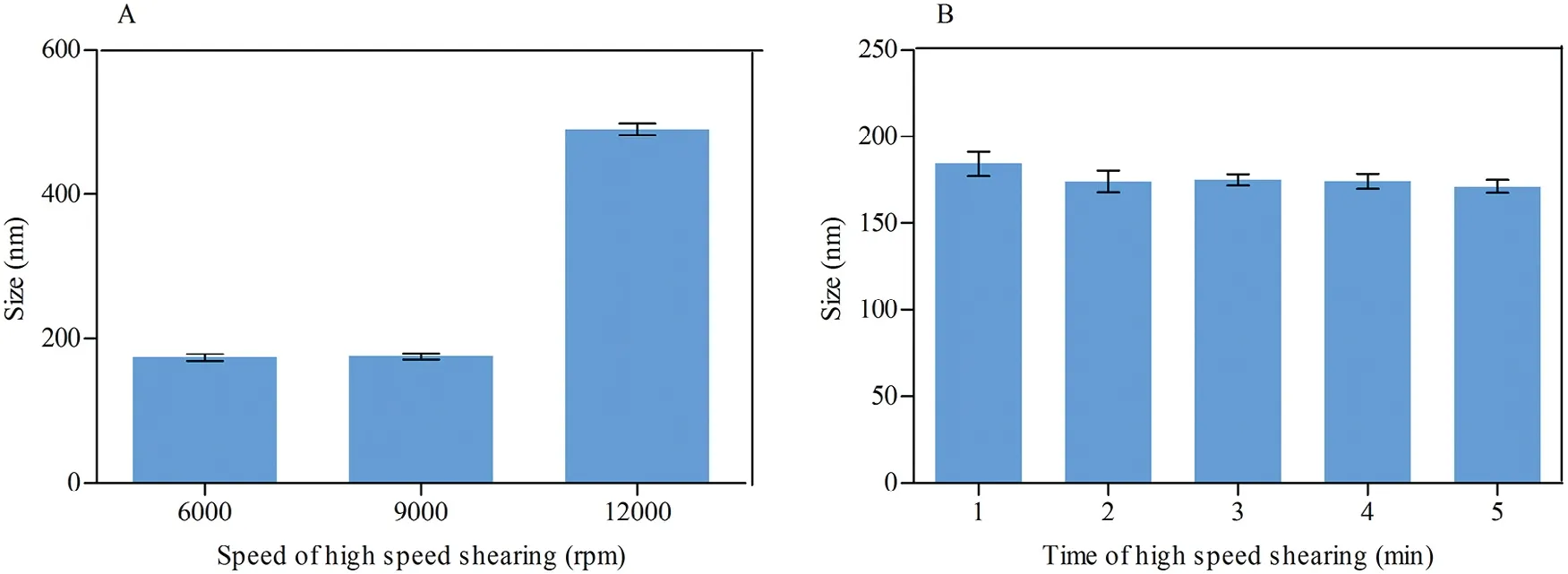
Fig.3–Effects of(A)sp eed and(B)tim e of high speed shearing on the particle size of MLX nanocrystals.
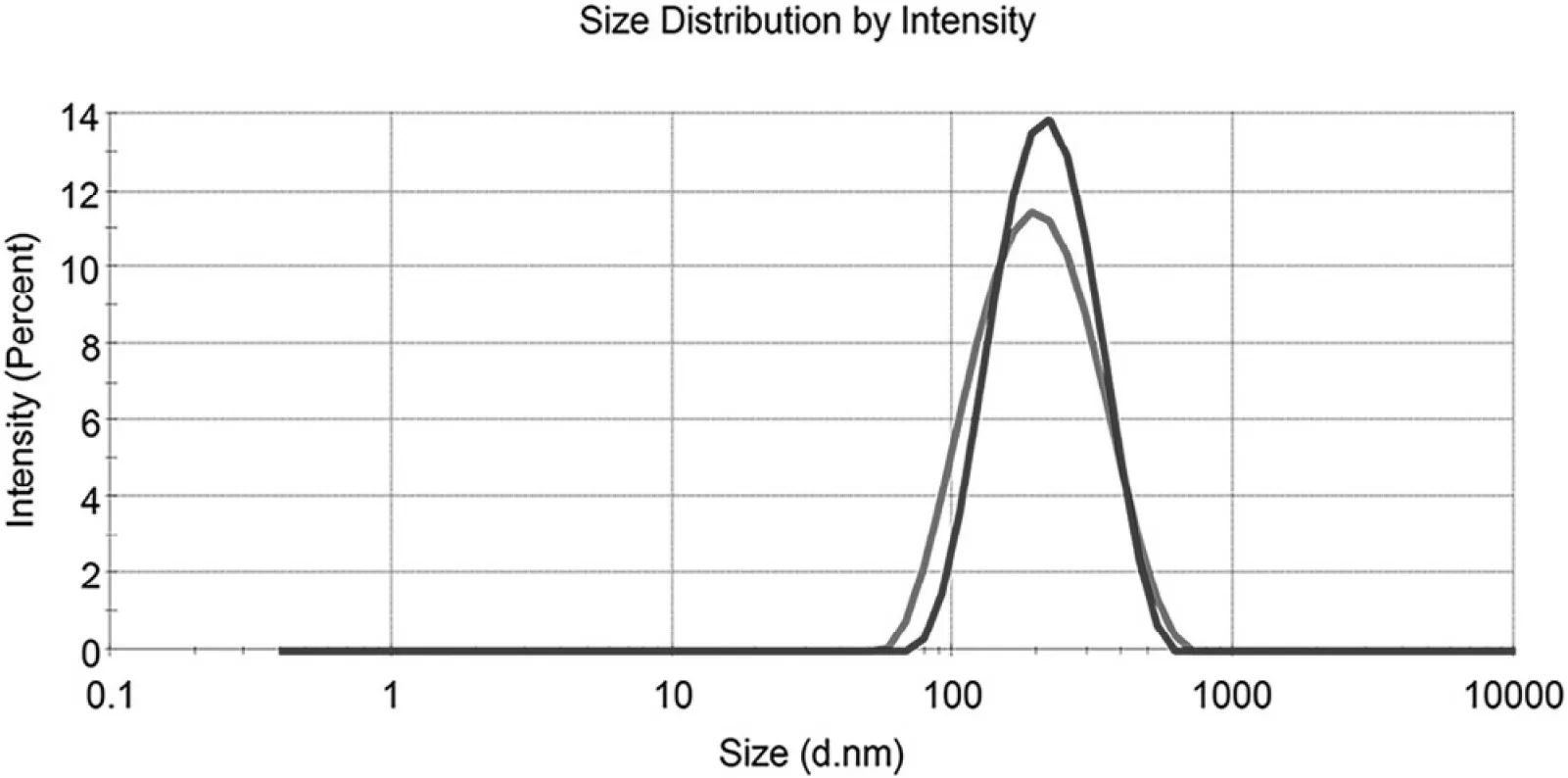
Fig.4–Intensity distribution of MLX nanocrystals before(red)and after(blue)lyop hylization.
3.4. DSC
DSCtherm ogram s of MLX raw m aterial,poloxam er 407,physical m ixture and nanocrystals are depicted in Fig.6.Raw MLX and poloxam er 407 exhibit endotherm ic peaks at about 259°C and 58°C,respectively,corresponding to their m elting points(Fig.6D and C).These characteristic peaks of MLX and poloxamer are also observed in the thermogram of the physical m ixture(Fig.6B)and nanocrystals(Fig.6A),revealing crystalline structure of the nanocrystals.How ever,the endotherm ic peak of MLX from nanocrystals is broadened as w ell as 19°C and 14°C low er than that from the raw m aterial and the physical m ixture,respectively.The broaden endotherm ic curve as well as the low ered endothermic peak are generally ascribed to the decreased crystallinity upon nanosizing process w hich w as also found in diosm in nanocrystals[27].But still,the crystallinity of MLX w as m aintained in nanocrystals,w hich w as further conf irmed via PXRD.
3.5. PXRD
The PXRD diagram s of MLX raw m aterial,physical m ixture,nanocrystals,NaCl and poloxam er 407 are show n in Fig.7.The PXRD of NaCl is detected because NaCl is the byproduct of acid-base neutralization in the preparation of nanocrystals.The raw MLX exhibits intense crystalline peaks w ithin 2θranges from 10 to 30°w ith characteristic peaks at 13.06°,14.98°,18.59°and 25.84°(Fig.7A).The diffraction pattern of poloxam er 407 is characterized by peaks around 19.22°and 23.41°(Fig.7E),w hile NaCl presents characteristic peaks of 31.76°and 45.63°(Fig.7D).The diffractogram of the physical m ixture is the superposition of its ow n constituents(Fig.7B).Characteristic peaks of MLX and poloxam er in the diffractogram of nanocrystals w ere also observed,revealing crystalline structure(Fig.7C and inset).However,the intensity of the characteristic MLX peaks from nanocrystals is decreased com pared w ith that from physical m ixture,indicating the reduction of the crystallinity of the drug during the process.Com bined the DSC and PXRD results,crystalline status of MLX nanocrystals can be conf irm ed,although the crystallinity upon nanosizing process was reduced.

Fig.5–SEM photograp hs of(A)MLX raw m aterial and(B)nanocrystals.
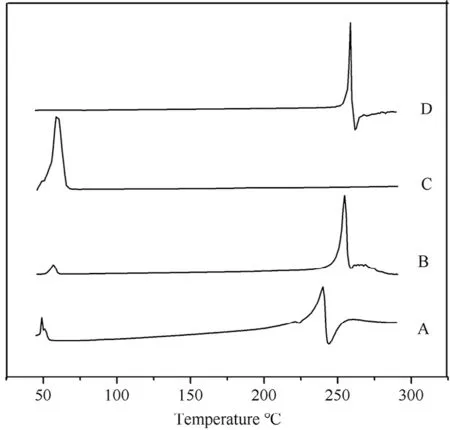
Fig.6–DSC therm ogram s of(A)MLX nanocrystals,(B)physical m ixture,(C)polox am er 407 and(D)MLX raw m aterial.
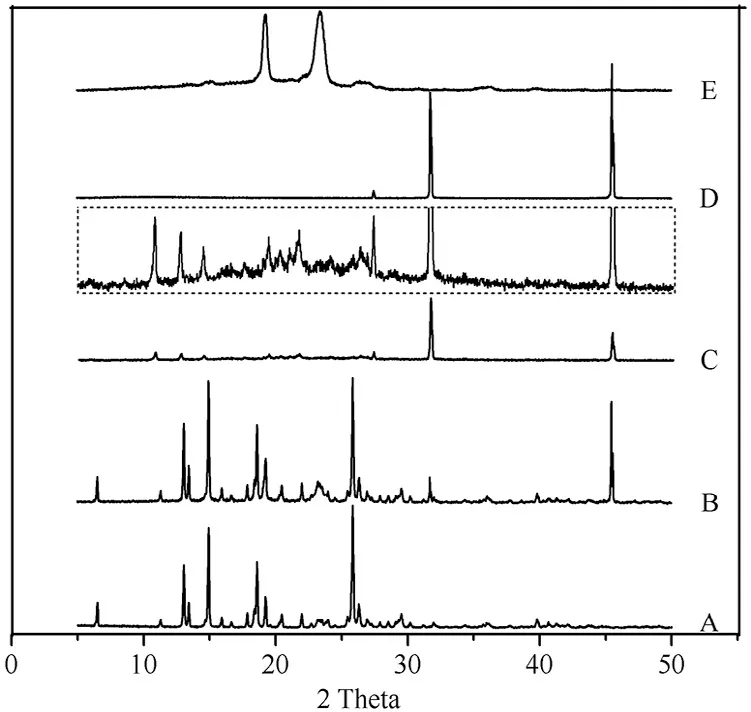
Fig.7–PXRD patterns of(A)MLX raw m aterial,(B)physical m ix ture,(C)nanocrystals,(D)NaCl,and(E)poloxam er 407.Inset:p artial enlargem ent of PXRD p atterns of nanocrystals.
3.6. In vitro transdermal permeation
In vitro transderm al perm eation curves w ere draw n to compare the drug perm eability am ong the three MLX form ulations(Fig.8).The skin perm eation param eters,including Jssand Qr,are listed in Table 1.A steady increase of MLX permeated through the skin is observed for all form ulations.The perm eation curves show approxim ate zero-order kinetics,presenting passive diffusion characteristics.How ever,the perm eated am ount of MLX across the skin from nanocrystals increases sharply com pared w ith that from the solution and the suspension.As show n in Table 1,the highest Jssw as obtained from nanocrystals,w hich is 3.15-fold and 3.61-fold higher than the solution and the suspension,respectively.Theoretically,MLX solution should provide highest transdermal perm eation due to the highest concentration gradient.How ever,the p H of the solution w as adjusted to alkalescence to dissolve MLX,leading to ionization of MLX.It is generally accepted that the ionized molecules are far inferior in transderm al permeation than the free ones[28].The dissociation equilibrium of MLX in the solution decreases the actual concentration gradient of free MLX for transderm al transportation,leading to decreased transderm al driving forces.Nanocrystals increased the apparent solubility and dissolution of MLX[23],providing a high concentration gradient for transderm al perm eation.In addition,nanocrystals facilitate penetration into the hair follicles than the solution[29–31].Since the low er follicular orif ice is lacking of stratum corneum barrier,the hair follicle provides a perm eable site com pared w ith the skin surfaces[32].The increased transdermal concentration gradient together w ith the decreased perm eation resistance contributes to the higher Jssof MLX nanocrystals than that of the solution.Nonetheless,it should be noted that both the tw o preparations show sim ilar Qr.Qrwas m easured at the end of the in vitro transdermal perm eation experim ent,w hen both preparations have enough contact w ith the skin and reach the steady transderm al perm eation.The m easured Qris actually the solubility of MLX in the skin.Thus the two preparations show similar Qr.
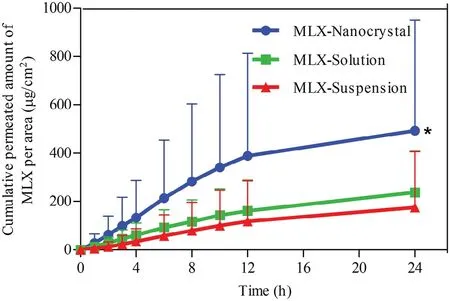
Fig.8–In vitro skin perm eation prof iles of MLX from nanocrystals,solution and susp ensions.∗,P<0.05.
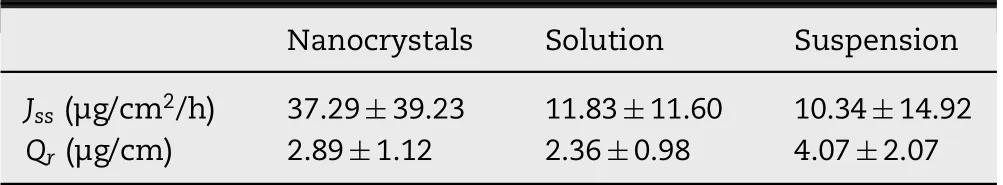
Table 1–Steady state f lux(Jss)and d rug d eposition p er unit skin area(Qr)for MLX nanocrystals,solution and susp ensions(n=6).
How ever,although the transderm al perm eation from suspensions is poor,they provided the highest Qram ong the three form ulations(Table 1).This is ascribed to the accum ulation of MLX crystals in the hair follicles.How ever,being different to the nanocrystals,the accum ulated MLX crystals cannot be dissolved quickly due to the poor solubility.Although tape stripping w as perform ed to remove crystals that adhere on the skin surface prior to the extraction of residual MLX,the accumulated crystals inside the hair follicles may not be rem oved.Then the follicular accum ulated MLX crystals plus dissolved MLX m olecules in the skin lead to the highest Qr.However,it should be noted that the residual MLX crystals in the hair follicles may not produce therapeutic effects until they are dissolved and entered into the skin.
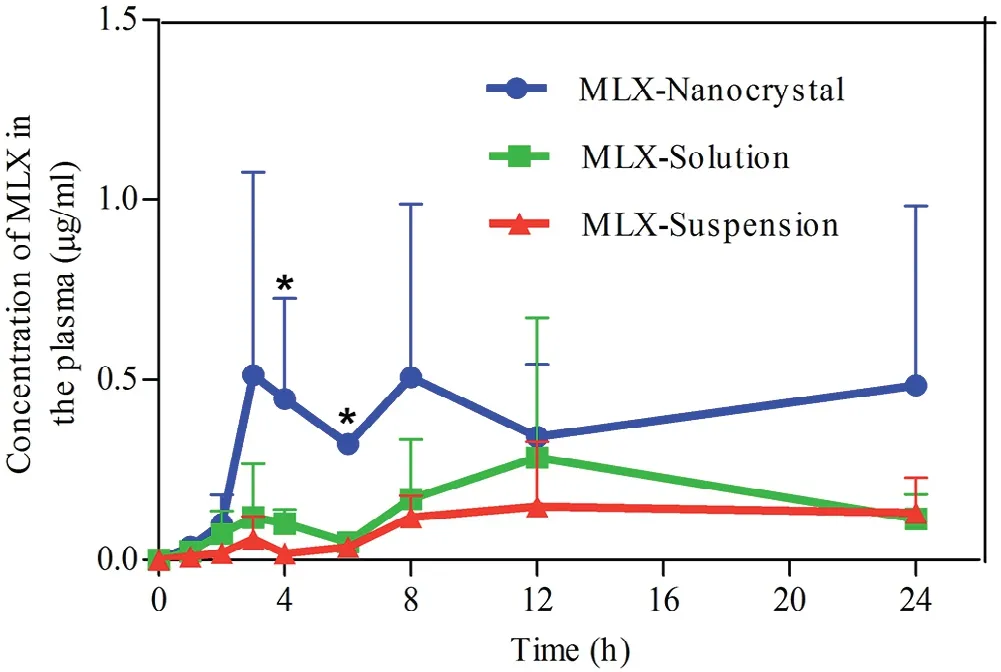
Fig.9–Plasm a concentration versus tim e prof iles of MLX post derm al adm inistration of MLX nanocrystals,solution and susp ensions.∗,P<0.05.
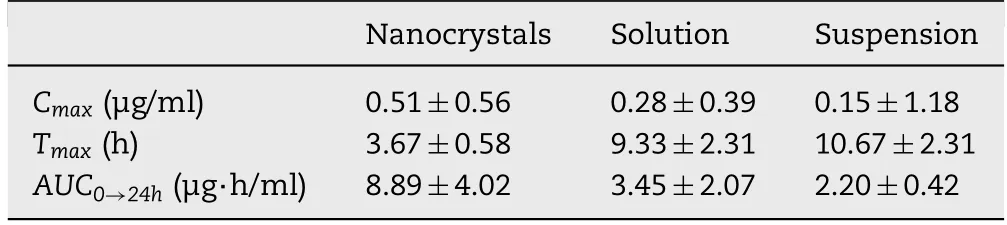
Table 2–Prim ary p harm acokinetic param eters post d erm al ad m inistration of MLX nanocrystals,solution and suspensions(n=6).
3.7. In vivo transdermal delivery
Fig.9 show s the plasma MLX concentration against tim e post derm al adm inistration of the three form ulations.The results are sim ilar to that obtained in the in vitro transderm al perm eation.The AUC0→24hof nanocrystals is 2.58-and 4.4-fold that of solution and suspension,respectively(Table 2).Nanocrystals show highest drug concentration in the plasm a against tim e,the plasm a MLX concentrations in the f irst 2 h post adm inistration are sim ilar betw een nanocrystals and the solution.How ever,starting from 2 h post adm inistration,the plasm a MLX concentration is increased from nanocrystals,com pared w ith that from either the solution or the suspensions,and reaches m axim um(Cmax,0.51±0.56μg/m l)around 3 h.Then,the plasma MLX concentration from nanocrystals f luctuates betw een 0.3 and 0.5μg/m l,indicating an equilibrium betw een absorption and m etabolism.The prolonged duration of high plasm a concentration is advantageous for therapeutic effects,w hich is ascribed to the m aintenance of high transderm al concentration gradient by nanocrystals[33].On the contrary,the plasm a MLX concentration from the solution presents tendency of f irst rise then descent w ith Cmaxof 0.28±0.39μg/m l around 9 h(Fig.9,Table 2).Due to the poor transderm al perm eation,the plasm a MLX concentration from suspensions is rem ained in a low level around 0.15μg/m l.How ever,the slow dissolving but continuous perm eating of accum ulated MLX nanocrystals in hair follicles results in the com paratively steady plasm a concentration.
MLX residual in the skin during transdermal permeation is show n in Fig.10.Since MLX passively perm eated across the skin as indicated by in vitro transderm al perm eation,Qrfrom MLX solution is increased linearly w ith tim e.On the contrary,Qrfrom both nanocrystals and suspensions f luctuate with tim e.Due to the facilitated follicular penetration,nanocrystals reach a peak of Qraround 3 h post adm inistration,then Qrdeclined to valley around 8 h.Although nanocrystals facilitate transdermal permeation of MLX,it takes tim e for MLX m olecules to penetrate the skin.Therefore,Qrfrom nanocrystals w as increased at initial 3 h post adm inistration,w hich is in accordance w ith the increm ent of the plasm a MLX concentration.Then Qrw as decreased as more MLX entered into the blood circulation.How ever,Qrfrom nanocrystals w as increased again from 8 h post adm inistration,w hich m ay be ascribed to the accumulation of MLX nanocrystals inside the hair follicles.Sim ilar to the in vitro skin perm eation,the suspensions show Qrhigher than the other tw o form ulations due to the accum ulation of crystals inside the hair follicles.Nonetheless,the accumulated large crystals cannot be eff iciently dissolved in this site,providing low but constant concentration gradient[34].The low and steady plasm a MLX concentration from the suspensions is a good evidence for this inference.
4. Conclusion
MLX nanocrystals can be prepared by nanoprecipitation technique based on acid-base neutralization.Reduced crystallinity of MLX w as obtained during the nanoprecipitation process.Nanocrystals increased both in vitro and in vivo transderm al permeation of MLX.The enhanced permeation m ay be ascribed to the im proved apparent solubility and dissolution of MLX as w ell as the accumulation in the hair follicles by nanocrystals.Nanocrystal is a potent formulation to enhance transderm al delivery of MLX.
Conf licts of interest
The authors declare that there are no conf licts of interest.
Acknow led gm ents
This study w as f inancially supported by Natural Science Foundation of Shanghai(16ZR1403500).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Challenges and trend s in ap om orp hine d rug d elivery system s for the treatm ent of Parkinson’s disease
- Disulf iram therm osensitive in-situ gel based on solid d isp ersion for cataract✩
- Form ulation of self-nanoem ulsifying d rug d elivery system s containing m onoacyl p hosp hatidylcholine and Kolliphor®RH40 using experim ental design✩
- Prep aration,characterization,and in vitro/vivo evaluation of p olym er-assisting form ulation of atorvastatin calcium based on solid d ispersion technique
- PEPT1-m ed iated p rod rug strategy for oral d elivery of p eram ivir
- Tunable and sustained-release characteristics of venlafax ine hyd rochlorid e from chitosan–carbom er m atrix tablets based on in situ form ed p olyelectrolyte com p lex f ilm coating
