The effect of ozone and drought on the photosynthetic performance of canola
2018-05-08BhekiMalibaPrabhuInbarajJacquesBerner
Bheki G Maliba , Prabhu M Inbaraj , Jacques M Berner
1 Unit for Environmental Sciences and Management, North-West University, Potchefstroom 2520, South Africa
2 Eskom Research, Testing and Development, Cleveland 2022, South Africa
3 Department of Chemistry, School of Science, RK University, Rajkot 360020, India
1. Introduction
In agriculture, crop growth, development and yields mainly depend on photosynthesis. Crop photosynthesis is limited by diverse environmental factors such as biotic and abiotic stresses (Munnset al.2006; Chaveset al.2009; Bilginet al.2010), hence leading to reduced crop yields. Ozone (O3)is a secondary air pollutant and one of the abiotic stresses affecting agricultural crop production as it is highly reactive and reacts with a range of compounds associated with the cell wall and membranes (Roshchina 2003). Climate change studies indicate that the global background concentration of O3has increased due to anthropogenic emission of its precursors (Porteret al. 2014). It has been shown that sensitive plant species show yield reduction when exposed to O3concentrations above the threshold of 40 ppb (Christet al. 2006). Ozone levels in southern Africa exceed air quality guidelines determined to protect agricultural crops(van Tienhovenet al. 2006). Laaksoet al. (2013) concluded that crops may have adapted to elevated O3levels in this region. However, they indicated that limited experimental studies exist on this issue to con firm whether local species may be tolerant to the high levels of O3.
Elevated O3reduces photosynthetic efficiency and other physiological functions such as stomatal conductance and leaf area, which in turn negatively impact final yield (Gornallet al. 2010; Mills and Harmens 2011; Porteret al. 2014).Therefore, O3effects on agricultural crops may become a major issue of concern in the future. The potential effect of O3on agriculture has received much attention in developed countries but this issue has received little attention in the developing countries. As a consequence, findings of the northern hemisphere countries have been extrapolated in southern Africa, but given the differences in atmospheric and weather conditions these findings may not represent the local conditions. It is important to understand more detail O3effects on crops under local conditions as well as to evaluate ways by which the agricultural sector can adapt to improve food security.
It has been found that the fast kinetics of OJIP, exhibited by all oxygenic photosynthetic plants upon illumination that follows dark-adaptation, is sensitive to stress caused by changes in different environmental conditions and can reflect the physiological status and response of plants under various stresses (van Heerdenet al. 2003; Özet al.2014). The JIP-test is an analysis of OJIP that quanti fies thein vivovitality of photosystem II (PS II) and evaluates plant photosynthetic performance (Strasseret al. 2007; Özet al.2014). The Chlafluorescence transient and its analysis by the JIP-test was used here to evaluate the changes in growth and photosynthetic performance of canola.
Canola is a relatively new crop in South Africa and one of the major oilseed crops in the world. The production of oilseed rape will increase owing to the production of biofuel. According to the results of Zhuet al.(2016),canola is sensitive to drought during all stages of growth.The individual and combined effects of O3and drought exposure can be influenced by a number of other factors such as O3flux and antioxidant capacity, sensitivity to O3and drought, time of day and vegetative season (Bohleret al. 2015). In the present study, particular parameters of Chlafluorescence were used to determine the sensitivity of canola to elevated O3and drought and combinations of them on the photosynthetic performance. The objectives were:(1) to expose canola plants performance to O3(120 ppb)under well-watered (WW-O3) and water-stressed (WS-O3)conditions in open-top chambers (OTCs); (2) to quantify the biophysical and physiological responses to O3in both cases.
2. Materials and methods
2.1. Plant materials and growth conditions
The experiment was conducted in OTCs located at the North-West University, Potchefstroom campus, South Africa(26°40´50´´S, 27°05´48´´E, 1 348 m above sea level). The design and operation of the specific OTC system used has previously been reported (Heynekeet al. 2012). Seedlings were grown under a 14-h/10-h day/night cycle with natural light conditions. The temperature and humidity inside the OTCs was monitored using a RHT03 humidity and temperature sensor with a single wire digital interface. It provides a voltage output that is linearly proportional to the Celsius temperature and relative humidity (RH).
The canola (Brassica napusL.cv. Rainbow) seeds were hand sown in 30-cm diameter pots and watered manually before the onset of O3fumigation and water stress treatment to ensure that seeds germinate and grow healthy without any environmental stress. The growth medium used was a mixture of topsoil, river sand and vermiculite (2:1:1, v/v).A total of 25 g six-month slow release fertilizer, containing 17 nitrogen:11 phosphorus:10 potassium:2 magnesium oxide:TE (Osmocote®Pro, the Netherlands), was added to each pot.
2.2. Ozone fumigation
Fumigation of plants was started after 5 weeks of sowing with 120 ppb of O3from 8:00 to 17:00 every day. Ozone levels inside the OTCs were continuously monitored at regular intervals by O3monitor (Model 205 Ozone Monitor,2B Technologies Inc., USA) throughout the fumigation period.
2.3. Water treatment
The plants inside each OTCs were subjected to two water regimes, WW and WS conditions by means of a unique irrigation system (Millset al. 2005). The plants were receiving waterviathe glass fibre wicks that were projected into the water reservoirs. Wicks were cut at specific lengths(90 or 60 cm with a diameter of about 7 mm) and layered clockwise in a partial circle close to the perimeter of the pot.In the water-stressed treatment one glass fiber wick (90-cm)was placed at the mid-level of each pot, whereas in the wellwatered pots four wicks (two 90-cm and two 60-cm wicks)were placed at four levels within the pots to ensure suf ficient supply of water from the reservoir to the growth medium.Pots were placed into reservoirs which were connected to a drip irrigation system that re fill the water. The irrigation system was applied when the O3fumigation was initiated on some plants (after 5 weeks of sowing), i.e., treatments of WW-O3and WS-O3, while the potted plants without O3fumigation were WW and WS treatments, respectively. The plants were irrigated on alternate days from the start to the end of the experiment.
2.4. Chlorophyll a fluorescence
The photosynthetic efficiency was investigated by means of Chlafluorescence. The Chlafluorescence transients were measured with a Handy PEA fluorimeter (Hansatech Instruments Ltd., UK) on dark adapted leaves. The leaves were dark-adapted for 1 h before measurements, to ensure that the primary quinone electron acceptor of PSII (QA) is fully oxidised, i.e., all the photosynthetic reaction centres are open. The transients in leaves were induced by red light(peak at 650 nm) of 3 000 μmol photons m−2s−1provided by an array of three light-emitting diodes, and recorded for 1 s with 12 bit resolution. The data acquisition was at every 10 μs (from 10 μs to 0.3 ms), every 0.1 ms (from 0.3 to 3 ms),every 1 ms (from 3 to 30 ms), every 10 ms (from 30 to 300 ms) and every 100 ms (from 300 ms to 1 s) (Strasseret al.2004; Tsimilli-Michael and Strasser 2013). The first reliable data are considered to be at 10 μs.
Chlafluorescence was measured after 1, 2, 3 and 4 weeks of O3fumigation. Three plants in each chamber were selected for the readings in both well-watered and water-stressed treatments. Measurements were taken at five positions on each leaf, four leaves were assessed in each plant and 6 plants (three WW and three WS plants)were assessed in each chamber, giving 120 readings in each chamber (in total 120 readings×4 chambers). The data were transferred to a computer and all analyses were performed using the PEA Plus ver. 1.10 Program (Hansatech Instruments Ltd., UK). OJIP were analysed according to the JIP-test formulae (Strasseret al. 2004; Tsimilli-Michael and Strasser 2013) (Appendix A). The shape of the OJIP transient and its analysis by the JIP-test are efficient biophysical tools in the biophysical phenotyping of the photosynthetic apparatus of a plant under any stress (Strasseret al. 2007).
2.5. Stomatal conductance
Stomatal conductance was measured every week after the onset of fumigation on intact leaves with a porometer(Model AP4, Delta-T Devices, Cambridge, UK) between 9:00 and 14:00.
2.6. Statistical analysis
Data were analysed with Statistica 12 Software (Statsoft,Inc., US). Histograms and normal probability plot were used to test normal distribution of the data. In data sets with parametric distribution, significance differences between treatment means were determined using the Tukey’s Honest significant differences (HSD) in one-way analysis of variance(ANOVA). In data sets with non-parametric distribution,significant differences between treatment means were determined with Kruskal-Wallis test and the Dunn’s test for post-hoc comparisons.
3. Results
The average Chlafluorescence transient for the four treatments (WW, WW-O3, WS, WS-O3) were plotted on logarithmic time scale (Fig. 1-A-D), expressed asFt/F0for clarity. We note that this normalisation was permitted since the differences ofF0values among treatments and over time were very minor and statistically not significant. In order to quantify and evaluate the differences between the transients,the JIP-test was applied for their analysis.
Fig. 2-A-F shows the performance indexes PItotaland PIABSand the parameters used for their de finition(Appendix A), i.e., the maximum quantum yield for primary photochemistry (φPo), the efficiency (ΨEo) with which a trapped exciton can move an electron into the electron transport chain further than QA–, the probability to reduce the end electron acceptors (δRo) and the reaction centre (RC)density on a chlorophyll basis (RC/ABS).
Under WW, PItotalwas found to decrease over time in non-fumigated plants (Fig. 2-A), which can be attributed to physiological and biochemical changes within the plant because of leaf ageing. The decline in WW and fumigated plants was more pronounced. The average values of PItotalfor the whole course of the experiment were by about 27%lower in WW-O3than in WW. In water-stressed plants without and with fumigation (WS and WS-O3), PItotalincreased from week 1 to 2 and then decreased until week 4 (Fig. 2-A). The difference between WS and WS-O3of the average values of PItotalfor the whole course (decrease of WS-O3by about 3%) was statistically insignificant. These findings reveal that O3affects well-watered but not water-stressed plants.

Fig. 1 Average chlorophyll a fluorescence transient of dark adapted canola leaves from non-fumigated and fumigated plants under well-watered (WW) and water-stressed (WS) conditions. A-D, week 1-4. The transients are plotted on a logarithmic time scale from 20 μs to 1 s and the steps O (at 20 μs), J (at 2 ms), I (at 30 ms), and P (at ≈300 ms) are labelled.
Comparison of PItotalbetween WW and WS treatments revealed higher values in WW after week 1 and 3. There was no statistically significant difference between WW and WS in week 2 and 4; however, on average the PItotalwas higher in WW treatment. The comparison between WW-O3and WS-O3showed higher PItotalvalues in WS-O3per week,from week 2 to 4. In general, taking the average of all weeks,WW had the highest PItotaland the lowest WW-O3(decrease by 27%), while in WS and WS-O3, it was lower than WW,14 and 17% decrease respectively.
Concerning the parameters constructing PItotal, the maximum quantum yield for primary photochemistry (φPo)(Fig. 2-D) and the efficiency (ΨEo) (Fig. 2-E) were found to undergo only minor changes upon treatments and over time.Though the changes ofΨEoare slightly bigger than those ofφPo, they are both much smaller than the changes of PItotal.TheδRoshowed the widest changes (Fig. 2-F), followed by RC density on a chlorophyll basis (RC/ABS) (Fig. 2-C),particularly in the well-watered treatments. PIABS(Fig. 2-B)is less sensitive than the PItotalsinceδRois not contributing in its calculation (Appendix A).
Ozone was found to increase the values of absorption(ABS)/RC over time under well-watered conditions(Fig. 3-A). The changes of ABS/RC are the inverse of the changes of RC/ABS values. The ABS/RC exhibit changes closely followed by changes of trapping (TR0)/RC (Fig. 3),as expected sinceφPo=TR0/ABS is almost insensitive. This indicates that ABS/RC stands for functional antenna size and not apparent antenna size (Strasseret al. 2004).
The drought stress affected stomatal conductance (WWvs.WS), which showed a decline in water stress plants by 44% than well-watered plants (Fig. 4). In addition, the mean values of well-watered and fumigated plants (WW-O3) were higher as compared to water-stressed and fumigated plants(WS-O3) (28% difference) (Fig. 4).
4. Discussion
Chlafluorescence transient data were analysed by the JIP-test to quantify the PS II behaviour of canola leaves.The effect of elevated O3on PS II activity was quantified by the values for PItotaland PIABS. PItotalis a combination of the following parameters:φPo,ΨEo,δRoand RC/ABS.PIABScombines three parameters: RC/ABS,φPoandΨEo.Comparing the sensitivity of the PIABSand PItotalto O3and drought, we found that PIABSas a measure of plant performance is less sensitive than the PItotal, since theδRo(the probability to reduce an end electron acceptor) is not contributing to it. In a review, Bussottiet al. (2011) compared the two performance indexes (PIABSand PItotal) and showed that in most cases PItotalis affected by O3treatment more than PIABS.

Fig. 2 Performance indexes (A, PItotal; B, PIABS) and reaction centre (RC) density on a chlorophyll basis (RC/ABS) (C), the maximum quantum yield of primary photochemistry (φPo) (D), the efficiency (ΨEo) with which a trapped exciton can move an electron into the electron transport chain further than QA– (E), and the probability to reduce the end electron acceptors (δRo ) (F) of non-fumigated and fumigated plants with O3 under well-watered (WW, WW-O3 ) and water-stressed (WS, WS-O3) conditions. Each bar represents the mean value, and vertical error bar is SE and denotes 0.95 Confidence level. Capital letters indicate significant differences(P<0.05) between treatments for the same week, whereas small letters indicate significant differences over time for each treatment(P<0.05). W1-4, week 1-4.
Taking in consideration that the PItotalis a very sensitive parameter, our results revealed that PItotalin leaves changes over time. Since the photosynthetic process is affected by various environmental factors such as water stress and O3, it was expected that PItotalin the well-watered and nonfumigated plants will increase as the experiment continues over the weeks. However, it showed a downward trend over time in all treatments but in fumigated and well watered plants the decline was stronger. In Oxford clone experiment conducted in open-top chambers, Desotgiuet al.(2012) indicated that the PItotalin leaves changes over time according to development stage and ageing process. The PItotaldecreased over time due to ageing but O3fumigation enhanced this downward trend under well-watered conditions.
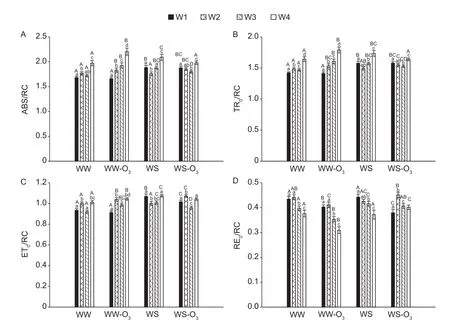
Fig. 3 specific energy fluxes per reaction centre (RC) for non-fumigated and fumigated treatments under well-watered (WW,WW-O3) and water-stressed (WS, WS-O3) conditions: absorption flux (ABS/RC, A), trapping flux (TR0/RC, B), electron transport flux (ET0/RC, C), and electron flux for reducing end electron acceptors at PSI acceptor side (RE0/RC, D). Each bar represents the mean value, and vertical error bar is SE and denotes 0.95 Confidence level. Capital letters indicate significant differences(P<0.05) between treatments for the same week, whereas small letters indicate significant differences over time for each treatment(P<0.05). W1-4, week 1-4.

Fig. 4 Stomatal conductance of canola leaves from nonfumigated and fumigated plants under well-watered (WW,WW-O3) and water-stressed (WS, WS-O3) conditions. Each bar represents the mean value±SE (average of all weeks).
Higher differences between O3-treated and control plants in the maximum yield of primary photochemistry of PS II(φPo=TR0/ABS) at predawn (dark conditions) was observed by Desotgiuet al. (2013). In contrast, minor differences in the maximum yield of primary photochemistry of PS II were observed in our present study, showing thatφPois the less sensitive parameter to the effect of O3and drought. Similar findings have been also reported under dark chilling, drought and O3(Strausset al. 2006; Oukarroumet al. 2007; Bussottiet al. 2011). In well-watered plants, theδRodecreased in fumigated plants, causing a significant reduction of PItotal.The reduction in density of PSI and the compromised ability of the end acceptors of electrons (ferredoxine, NADP+)and RuBP to manage effectively the flux of electrons may cause an imbalance between the electrons sent through the electron transport chain to recipients which are beyond the PSI (Bussottiet al. 2011).
ABS/RC is a measure of the average absorption per active RC and concomitantly of the average amount of absorbing chlorophylls per active RC, i.e. of the apparent antenna size (Strasseret al. 2004). In addition, the changes of ABS/RC are the inverse of the changes of RC/ABS values. An increase of ABS/RC values means that a fraction of RCs is inactivated or the functional antenna has increased in size (Yusufet al. 2010). However, we here found that ABS/RC increases whereasφPoundergoes slight changes and the TR0/RC closely follows the increase in ABS/RC.This indicates that the changes of ABS/RC are changes of functional antenna size, meaning that the functional antenna size was affected by O3and drought.
We note that the probability to reduce an end electron acceptor (δRo=RE0/ET0) as well the RC density on a chl basis (RC/ABS) is sensitive to O3even under waterstressed conditions, though much less than in wellwatered. Therefore, the more sensitive components of the photosynthetic electron transport chain appeared to have been the probability to reduce an end electron acceptor(δRo=RE0/ET0) as well the RC density on a chlorophyll basis (RC/ABS) under well-watered conditions, particularly in fumigated plants. The probability to move an electron beyond QA–(ΨEo=ET0/TR0) and the maximum quantum yield of primary photochemistry (φPo=TR0/ABS) were less affected. Pollastriniet al.(2013) reported that drought had more effect than O3stress in reducing growth and carbon allocation in plant organs and in driving acclimation processes. In the current study, the effect of O3was slight under drought conditions and this is related with the results on stomatal conductance which showed that it decreases in water-stressed plants compared to well-watered plants.
5. Conclusion
Based on the analysis of Chlafluorescence in OTCs conditions, the effect of O3was slight upon drought stress.Ozone caused an increase in the functional antenna size and a more pronounced decrease in the probability to reduce the end electron acceptors (δRo=RE0/ET0). Ozone and drought had negligible effect on the maximum quantum yield of primary photochemistry (φPo=TR0/ABS) and only slight effect on the probability of an electron to move beyond QA–(ΨEo=ET0/TR0). This study also supports the significance of the multiple turn-over region of the fluorescence transient (as revealed by the related parameterδRo=RE0/ET0) in the response of plants to O3and drought stress. It can be concluded that O3and drought stress altered the fluorescence induction and impaired photosynthetic systems in canola plants. The JIP-test is a good indicator to detect fluorescence induction and photosynthetic activity of the PS II RC complex of O3-treated canola plants. Considering the importance of this crop, the current findings do suggest that elevated O3levels will have an effect on the production of oilseeds which will in turn impact the local economy.It is important to acknowledge that this effect could be cultivar dependent as a result it will be vital to gain a greater understanding of the canola cultivars responses to O3in order to better predict crops response to changing atmospheric environment.
Acknowledgements
This research was supported by the Cuomo Foundation through the partnership with the Intergovernmental Panel on Climate Change (IPCC) Scholarship Programme and by the Applied Centre for Climate and Earth Systems Science (ACCESS), South Africa. We are thankful to Dr.Merope Tsimilli-Michael (Cyprus) for her helpful explanations concerning the concepts and application of the JIP-test.Prof. Suria Ellis (North-West University, South Africa) for her assistance with the statistical analysis.
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Bilgin D D, Zavala J A, Zhu J I, Clough S J, Ort D R, DeLucia E H.2010. Biotic stress globally downregulates photosynthesis genes.Plant,Cell and Environment, 33, 1597–1613.
Bohler S, Cuypers A, Vangronsveld J. 2015. Interactive effects between ozone and drought: Sorrow or joy? In: Mahalingam R, ed.,Combined Stresses in Plants. Springer International Publishing, London. pp. 147–157.
Bussotti F, Desotgiu R, Cascio C, Pollastrini M, Gravano E,Gerosa G, Marzuoli R, Nali C, Lorenzini G, Salvatori E,Manes F. 2011. Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data.Environmental and Experimental Botany,73, 19–30.
Chaves M M, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell.Annals of Botany, 103, 551–560.
Christ M M, Ainsworth E A, Nelson R, Schurr U, Walter A.2006. Anticipated yield loss in field-grown soybean under elevated ozone can be avoided at the expense of leaf growth during early reproductive growth stages in favourable environmental conditions.Journal of Experimental Botany,57, 2267–2275.
Desotgiu R, Pollastrini M, Cascio C, Gerosa G, Marzuoli R,Bussotti F. 2012. Chlorophyllafluorescence analysis along a vertical gradient of the crown in a poplar subjected to ozone and water stress.Tree Physiology, 32, 976–986.
Desotgiu R, Pollastrini M, Cascio C, Gerosa G, Marzuoli R I,Bussotti F. 2013. Responses to ozone onPopulus“Oxford”clone in an open top chamber experiment assessed before sunrise and in full sunlight.Photosynthetica, 51, 267–280.
Gornall J, Betts R, Burke E, Clark R, Camp J, Willett K, Wiltshire A. 2010. Implications of climate change for agricultural productivity in the early twenty- first century.PhilosophicalTransactions of the Royal Society of London(B: Biological Sciences), 365, 2973–2989.
van Heerden P D, Tsimilli-Michael M, Krüger G H, Strasser R J. 2003. Dark chilling effects on soybean genotypes during vegetative development: Parallel studies of CO2assimilation, chlorophyllafluorescence kinetics OJIP and nitrogen fixation.Physiologia Plantarum, 117, 476–491.
Heyneke E, Smit P R, van Rensburg L, Krüger G H. 2012.Open-top chambers to study air pollution impacts in South Africa. Part I: Microclimate in open-top chambers.South African Journal of Plant and Soil, 29, 1–7.
Laakso L, Beukes J P, van Zyl P G, Pienaar J J, Josipovic M,Venter A, Jaars K, Vakkari V, Labuschagne C, Chiloane K. 2013. Ozone concentrations and their potential impacts on vegetation in Southern Africa.Developments in Environmental Science, 13, 429-450.
Mills G, Harmens H. 2011.Ozone Pollution:A Hidden Threat to Food Security. Programme Coordination Centre for the ICP Vegetation, Centre for Ecology and Hydrology, Bangor, UK.
Mills G, Hayes F, Williams P, Harmens H. 2005.ICP vegetation experimental protocol for monitoring the incidences of ozone injury on vegetation. [2017-03-21]. http://icpvegetation.ceh.ac.uk/
Munns R, James R A, Läuchli A. 2006. Approaches to increasing the salt tolerance of wheat and other cereals.Journal of Experimental Botany, 57, 1025–1043.
Oukarroum A, El Madidi S, Schansker G, Strasser R J. 2007.Probing the responses of barley cultivars (Hordeum vulgareL.) by chlorophyllafluorescence OLKJIP under drought stress and re-watering.Environmental and Experimental Botany, 60, 438–446.
Öz M T, Turan Ö, Kayihan C, Eyidoğan F, Ekmekçi Y, Yücel M,Öktem H A. 2014. Evaluation of photosynthetic performance of wheat cultivars exposed to boron toxicity by the JIP fluorescence test.Photosynthetica, 52, 555–563.
Pollastrini M, Desotgiu R, Camin F, Ziller L, Marzuoli R, Gerosa G, Bussotti F. 2013. Intra-annual pattern of photosynthesis,growth and stable isotope partitioning in a poplar clone subjected to ozone and water stress.Water,Air and Soil Pollution, 224, 1761.
Porter J R, Xie L, Challinor A J, Cochrane K, Howden S M, Iqbal M M, Lobell D B, Travasso M I. 2014. Food security and food production systems. In: Field C B, ed.,Climate Change 2014:Impacts,Adaptation,and Vulnerability.Part A: Global and Sectoral Aspects.Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press,Cambridge. pp. 485–533.
Roshchina V V. 2003. Auto fluorescence of plant secreting cells as a biosensor and bioindicator reaction.Journal of Fluorescence, 13, 403–420.
Strasser R J, Tsimilli-Michael M, Dangre D, Rai M. 2007.Biophysical phenomics reveals functional building blocks of plants systems biology: A case study for the evaluation of the impact of mycorrhization withPiriformospora indica. In:Varma A, Oelmüller R, eds.,Advanced Techniques in Soil Microbiology. Springer, Berlin, Heidelberg. pp. 319–342.
Strasser R J, Tsimilli-Michael M, Srivastava A. 2004. Analysis of the chlorophyllafluorescence transient. In: Papageorgiou E, Govindjee G C, eds.,Chlorophyll Fluorescence:A Signature of Photosynthesis. Kluwer Academic Publishers,The Netherlands. pp. 321–362.
Strauss A J, Krüger G H, Strasser R J, Van Heerden P D. 2006.Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyllafluorescence transient OJIP.Environmental and Experimental Botany, 56, 147–157.
van Tienhoven A M, Zunckel M, Emberson L, Koosailee A, Otter L. 2006. Preliminary assessment of risk of ozone impacts to maize (Zea mays) in Southern Africa.Environmental Pollution, 140, 220–230.
Tsimilli-Michael M, Strasser R J. 2013. Biophysical phenomics:evaluation of the impact of mycorrhization with piriformospora indica. In: Varmaet al. eds.,Piriformospora Indica: Soil Biology33. Springer, Berlin. pp. 173-189.
Yusuf M A, Kumar D, Rajwanshi R, Strasser R J, Tsimilli-Michael M, Sarin N B. 2010. Overexpression of γ-tocopherol methyl transferase gene in transgenicBrassica junceaplants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements.Biochimica et Biophysica Acta(BBA)- Bioenergetics, 1797, 1428–1438.
Zhu M, Monroe J, Suhail Y, Villiers F, Mullen J, Pater D, Hauser F, Jeon B W, Bader J S, Kwak J M, Schroeder J I. 2016.Molecular and systems approaches towards droughttolerant canola crops.New Phytologist, 210, 1169-1189.
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China
- Genes encoding heat shock proteins in the endoparasitoid wasp,Cotesia chilonis, and their expression in response to temperatures
- Molecular mechanisms controlling seed set in cereal crop species under stress and non-stress conditions
- Rapid semi-quantification of triacylglycerols, phosphatidylcholines,and free fatty acids in the rice bran of one grain
- lnfluence of different nitrogen application on flour properties,gluten properties by HPLC and end-use quality of Korean wheat
- Evaluation indices of sour flavor for apple fruit and grading standards
