Rapid semi-quantification of triacylglycerols, phosphatidylcholines,and free fatty acids in the rice bran of one grain
2018-05-08JunkoNagamotoAiSawazakiMotonoriMiyagoBungoShirouchiMitsukazuSakataTONGLitaoToshihiroKumamaruMasaoSato
Junko Nagamoto, Ai Sawazaki, Motonori Miyago, Bungo Shirouchi, Mitsukazu Sakata, TONG Litao, Toshihiro Kumamaru, Masao Sato
1 Laboratory of Nutrition Chemistry, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School, Kyushu University, Fukuoka 812-8581, Japan
2 Laboratory of Plant Genetic Resources, Institute of Genetic Resources, Kyushu University, Fukuoka 812-8581, Japan
1. lntroduction
In Asia, rice bran oil is extracted from rice bran, which is composed of the embryo and aleurone layer, using hexane and then used as cooking oil (Pal and Pratap 2017).However, when triacylglycerols (TAGs), the main component of rice bran oil, are excluded, the crude oil contains only phospholipids and free fatty acids (FFAs) that cannot be used as re fined oil. FFAs are produced by lipase present in rice bran (Bhattacharyya and Bhattacharyya 1989). It is possible to produce rice oil more efficiently when rice bran is rich in TAG but poor in phospholipids and FFAs.For screening rice lines suitable for rice oil production, we attempted to rapidly determine the TAG, phosphatidylcholine(PC) which is a major phospholipid class, and FFA contents in the rice bran obtained from one grain using a microplate assay method.
2. Materials and methods
2.1. Materials
Glyceryl trioleate (≥99% purity) was purchased from Sigma Aldrich (Tokyo, Japan) and then used as a TAG standard.COATSOME NC-20 (soybean PC, 90% purity) was purchased from Wako Pure Chemical Industries, Ltd. (Tokyo,Japan) and then used as a PC standard. Oleic acid (≥99%purity) was purchased from Sigma Aldrich and then used as an FFA standard. ADAM®(9-anthryldiazomethane) (Nimura and Kinoshita 1980; Roemen and van der Vusse 1991) was purchased from Funakoshi Co., Ltd. (Tokyo, Japan) and then used as a fluorescent reagent for FFA determination. Triton X-100, Tween 40, and Nonidet P-40 were purchased from Nacalai Tesque, Inc. (Kyoto, Japan) (Fig. 1).
2.2. Methods
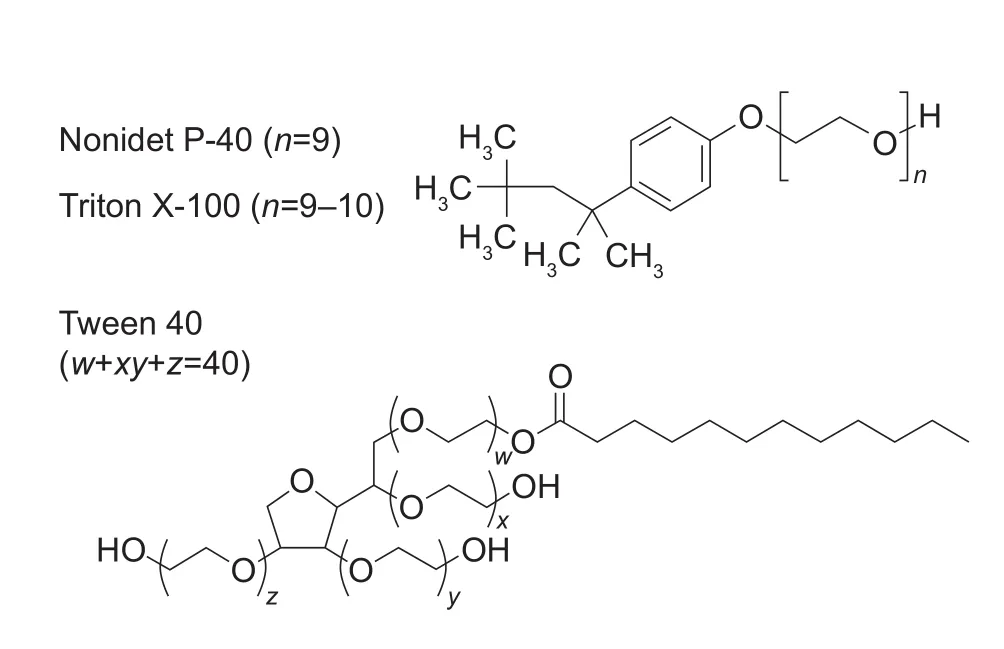
Fig. 1 Structures of the surfactants used in this study.
Rice bran including the rice germ and aleurone layer of unpolished rice was scraped off with a scalpel and collected in a microcentrifuge tube placed on ice. The obtained bran was weighed in an electronic precision balance. The bran samples were transferred to a glass 96-well plate, and 250 μL of chloroform:methanol (2:1,v/v) solution (CM solution) was added to the wells (Folchet al. 1957). For the TAG calibration curve, 10 mg glyceryl trioleate mL–1CM solution; for the PC calibration curve,0.0018 mg COATSOME NC-20 mL–1CM solution; and for the FFA calibration curve, 2.78 mg oleic acid mL–1CM solution were prepared. Three solutions were equivalently mixed and the solution was used as a standard solution.To each well of the plate, 250 μL of the standard solution was added at different concentrations (5- to 200-fold dilution). The glass plate was incubated at 45°C for 30 min, and then dried with nitrogen gas. To each well, 70 μL of an ADAM staining solution adjusted to 0.2 mg mL–1with CM solution was added. The plate was shaken at 1 000 r min–1for 3 min with a plate shaker and then left for 2 min.FFA contents were measured using a fluorescence plate reader (FLx800, BioTek®Instruments, Inc., Vermont, USA).The excitation and emission wavelength filters were set at 360 and 410 nm, respectively. After FFA determination,50 μL of 5% Nonidet P-40 in methanol was added to each well, and then the plate was shaken with the shaker at 1 000 r min–1for 3 min and dried with nitrogen gas. We also examined the effect of three different surfactants(Triton X-100, Tween 40, and Nonidet P-40, as shown in Fig. 1) on TAG determination (Table 1 and Figs. 2–3). We selected Nonidet P-40 for subsequent experiments, based on the results (Table 1 and Fig. 3). To each well, 100 μL of deionized water was added and then the plate was shaken with the shaker at 1 000 r min–1for 3 min. For TAG determination, 20 μL from each well was transferred into its corresponding well of an empty plate. The remaining 80 μL of solution in the original wells was used for PC determination. To the wells for TAG measurement, 200 μL of chromogenic reagent for the Triglyceride E-test Wako(Wako Pure Chemical Industries, Ltd.) (Spayd and Bruschi 1978) was added, and 180 μL of chromogenic reagent for the Phospholipid C-test Wako (Wako Pure Chemical Industries, Ltd.) (Takayamaet al. 1977) was added to the PC determination wells. The plates were shaken with the shaker at 1 000 r min–1for 3 min. All wells were measured at 600 nm using a spectrophotometer (Multiskan GO, Thermo Fisher Scientific Co., Ltd., Yokohama, Japan) set at 37°C for the TAG and PC determinations. To con firm the validity of our method,the TAG contents of the three rice lines (A, B, and C) used in this study were compared with previous measurements using large amounts of rice grain (Sakataet al. 2016).

Table 1 Correlation coefficients between absorbance and triolein content (7–150 mg L–1) using three different surfactant concentrations
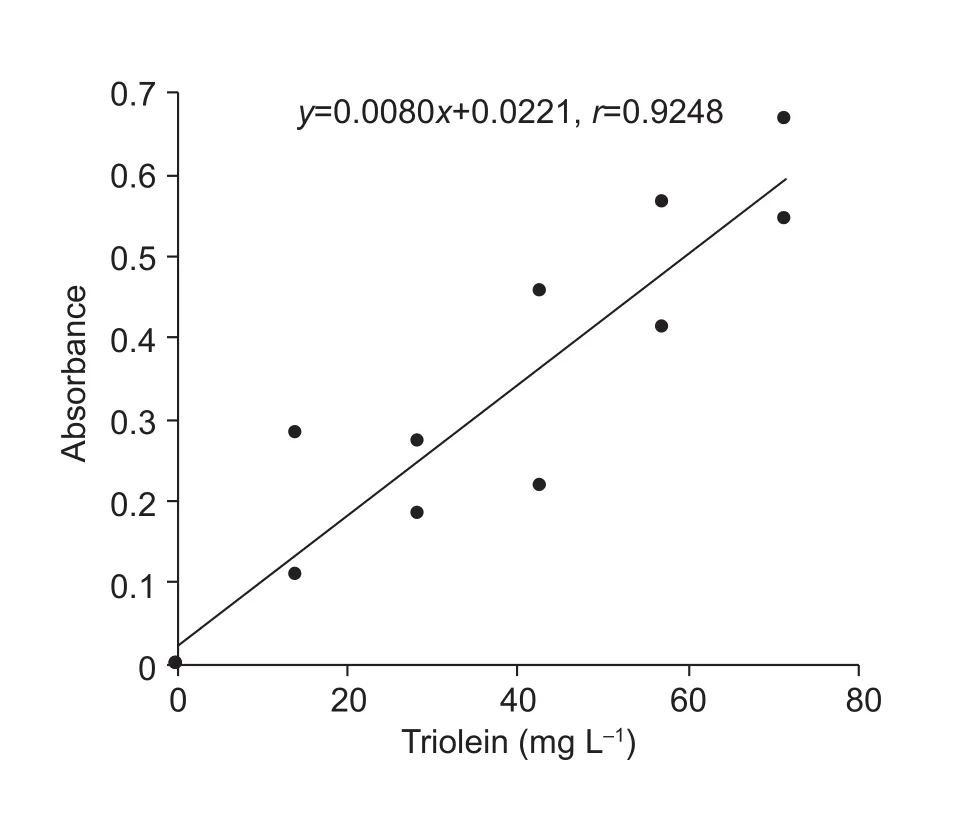
Fig. 2 Relationship between absorbance and concentration of triolein using Triton X-100 as a surfactant.

Fig. 3 Calibration curve for triacylglycerol (TAG) measurement using different concentrations of surfactants at 2.5% (A), 5%(B) and 10% (C), respectively.
3. Results and discussion
3.1. ln fluence of surfactants on TAG measurement
For measuring the amount of TAGs, the type of surfactant used in the lipid dissolving water was examined. As shown in Fig. 2, an effective calibration curve with the triolein standard was not obtained using Triton X-100 as a surfactant. Next,the nonionic surfactants, Nonidet P-40 and Tween 40, which are considered not to inhibit enzymatic reactions, were used. As shown in Table 1, the linear relationship with a correlation coefficient of 0.9993 was obtained with 2.5%Nonidet P-40 in the TAG (triolein) concentration range of 7–150 mg L–1. The structures of Triton X-100 and Nonidet P-40 are quite similar (Fig. 1). Triton X-100 and Nonidet P-40 contain polyoxyethylene chains. A difference in the length of their polyoxyethylene chains affects the average number of monomers per micelle. A previous study showed that the surfactant monomer numbers per micelle of Triton X-100 and Nonidet P-40 were 9.6 and 9.0, respectively(Arnold and Linke 2007). This suggests that these surfactant properties enhanced the reactivity of lipoprotein lipase (LPL),which is found in the commercial enzyme assay kit, to TAGs.TAGs are located in the center of micelles as a “core lipid”(Scanu 1979; Assman 1982). In nature, the LPL contained in this commercial enzyme assay kit degrades TAGs in lipoproteins (Wong and Schotz 2002). Researchers in the field of nutrition have adapted this kit in order to measure TAG contents in lipid extracts from animal tissues (Carret al. 1993; Sugawara and Miyazawa 2001). Triton X-100 has been used as a surfactant for these measurements. In other words, it is said that micelles made with this surfactant mimic a lipoprotein serum. Therefore, phospholipid (PL) is placed on the micelle surface together with the surfactant,and TAGs are placed at the center of the micelle (Scanu 1979; Assman 1982). Furthermore, correlation coefficients of the calibration curves for PC (5–70 mg L–1) and FFA(8–200 mg L–1) measurements were 0.9990 and 0.9987,respectively (Fig. 4). Under these conditions, it is thought that the lipid components of rice bran can be measured from the same extract. Based on this result, we surmised that 2.5% Nonidet P-40 is present in the lipid-dissolved solution.
3.2. Quantification of TAGs, PCs, and FFAs in actual rice bran samples using this method and validation of this method
To evaluate the validity of this method, we directly compared the TAG contents in actual rice bran samples measured using this method with previous measurements using large amounts of rice grain (Sakataet al. 2016). We did this using three rice lines with different TAG contents. As shown in Fig. 5, the results of this method showed good correspondence with those of previous reports using large amounts of rice grain (r=0.9881). The PC content of rice bran is estimated to be 5–6 mg g–1rice bran when the crude lipid content is around 100 mg g–1of rice bran (Liuet al.2013). To measure the PC contents exactly, it is necessary to use thin layer chromatography (TLC) separation of the PL classes, but because the amount of PCs is high in rice bran, a considerable amount of rice is necessary. Generally,the levels of each PL class are determined by quantification of phosphorus according to the method described by Rouseret al. (1966). The detection sensitivity of Rouser’s method is low, and the detection limit is approximately 0.25 μg. Taken together, it is difficult to visualize PC bands on the TLC plate if the PC contents in the rice bran are low. The FFA contents in rice brans are highly variable(Bhattacharyya and Bhattacharyya 1989). An accurate quantitative method for FFAs is to quantify the fatty acid compositionviagas-liquid chromatography (GLC) with an internal standard after fractionation by TLC. However, this method has a disadvantage in that an appropriate internal standard cannot be found. Rice has minor fatty acids that can be separated by GLC, even though their amounts are different (Lugay and Juliano 1964; Shin and Godber 1996;Zhouet al. 2003). Many researchers use odd-chain fatty acids as an internal standard for GLC, but they also exist in rice bran (Yilmazet al. 2014; MECSST 2015). In addition to the analysis of fatty acid composition by GLC, the amount of FFAs in edible oil is usually measured as an acid value(AV) (Horwitzet al. 1976). Because this procedure involves titration, a large sample of rice bran is required. Therefore,to evaluate the validity of FFA contents in rice bran measured using our method, it is necessary to measure FFA contents extracted from a large amount of bran using ADAM and then to compare the results.
4. Conclusion
Three lipid components of rice bran can be measured by the microplate assay method. It is thought that it can be used for screening oil-rich rice lines. For the quantification of FFAs and PCs, it is necessary to compare this method with a more advanced method.
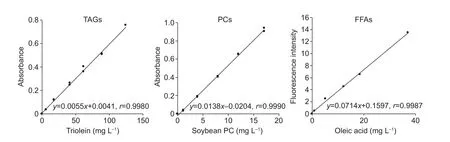
Fig. 4 Relationship between absorbance and concentrations of triolein, soybean PC, and oleic acid (using Nonidet P-40 as a surfactant). TAGs, triacylglycerols; PCs, phosphatidylcholines; FFAs, free fatty acids.

Fig. 5 Quantities of triacylglycerols (TAGs), phosphatidylcholines (PCs), and free fatty acids (FFAs) per gram rice bran. Gray columns indicate the data of the large-scale extraction method for TAGs only. Blank columns indicate the data of the method in the present study. The data are expressed as the mean±SEM (n=3).
Acknowledgements
This work was supported by the Ministry of Agriculture,Forestry and Fisheries, MAFF, Tokyo, Japan (27001B). We would like to thank Editage (www.editage.jp) for the English language editing.
Arnold T, Linke D. 2007. Phase separation in the isolation and purification of membrane proteins.Biotechniques, 43,427–434.
Assman G. 1982.Lipid Metabolism and Atherosclerosis.Schattauer Verlag, Stuttgart. p. 14.
Bhattacharyya S, Bhattacharyya D K. 1989. Biorefining of high acid rice bran oil.Journal of the American Oil Chemists’Society, 66, 1469–1471.
Carr T P, Andresen C J, Rudel L L. 1993. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts.Clinical Biochemistry,26, 39–42.
Folch J, Lees M, Sloane-Stanley G H. 1957. A simple method for the isolation and purification of total lipids from animal tissues.Journal of Biological Chemistry, 226, 497–509.
Horwitz W. 1976. Methods of analysis approved by the codex alimentarius commission.Journal Association of Official Analytical Chemists, 59, 658–661.
Liu L, Waters D L, Rose T J, Bao J, King G J. 2013. Phospholipids in rice: Significance in grain quality and health benefits: A review.Food Chemistry, 139, 1133–1145.
Lugay J C, Juliano B O. 1964. Fatty acid composition of rice lipids by gas-liquid chromatography.Journal of the American Oil Chemists’ Society, 41, 273–275.
MECSST (Ministry of Education, Culture, Sports, Science and Technology of Japan). The 7th revised edition of standard tables of food composition in Japan 2015. [2017-09-26].http://www.mext.go.jp/a_menu/syokuhinseibun/1365451.htm (in Japanese)
Nimura N, Kinoshita T. 1980. Fluorescent labeling of fatty acids with 9-anthryldiazomethane (ADAM) for high performance liquid chromatography.Analytical Letters, 13, 191–202.
Pal Y P, Pratap A P. 2017. Rice bran oil: A versatile source for edible and industrial applications.Journal of Oleo Science,66, 551–556.
Roemen T H, van der Vusse G J. 1991. Assessment of fatty acids in cardiac tissue as 9-anthryldiazomethane esters by high-performance liquid chromatography.Journal of Chromatography, 570, 243–251.
Rouser G, Siakotos A N, Fleischer S. 1966. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots.Lipids, 1, 85–86.
Sakata M, Seno M, Matsusaka H, Takahashi K, Nakamura Y,Yamagata Y, Angeles E R, Mochizuki T, Kumamaru T, Sato M, Enomoto A, Tashiro K, Kuhara S, Satoh H, Yoshimura A. 2016. Development and evaluation of rice giant embryo mutants for high oil content originated from a high-yielding cultivar ‘Mizuhochikara’.Breed Science, 66, 425–433.
Scanu A M. 1979.The Biochemistry of Atherosclerosis. Marcel Dekker, New York. p. 3.
Shin T S, Godber J S. 1996. Changes of endogenous antioxidants and fatty acid composition in irradiated rice bran during storage.Journal of Agricultural and Food Chemistry,44, 567–573.
Spayd R W, Bruschi B. 1978. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide.Clinical Chemistry, 24, 1343–1350.
Sugawara T, Miyazawa T. 2001. Beneficial effect of dietary wheat glycolipids on cecum short-chain fatty acid and secondary bile acid pro files in mice.Journal of Nutritional Science and Vitaminology, 17, 299–305.
Takayama M, Itoh S, Nagasaki T, Tanimizu I. 1977. A new enzymatic method for determination of serum cholinecontaining phospholipids.Clinica Chimica Acta, 79, 93–98.
Wong H, Schotz M C. 2002. The lipase gene family.Journal of Lipid Research, 43, 993–999.
Yılmaz N, Tuncel N B, Kocabıyık H. 2014. Infrared stabilization of rice bran and its effects on γ-oryzanol content, tocopherols and fatty acid composition.Journal of the Science of Food and Agriculture, 94, 1568–1576.
Zhou Z, Blanchard C, Helliwell S, Robards K. 2003. Fatty acid composition of three rice varieties following storage.Journal of Cereal Science, 37, 327–335.
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China
- Characterization of two novel heat shock protein 70s and their transcriptional expression patterns in response to thermal stress in adult of Frankliniella occidentalis (Thysanoptera: Thripidae)
- Molecular mechanisms controlling seed set in cereal crop species under stress and non-stress conditions
- lnfluence of different nitrogen application on flour properties,gluten properties by HPLC and end-use quality of Korean wheat
- Evaluation indices of sour flavor for apple fruit and grading standards
- Relationship of chemical properties of different peanut varieties to peanut butter storage stability
