Molecular mechanisms controlling seed set in cereal crop species under stress and non-stress conditions
2018-05-08LIHuiyongThomasbberstedt
LI Hui-yong , Thomas Lübberstedt
1 Cereal Crops Research Institute, Henan Academy of Agricultural Sciences, Zhengzhou 450002, P.R.China
2 Department of Agronomy, Iowa State University, Ames, IA 50010, USA
1. Introduction
Food security is of global concern, especially in developing countries (Rosegrant and Cline 2003). The six most widely grown crops in the world are maize, rice, wheat, soybean,barley, and sorghum. These crops are grown for seed on more than 40% of global cropland area, and account for 55% of non-meat calories and over 70% of animal feed. Among all seed crop species, cereal crops are most important for humans, which provide over 70% of the food consumed by humans. Though the seed yield per land area has increased during the last decades, 20–40% of the world’s potential crop production still is lost because of weeds, pests, diseases, and abiotic stress factors according to the FAO (2015). Even in major cereal crops producing countries, rice, wheat, and maize still have a 10–15% yield gap between actual and potential seed yields (Tollenaar and Lee 2002; Cassmanet al. 2003; Grassiniet al. 2011).However, there can be about 50% yield gap in specific crop planting areas. For example, the average maize yield in the US is 10.7 Mg ha–1, but the maximum yields exceed 18.8 Mg ha–1. Maize yield in China has the potential to increase dramatically due to the introduction of hybrids that are better adapted to denser planting conditions, mechanization, and improvements in seed technology (Liet al. 2011). Yield potential is defined as the yield of a cultivar grown under non-limiting abiotic and biotic conditions in an environment to which it is adapted. Causes for the yield gap are unfavorable abiotic or biotic growth conditions, limited water and nutrient supply, or exposure of plants to abiotic and/or biotic stress.Increased seed yield of cereal crops is predominantly the result of improved stress tolerance (Duvick 1997; Fasoula and Fasoula 2002).
In cereal crops, the process of seed formation can be divided into three stages: seed set, seed growth, and seed maturation (Ruanet al. 2012). Seed set is established during and soon after fertilization with two genetically identical male sperm cells. One male sperm cell fuses with the haploid egg cell, resulting in a diploid embryo. The other sperm cell fuses with two (female) haploid central cells,resulting in a triploid endosperm. This double fertilization event initiates seed development. Embryo and endosperm are surrounded by a maternally derived seed coat, which provides a shield for the developing seed (Köhler and Makarevich 2006). The newly formed seed then undergoes cell expansion and accumulation of storage products, mainly proteins, starch, and oils, which are typical features of growth and maturation stages (Weberet al. 2005).
During the process of seed formation in cereal crops,seed set features a transition from fertilized ovules to seed,which has a profound impact on later seed developmental stages, and determines seed number by determining cell numbers, and seed yield potential (Fig. 1). Seed set is more sensitive to internal or external stresses compared to later stages of seed development or vegetative growth (Boyer and McLaughlin 2007; Suwaet al. 2010; Zinnet al. 2010).Stresses include insufficient supply of nutrients, drought,heat, cold, high plant density, presence of weeds, plant diseases, and insect pests, which often induce floral or seed abortion and irreversible seed yield losses.
Maize and rice are the two most important cereal crops in the world, which have different floral architectures(monoeciousvs. hermaphrodite species, respectively) and photosynthetic pathways (C4vs. C3, respectively). Here,we focus on discussing genetic mechanisms controlling seed set in maize and rice at four developmental stages:(1) development of floral structure; (2) formation of viable gametes; (3) double fertilization; and (4) seed development and abortion.
2. Development of male and female floral architectures

Fig. 1 The major development stage and seed yield component in cereals.
Seed set is influenced by the floral architecture in cereal crops, which depends on the number and arrangement of floral branches (Vollbrechtet al. 2005). Variation in branching patterns lead to diversity in inflorescences and seed number (Satoh-Nagasawaet al. 2006). Maize possesses two types of inflorescences: the tassel and the ear. The tassel is a terminal, staminate inflorescence. The ear is a pistillate inflorescence produced on a lateral branch,which is a major yield trait controlled by the developmental fate of axillary shoot meristems (Satoh-Nagasawaet al.2006). The units of rice floral structure are spikelets and florets. Spike (an unbranched in florescence in which stalkless spikelets are arranged on an elongated axis)initiation and spikelet (contains one or more florets enclosed by two glumes) formation is the first phase of reproductive growth. This process provides the developmental basis for spike differentiation, which contributes to altering final seed number and yield potential (Sreenivasulu and Schnurbusch 2012). In the past decades, various major genes or QTL affecting the development of floral architecture have been cloned. In maize, inflorescence branching is regulated by threeRAMOSAgenes. RAMOSA1 (RA1) is a plant specific epidermal patterning factor-like protein (EPF-like protein),regulating the branching architecture of maize in florescence.InRA1mutants, both the tassel and ear become more branched. RAMOSA2 (RA2) encodes a lateral organ boundaries-domain protein (LOB-domain protein) whose RNA is expressed at the edge of the bract and meristem early in inflorescence development. RAMOSA3 (RA3)encodes a trehalose 6-phosphate phosphatase and is expressed in discrete domains subtending axillary in florescence meristems.RA2andRA3act upstream ofRA1,andRA3may act in parallel withRA2(Vollbrechtet al.2005; Satoh-Nagasawaet al. 2006). In rice, grain number 1a (Gn1a), encoding a cytokinin oxidase in rice (OsCKX2),wealthy farmers panicle1 (WFP1), encodingOsSPL14,and dense and erect panicle1 (OsDEP1), encoding a truncated phosphatidylethanolamine-binding protein are three important genes controlling the development of floral organs. Reduced expression ofOsCKX2causes cytokinin accumulation in in florescence meristems and increases the number of reproductive organs, resulting in enhanced grain yield (Ashikariet al. 2005). In contrast, increased expression ofOsDEP1enhances meristematic activity,resulting in a reduced length of in florescence internodes,an increased grain number per panicle and consequently,increased grain yield. Increased expression ofOsDEP1reduces the level ofOsCKX2expression, suggesting thatOsDEP1acts upstream ofOsCKX2to control cytokinin homeostasis in panicle meristems (Huanget al. 2009).Increased expression ofOsSPL14in the reproductive stage promotes panicle branching and grain yield, and also controls shoot branching at the vegetative stage (Miuraet al. 2010). Although those genes are involved in different pathways to control the development of floral architecture,they play important roles in regulating seed number and arrangement.
Flowering time is also an important selection criterion in cereal breeding, which not only influences plant demand for resources and a plant’s ability to capture resource for growth(Donget al. 2012), but also influences floral development and seed set. Among cereal crops, winter wheat relies on vernalization for flowering. Rice is a short day plant, and flowering time is day length sensitive. Maize undergoes the transition to flowering after a fixed number of leaves has been produced (Bortiri and Hake 2007; Itohet al.2010). Tropical maize is an exception, which is photoperiod sensitive for flowering time, and unadapted to temperate latitudes. However, the basic genetic mechanisms controlling flowering time are largely conserved among cereals (Lagercrantz 2009; Songet al. 2010). For example,the FRUITFUL1 (FUL1)/VERNALIZATION1 (VRN1) protein is associated with specifying competence to flower initiation and transition after a cold treatment (vernalization) in wheat(Danyluket al. 2003; Muraiet al. 2003; Loukoianovet al.2005). In maize,ZMM4is a MADS-box gene in the FUL1 family that regulates floral transition and in florescence development, which can be activated after floral transition in early developing inflorescences. Over-expression ofZMM4leads to early flowering in transgenic maize (Danilevskayaet al. 2008). In rice, overexpression ofOsMADS18can induce early flowering, accelerate the formation of axillary shoot meristem (Fornaraet al. 2004). Although evidence from various studies supports a complex gene network responsible for floral transition and floral development in cereals (Yanoet al. 2001; Izawaet al. 2003; Bosset al.2004; Bernier and Perilleux 2005), the function ofVRN1in wheat,ZMM4in maize, andOsMADS18in rice is similar to thatAPETALA1(AP1) inArabidopsis.AP1plays a central role in the transition from floral induction to flower formation(Wellmer and Riechmann 2010).
In addition, water stress during flower induction and in florescence development leads to a delay in flowering(anthesis), or even to complete inhibition (Mahalakshmi 1985; Wopereis 1996; Winkel 1997). High temperatures during floret formation cause complete or partial abortion(Sanini and Aspinall 1982), in which the main effect of heat stress is reduction of kernel number (Fischer 1985).
3. Formation of viable (male and female)gametes
In cereal crops, pollen and ovary development greatly depend on an adequate import or utilization of photoassimilates,mainly in the form of sucrose and starch. For pollen to be viable, it is necessary to synthesize sufficient starch,cellulose, and callose, which are main components for building internal pollen wall tubes. Starch, cellulose, and callose are all polymerized from glucose inα-1,4,β-1,4,andβ-1,3linkages, respectively (Kudlickaet al. 1997; Caiet al.2011). Glucose can be derived from sucrose hydrolysis and starch degradation by cell wall invertase (CWIN) and α-amylase, respectively (Fig. 2). The CWIN-mediated metabolic pathway is the main route to produce glucose.Reduction in CWIN expression or sucrose content leads to male sterility and seed abortion in wheat under drought stress (Koonjulet al. 2005) and in rice under cold stress(Oliveret al. 2007). Sucrose-rich pollen survives for a longer time than sucrose-poor pollen in maize and other crops(Hoekstraet al. 1989; Buitiniket al. 1996; Pacini 1996).Thus, sucrose supply and CWIN activity are key to male fertility and seed set.
Many studies also showed that viability of pollen is related to its water content and the humidity of atmosphere. Pollen needs strong protection (anther) against desiccation in time. Low water content of pollen can affect pollen growth speed and survival. However, anthers will not open at high air humidity, which can lead to premature loss of viability(Aylor 2003).
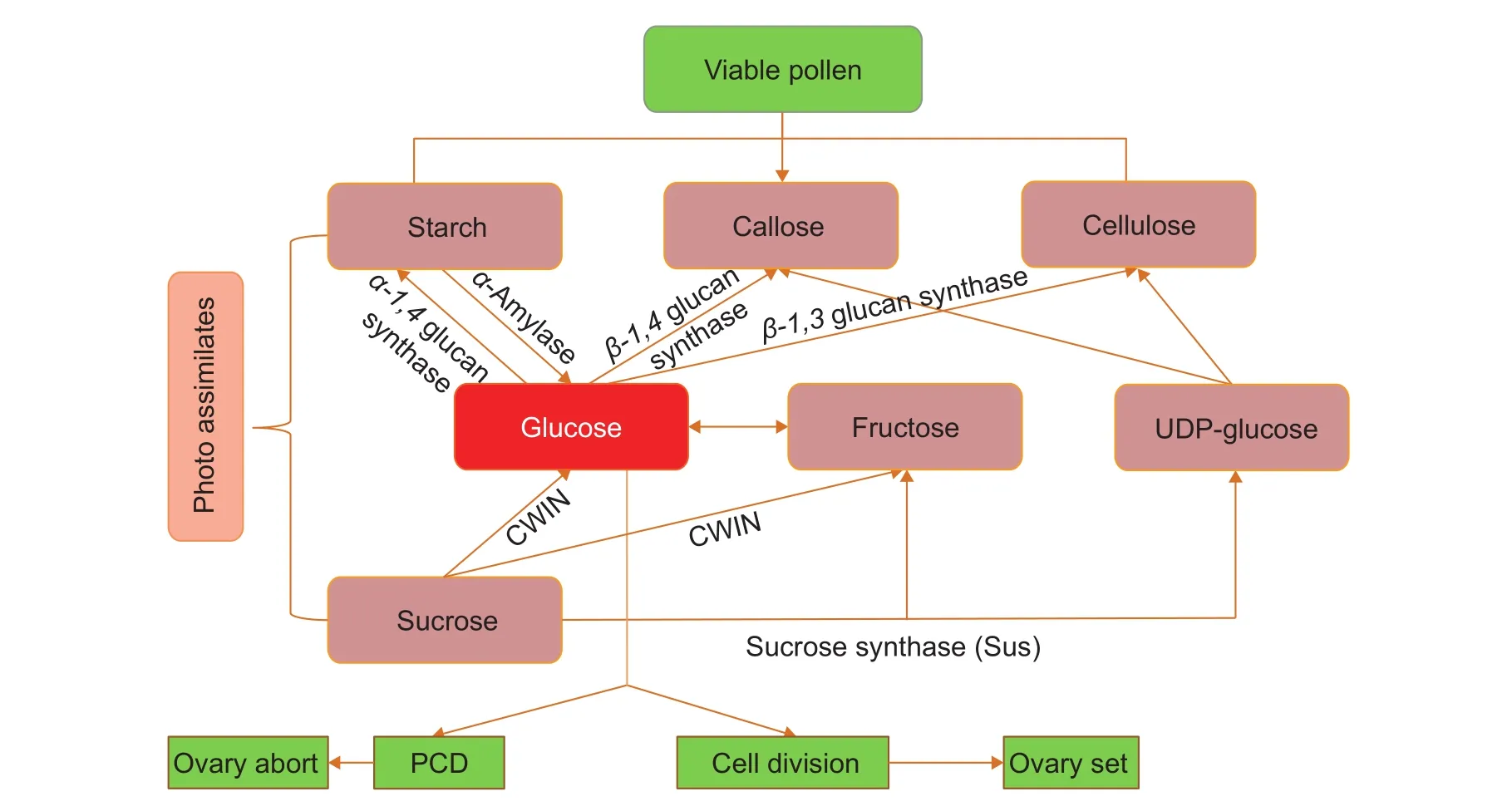
Fig. 2 The sugar regulated mechanism for pollen and ovary fertilization. CWIN, cell wall invertase; PCD, programmed cell death.
Ovary abortion has similar molecular mechanisms as pollen sterility (Ruanet al. 2010). In maize ovaries, phloem-imported sucrose supplies carbon for starch accumulation in ovary walls and to generate high glucose concentrations by CWIN in pedicels (Mclaughlinet al. 2004). Upon imposing a water deficit 5 days before an thesis, sucrose import is blocked due to inhibited leaf photosynthesis and remobilized ovary wall starch reserves. However, these become rapidly depleted, if drought persists for several days.Concomitantly, CWIN activities and glucose concentrations decrease, leading to ovary abortion and yield loss. A CWIN glucose signaling pathway is the primary genetic mechanism controlling maize ovary abortion (Mclaughlinet al. 2004). Glucose can repress the programmed cell death (PCD) pathway and promote cell division of filial cells(Ruanet al. 2012). Under stress conditions, photosynthetic activity is reduced (Schussler and Westgate 1991; Chaveset al. 2003), which results in decrease of glucose levels,followed by pollen sterility in anthers and activation of a PCD pathway leading to seed abortion in ovaries (Fig. 2).In summary, glucose is a key factor for pollen fertility and ovary development and, therefore, seed set in cereal crop species (Fig. 2).
4. Double fertilization
For successful seed set, pollen and ovaries in cereal crops must remain viable, pollen tubes must grow properly and release two sperm cells into the ovular embryo sac for double fertilization to produce embryo and endosperm.In maize, heat stress (>38°C) can lead to reduced pollen germination ability and pollen tube elongation, resulting in reduced seed set and seed production (Fischer 1985;Schoperet al. 1987). Increased temperature during the mid-anthesis period decreased the grain number per ear in spring wheat (Ferriset al. 1998), demonstrating heat sensitivity of fertilization and seed set. High-temperature stress at flowering reduces spikelet fertility in rice. Sterility is caused by poor anther dehiscence and low pollen grain production, and hence a low number of germinating pollen grains on the stigma (Matsui and Omasa 2002; Prasadet al. 2006).
During flower induction and in florescence development,drought stress leads to failure of panicle exertion and anther dehiscence in rice and sorghum (Ekanayakeet al. 1990;Craufurd and Peacock 1993; Wopereiset al. 1996), which leads to a substantial reduction in seed set. In maize,seed abortion is highly dependent on the timing of water stress. Low water availability before pollination results in seed abortion even if sufficient water was available at the time of pollination. Drought stress inhibits maize pistillate flower development, pistil integrity, ovule functions, and grain weight (Westgate 1994), while timing of male in florescence development and pollen shed is less affected, resulting in increased anthesis-silking intervals (ASI). An extended ASI reduces the chance that female spikelets will be selfpollinated and contribute to incomplete pollination (Fuad-Hassanet al. 2008), and thus increases the risk of yield reduction (Monneveuxet al. 2006; Brekkeet al. 2011).
In summary, pollen development is more prone to heat stress than that of ovaries. However, fertility of ovaries has a greater influence on seed set than that of pollen under drought stress in cereals (Boyer and McLaughlin 2007;Barnabáset al. 2008). Cereal breeding must develop cultivars tolerating multiple types of stresses impacting seed production (Tester and Bacic 2005). Low ASI under abiotic stress conditions is, therefore, important breeding goal.
In nature, self-incompatibility (SI) is a genetic mechanism to prevent self-fertilization by inhibiting the germination of pollen on stigmas, or the elongation of pollen tubes.Several grass species share a common incompatibility mechanism controlled by two unlinked loci, S and Z (Liet al. 1997). SI promotes cross-fertilization to produce heterozygous and vigorous plants. SI can be inactivated by high temperatures leading to pseudocompatibility in perennial ryegrass andIpomoea fistulosa(Prabhaet al.1982; Wilkins and Thorogood 1992). Pseudocompatibility will lead to self-pollination, and inbred offspring and thus directly and indirectly reduces seed set and seed yield.Although SI has not been reported in cereal crops including maize, rice, wheat, and barley, breeders have tried to utilize SI in F1hybrid breeding systems (Do Cantoet al. 2016).SI is also widespread in grasses and other plant families.
5. Seed development and abortion
Seed development is regulated by the interplay of phytohormones. Gibberellic acid (GA3) increases source strength by improving photosynthetic efficiency by influencing photosynthesis related enzymes (i.e., Rubisco, fructose-1,6-biphosphatase, and sucrose phosphate synthase), leaf area, light interception, and phloem loading (Iqbalet al.2011). GA3also increases sink strength by promoting cell division, general growth, and carbohydrate import by inducing sucrolytic activities, namely CWIN, a key enzyme in the regulation of phloem unloading (Roitsch and González 2004). GA3signaling is involved in metabolic adjustment for maintaining source-sink relations, increasing the efficiency of assimilate production and transport under limiting environmental conditions (Achardet al. 2006). However,most studies indicate that GA3signaling in response to stress reduces growth to allow plant adaptation and survival.Cytokinin (CK) levels play a key regulatory role for plant growth and survival. Optimal CK levels are necessary to increase not only leaf longevity and photosynthetic capacity (source strength) but also growth of sink organs(sink strength) under abiotic stress (Haet al. 2012; Zalabáket al. 2013). Constitutive over-expression of the CK-degrading enzyme cytokinin oxidase (CKX) or inhibition of the CK-biosynthetic IPT1, IPT3, IPT5, and IPT7 genes resulted in CK deficiency and enhanced drought and salt stress-tolerant phenotypes inA. thaliana(Nishiyamaet al.2011). However, in rice, reduced expression ofOsCKX2causes cytokinin accumulation in in florescence meristems and increases the number of reproductive organs, resulting in enhanced grain yield (Ashikariet al. 2005). Auxins influence carbon partitioning and stimulate mobilization of carbohydrates in leaves and the upper stem and increase translocation of assimilates towards sink organs (Smith and Samach 2013). Auxins regulate the activity of CWIN and thus sucrose allocation in sink organs (Albaceteet al.2008). ABA has been implicated in male sterility of tomato,rice, and wheat (Morgan 1980; Morgan and King 1984;Westgateet al. 1996). Exogenous ABA application inhibits the activity of invertases and monosaccharide transporters to reduce sucrose content, leading to pollen sterility and PCD in barley (Parishet al. 2012). However, some studies in cereals reported positive correlations between grain ABA content and efficient seed filling by optimizing faster remobilization events from stem reserves (nonstructural carbohydrates), a critical factor in sustaining grain filling and grain yield under drought stress (Yanget al. 2004). Ethylene is often regarded as a growth inhibitor (Pieriket al. 2007).Male gametophyte development is susceptible to ethylene at the stage of pollen mitosis. Ethylene has been used to manipulate the development of pollen grains by application of an ethylene inhibitor, to promote dry mass accumulation and concentrations of starch and reducing sugars in anthers of basal spikelets leading to improved seed set in rice (Naik and Mohapatra 1999). In summary, seed set is controlled by a phytohormone interplay, controlling the balance between source and sink strength under both optimal and stress conditions.
Seed development greatly depends on adequate supply of photosynthetic products and nutrient use efficiency.Water deficiency results in inhibition of photosynthesis,and reduces nutrient supply to generative organs. At the same time, water shortage limits N uptake from soil and decreases the nitrate concentration in xylem, affecting ABA accumulation in leaves, which leads to altered ear structure and yield reduction (Carcovaet al. 2000). In addition, high planting density decreases light penetration into the canopy, reducing photosynthetic capacity (Hammeret al. 2009), which then affects the final kernel number and characteristics (Borraset al. 2003). Many studies reported that grain number and weight both decreased in maize as plant density increased, suggesting a complex interaction between the sink and assimilate supply (Sangoiet al. 2002; Hashemiet al. 2005; Lashkariet al. 2011).Under full sunlight, plants are exposed to relatively equal fluxes of light with wavelengths of 600–700 nm (red light)and 700–800 nm (far-red light) (Holmesand Smith 1977).When plant density increases, red light is intercepted,while far-red light is largely reflected, creating a far-red light enriched environment. Under this condition, a series of morphological changes take place, including increased plant height, decreased leaf blade area (Smith 1995), decreased stem diameter (Lashkariet al. 2011), and delayed pollen shed and silking (Tokatlidis and Koutroubas 2004). Plant growth rate is reduced during reproductive stages (Rossiniet al. 2011), resulting in partitioning of more assimilates towards vegetative instead of reproductive growth (Kebrom and Brutnell 2007). In addition, the axillary position of ears in maize is subject to apical dominance, assimilates are partitioned to the shoot rather than the ear under high planting density (Sangoiet al. 2002), which increases the risk that ears will have poor or not seed set.
Biotic stress occurs as a result of damage to plants by bacteria, viruses, fungi, parasites, insects, and weeds.Plants are under constant assault by pathogens and competitors during their development (Pimentel 1991),and evolved a myriad of defenses to meet requirements for cellular maintenance, growth, and reproduction (Tianet al.2003; Bergeret al. 2007). Although defense response to different biotic stressors is highly variable, the transcriptional response to biotic stress is highly coordinated, which means that biotic stress globally down-regulates photosynthesis genes (Zouet al. 2005; Bergeret al. 2007; Bilginet al.2010). In order to trigger defenses to dissuade biotic stresses, plants will newly allocate resources from growth to defense by a reduction of photosynthetic capacity in leaf tissues (Nabityet al. 2009), which leads to reduced carbon assimilation, resulting in poor seed set. Abiotic and biotic stresses factors are most likely to occur simultaneously under field conditions, which has a devastating impact on crop productivity. Combined effects of these stresses are greater than the effects of single stress factors alone(Suzukiet al. 2014). Moreover, studies still found that abiotic stresses can reduce the resistance of plants to biotic stresses (Szittyaet al. 2003). Groszmannet al.(2015) suggests that a suppression of defense response gene activities are important for generating the hybrid vigor phenotype. So over-expression of defense and stress response genes will reduce seed production or hybrid vigor in cereal crops.
In contrast to sexual seed formation, apomixis is an evolutionary mechanism of seed formation without fertilization, which can occur by various genetic regulators reported for more than 300 species in 30 out of 460 angiosperm families (Kandemir and Saygili 2015). Although these different mechanisms have not yet been fully elucidated, it is now generally agreed that apomixis is a qualitative trait controlled by a few genes (Hannaet al. 1998;Barcaccia and Albertini 2013).Fertilization-independent seed(FIS) mutants inA. thalianahave the ability to form seed-like structures without fertilization (Chaudhuryet al.1997), which give significant clues about the genetic mechanism of apomixis. Three important genes have been identified and cloned inA. thaliana, which includes theFIEgene (Fertilization Independent Endosperm, encoding a WD type POLYCOMB protein) (Ohadet al. 1996), theMEDEAgene (encoding a SET domain type POLYCOMB protein) (Grossniklauset al. 1998), and theFIS2gene(Fertilization Independent Seed 2, encoding a zinc finger protein) (Chaudhuryet al. 1997). In recent years, apomixis has been seen as a way of multiplying superior genotypes clonally by seed. However, apomixis has not been found in cereals (Bashaw 1980; van Dijket al. 2016). Recently,a apomixis gene,ASGR-BABY BOOM-like inPennisetum(PsASGR-BBML) has been cloned and introduced into rice and the resulting plants developed embryos from egg cell without fertilization. But the negative feature is low seed set in articifially induced apomictic plant (Ozias-Akins and Connor 2015). Although transfer of apomixis to crop species through wide crosses has many additional hurdles so far(Kandemir and Saygili 2015), understanding the function of natural apomixis genes and the introduction of apomixis into cereal crop species would significantly alter breeding strategies of agricultural crop species.
6. Future prospects for studying seed set in cereal crop species
Seed set is a complex quantitative trait, which is affected by various environmental factors. However, it is critically important for cereal crop yield. Substantial progress has been made in understanding the biology of seed set.Several genetic pathways have been identified to control seed set in cereal crops. Moreover, the benefits of whole-genome sequence information generated in rice, maize, andArabidopsiscan be extended using synteny and collinearity relationships among cereal crop species. For example,rice chromosome 1 shows regions of sequence similarity with chromosomes 3, 6, and 8 in maize (Salseet al. 2004)and homoeologous group 3 chromosomes in bread wheat(Mooreet al. 1995), where some QTLs for grain yield and other agronomic traits have been mapped (Dilbirligiet al.2006). Gn1a (OsCKX2) in rice might correspond to these maize and wheat QTL, and orthologousCKXgenes might regulate yield in other cereal crops (Ashikariet al. 2005).Once identified through synteny, these genes for seed set can be manipulated to improve grain yield. However, it is still unclear how genetic and non-genetic factors coordinate control of seed set in cereal crops. As we know, seed yield improvement in cereal crops may have been the result of an improved genetic×agronomic management interactions, rather than the result of either genetic and/or agronomic improvementper se(Tollenaar and Lee 2002).For example, grain yield of USA maize hybrids from the 1930s to 1990s did not differ when plants were grown under low-density conditions, where competition among plants for soil moisture, soil nutrients, and incident solar radiation was negligible (Duvick 1997). Thus, yield improvement in cereal crops has been associated with increased stress tolerance. Under environmental stress, plants reallocate resources to defense of biotic and abiotic stresses. Today,the main challenge is to decrease the yield gap between the potential seed yield and realized seed yield. Future research is required on tradeoffs between growth processes and defense response to stress. More generally, there is a need to establish a genetic framework for seed set and to better de fine and prioritize the molecular mechanisms and factors controlling seed set.
Acknowledgements
Authors would like to thank the National Key Research and Development Program of China (2016YFD100103), the Major Science and Technology Projects in Henan Province,China (161100110500, 151100111000), the Science Foundation for the Excellent Youth Scholars of Henan Academy of Agricultural Sciences, China (2016YQ04), the International Cooperation Project in Henan Province, China(162102410034), as well as USDA’s National Institute of Food and Agriculture (IOW04314, IOW01018), the RF Baker Center for Plant Breeding and K. J. Frey Chair in Agronomy at Iowa State University for funding this work.
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Straeten D, Peng J, Harberd N P. 2006.Integration of plant responses to environmentally activated phytohormonal signals.Science, 311, 91–94.
Albacete A, Ghanem M E, Martínez-Andújar C, Acosta M,Sánchez-Bravo J, Martínez V, Lutts S, Dodd I C, Pérez-Alfocea F. 2008. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato(Solanum lycopersicumL.) plants.Journal of Experimental Botany, 59, 4119–4131.
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T,Nishimura A, Angeles E R, Qian Q, Kitano H, Matsuoka M. 2005. Cytokinin oxidase regulates rice grain production.Science, 309, 741–745.
Aylor D E. 2003. Rate of dehydration of corn (Zea maysL.)pollen in the air.Journal of Experimental Botany, 54,2307–2312.
Barcaccia G, Albertini E. 2013. Apomixis in plant reproduction:A novel perspective on an old dilemma.Plant Reproduction,26, 159–179.
Barnabás B, Jäger K, Fehér A. 2008. The effect of drought and heat stress on reproductive processes in cereals.Plant,Cell& Environment, 31, 11–38.
Bashaw E C. 1980. Apomixis and its application in crop improvement.Hybridization of Crop Plants.[2017-03-15].https://dl.sciencesocieties.org/publications/books/abstracts/acsesspublicati/hybridizationof/45
Berger S, Sinha A K, Roitsch T. 2007. Plant physiology meets phytopathology: Plant primary metabolism and plantpathogen interactions.Journal of Experimental Botany,58, 4019–4026.
Bernier G, Perilleux C. 2005. A physiological overview of the genetics of flowering time control.Plant Biotechnology Journal, 3, 3–16.
Bilgin D D, Zavala J A, Zhu J, Clough S J, Ort D R, Delucia E H.2010. Biotic stress globally downregulates photosynthesis genes.Plant,Cell & Environment, 33, 1597–1613.
Borrás L, Maddonni G A, Otegui M E. 2003. Leaf senescence in maize hybrids: Plant population, row spacing and kernel set effects.Field Crops Research, 82, 13–26.
Bortiri E, Hake S. 2007. Flowering and determinacy in maize.Journal of Experimental Botany, 58, 909–916.
Boss P K, Bastow R M, Mylne J S, Dean C. 2004. Multiple pathways in the decision to flower: Enabling, promoting,and resetting.The Plant Cell, 16(Suppl.), S18–S31.
Boyer J S, McLaughlin J E. 2007. Functional reversion to identify controlling genes in multigenic responses: Analysis of floral abortion.Journal of Experimental Botany, 58, 267–277.
Brekke B, Edwards J, Knapp A. 2011. Selection and adaptation to high plant density in the Iowa stiff stalk synthetic maize(Zea maysL.) population: II. Plant morphology.Crop Science, 51, 2344–2351.
Buitink J, Wakers B, Hoekstra F A, Crane J. 1996. Storage behavior ofTypha latifoliapollenat low water content:Interpretation on the basis of water activity and glass concept.Physiologia Plantarum, 103, 145–153.
Cai G, Faleri C, Del Casino C, Emons A M C, Cresti M. 2011.Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules.Plant Physiology, 155, 1169–1190.
Do Canto J, Studer B, Lubberstedt T. 2016. Overcoming selfincompatibility in grasses: A pathway to hybrid breeding.Theoretical and Applied Genetics, 129, 1815–1829.
Carcova J, Uribelarrea M, Otegui M, Westgate M E. 2000.Synchronous pollination within and between ears improves kernel set in maize.Crop Science, 40, 1056–1061.
Cassman K G, Dobermann A, Walters D T, Yang H. 2003.Meeting cereal demand while protecting natural resources and improving environmental quality.Annual Review of Environment and Resources, 28, 315–358.
Chaudhury A M, Ming L, Miller C, Craig S, Dennis E S, Peacock W J. 1997. Fertilization-independent seed development inArabidopsis thaliana.Proceedings of the National Academy of Sciences of the United States of America, 94, 4223–4228.
Chaves M M, Maroco J P, Pereira J S. 2003. Understanding plant responses to drought - from genes to the whole plant.Functional Plant Biology, 30, 239–264.
Craufurd P Q, Peacock J M. 1993. Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield.Experimental Agriculture, 29, 77–86.
Danilevskaya O N, Meng X, Selinger D A, Deschamps S,Hermon P, Vansant G, Gupta R, Ananiev E V, Muszynski M G. 2008. Involvement of the MADS-box geneZMM4in floral induction and in florescence development in maize.Plant Physiology, 147, 2054–2069.
Danyluk J, Kane N A, Breton G, Limin A E, Fowler D B, Sarhan F. 2003.TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals.Plant Physiology, 132, 1849–1860.
Van Dijk P J, Rigola D, Schauer S E. 2016. Plant breeding:Surprisingly, less sex is better.Current Biology, 26, R122–R124.
Dilbirligi M, Erayman M, Campbell B T, Randhawa H S,Baenziger P S, Dweikat I, Gill K S. 2006. High-density mapping and comparative analysis of agronomically important traits on wheat chromosome 3A.Genomics, 88,74–87.
Dong Z, Danilevskaya O, Abadie T, Messina C, Coles N,Cooper M. 2012. A gene regulatory network model for floral transition of the shoot apex in maize and its dynamic modeling.PLoS ONE, 7, e43450.
Duvick D N. 1997. What is yield? In:Proceedings of a Symposium. El Batan, Mexico. pp. 25–29.
Ekanayake I J, Steponkus P L, de Datta S K. 1990. Sensitivity of pollination to water de ficits at antheis in upland rice.Crop Science, 30, 310–315.
FAO (Food and Agriculture Organization of the United Nations).2015. FAOSTAT. [2017-03-15]. http://faostat.fao.org
Fasoula V A, Fasoula D A. 2002. Principles underlying genetic improvement for high and stable crop yield potential.Field Crops Research, 75, 191–209.
Ferris R, Ellis R H, Wheeler T R, Hadley P. 1998. Effect of high temperature stress at anthesis on grain yield and biomass of field-grown crops of wheat.Annals of Botany, 82, 631–639.
Fornara F, Pařenicová L, Falasca G, Pelucchi N, Masiero S,Ciannamea S, Lopez-Dee Z, Altamura M M, Colombo L,Kater M M. 2004. Functional characterization ofOsMADS18,a member of the AP1/SQUA subfamily of MADS box genes.Plant Physiology, 135, 2207–2219.
Fischer R A. 1985. Number of kernels in wheat crops and the influence of solar radiation and temperature.The Journal of Agricultural Science, 105, 447–461.
Fuad-hassan A, Tardieu F, Turc O. 2008. Drought-induced changes in anthesis-silking interval are related to silk expansion: A spatio-temporal growth analysis in maize plants subjected to soil water de ficit.Plant,Cell &Environment, 31, 1349–1360.
Grassini P, Thorbum J, Burr C, Cassman K G. 2011. High yield irrigated maize in the Western US Corn Belt: I. On-farm yield, yield potential, and impact of agronomic practices.Field Crops Research, 120, 142–150.
Grossniklaus U, Vielle-Calzada J P, Hoeppner M A, Gagliano W B. 1998. Maternal control of embryogenesis by MEDEA,a polycomb group gene inArabidopsis.Science, 280,446–450.
Groszmann M, Gonzalez-Bayon R, Lyons R L, Greaves I K,Kazan K, Peacock W J, Dennis E S. 2015. Hormone-regulated defense and stress response networks contribute to heterosis inArabidopsisF1hybrids.Proceedings of the National Academy of Sciences of the United States of America, 112, E6397–E6406.
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran L S P. 2012. Cytokinins: metabolism and function in plant adaptation to environmental stresses.Trends in Plant Science, 17, 172–179.
Hammer G L, Dong Z, McLean G, Doherty A, Messina C,Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M. 2009.Can changes in canopy and/or root system architecture explain historical maize yield trends in the US Corn Belt?Crop Science, 49, 299–312.
Hanna W, Roche D, Ozias-Akins P. 1998. Apomixis in crop improvement: Traditional and molecular approaches. In:Advances in Hybrid Rice Technology:Proceedings of the 3rd International Symposium on Hybrid Rice,14–16 November 1996. International Rice Research Institute,Hyderabad, India. p. 283.
Hashemi A M, Herbert S J, Putnam D H. 2005. Yield response of corn to crowding stress.Agronomy Journal, 97, 839–846.
Hoekstra F A, Crowe L M, Crowe J H. 1989. Differential dessiccation sensitivity of corn andPennisetumpollen linked to sucrose content.Plant,Cell & Environment, 12,83–91.
Holmes M G, Smith H. 1997. The function of phytochrome in the natural environment - IV. Light quality and plant development.Photochemistry and Photobiology, 25,551–557.
Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G M, Chu C C, Li J Y, Fu X D. 2009. Natural variation at theDEP1locus enhances grain yield in rice.Nature Genetics, 41, 494–497.
Iqbal N, Nazar R, Khan M I R, Masood A, Khan N A. 2011.Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions.Current Science, 100, 998–1007.
Itoh H, Nonoue Y, Yano M, Izawa T. 2010. A pair of floral regulators sets critical day length forHd3aflorigen expression in rice.Nature Genetics, 42, 635–638.
Izawa T, Takahashi Y, Yano M. 2003. Comparative biology comes into bloom: Genomic and genetic comparison of flowering pathways in rice andArabidopsis.Current Opinion in Plant Biology, 6, 113–120.
Kandemir N, Saygili I. 2015. Apomixis: New horizons in plant breeding.Turkish Journal of Agriculture and Forestry, 39,549–556.
Kebrom T H, Brutnell T P. 2007. The molecular analysis of the shade avoidance syndrome in the grasses has begun.Journal of Experimental Botany, 58, 3079–3089.
Koonjul P K, Minhas J S, Nunes C, Sheoran I S, Saini H S.2005. Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat.Journal of Experimental Botany,56, 179–190.
Köhler C, Makarevich G. 2006. Epigenetic mechanisms governing seed development in plants.EMBO Reports,7, 1223–1227.
Kudlicka K, Brown Jr R M. 1997. Cellulose and callose biosynthesis in higher plants (I. Solubilization and separation of (1-> 3)-and (1-> 4)-[beta]-glucan synthase activities from mung bean).Plant Physiology, 115, 643–656.
Lagercrantz U. 2009. At the end of the day: A common molecular mechanism for photoperiod responses in plants?Journal of Experimental Botany, 60, 2501–2515.
Lashkari M, Madani H, Ardakani M R, Golzardi F, Zargari K.2011. Effect of plant density on yield and yield components of different corn (Zea maysL.) hybrids.American-Eurasian Journal of Agricultural & Environmental Sciences, 10,450–457.
Li X, Paech N, Nield J, Hayman D, Langridge P. 1997. Selfincompatibility in the grasses: Evolutionary relationship of theSgene fromPhalaris coerulescensto homologous sequences in other grasses.Plant Molecular Biology, 34,223–232.
Li Y, Ma X, Wang T, Li Y, Liu C, Liu Z Z, Sun B C, Shi Y S, Song Y C, Carlone M, Bubeck D, Bhardwaj H, Whitaker D, Wilson W, Jones E, Wright K, Sun S K, Niebur W, Smith S. 2011.Increasing maize productivity in China by planting hybrids with germplasm that responds favorably to higher planting densities.Crop Science, 51, 2391–2400.
Loukoianov A, Yan L, Blechl A, Sanchez A, Dubcovsky J.2005. Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat.Plant Physiology, 138,2364–2373.
Mahalakshmi V, Bidinger F R. 1985. Water stress and time of floral initiation in pearl millet.The Journal of Agricultural Science, 105, 437–445.
Matsui T, Omasa K. 2002. Rice (Oryza sativaL.) cultivars tolerant to high temperature at flowering: Anther characteristics.Annals of Botany, 89, 683–687.
McLaughlin J E, Boyer J S. 2004. Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials.Annals of Botany,94, 675–689.
Miura K, Ikeda M, Matsubara A, Song X J, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. 2010.OsSPL14promotes panicle branching and higher grain productivity in rice.Nature Genetics, 42, 545–549.
Monneveux P, Sanchez C, Beck D, Edmeades G O. 2006.Drought tolerance improvement in tropical maize source populations.Crop Science, 46, 180–191.
Moore G, Foote T, Helentjaris T, Devos K, Kurata N, Gale M.1995. Was there a single ancestral cereal chromosome?Trends in Genetics, 11, 81–82.
Morgan J. 1980. Possible role of abscisic acid in reducing seed set in water-stressed wheat plants.Nature, 285, 655–657.
Morgan J, King R. 1984. Association between loss of leaf turgor,abscisic acid levels and seed set in two wheat cultivars.Functional Plant Biology, 11, 143–150.
Murai K, Miyamae M, Kato H, Takumi S, Ogihara Y. 2003.WAP1, a wheatAPETALA1homolog, plays a central role in the phase transition from vegetative to reproductive growth.Plant and Cell Physiology, 44, 1255–1265.
Nabity P D, Zavala J A, DeLucia E H. 2009. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory.Annals of Botany, 103, 655–663.
Naik P K, Mohapatra P K. 1999. Ethylene inhibitors promote male gametophyte survival in rice.Plant Growth Regulation,28, 29–39.
Nishiyama R Y, Watanabe Y, Fujita Y, Le D T, Kojima M,Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmülling T, Tran L P. 2011.Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis.The Plant Cell, 23, 2169–2183.
Ohad N I R, Margossian L, Hsu Y C, Williams C, Repetti P,Fischer R L.1996. A mutation that allows endosperm development without fertilization.Proceedings of the National Academy of Sciences of the United States of America, 93, 5319–5324.
Oliver S N, Dennis E S, Dolferus R. 2007. ABA regulates apoplastic sugar transport and is a potential signal for coldinduced pollen sterility in rice.Plant and Cell Physiology,48, 1319–1330.
Ozias-Akins A, Connor J A. 2015.Gene for Induction of Parthenogenesis,a Component of Apomictic Reproduction Patent Application. U.S. Patent, Application No.WO/2015/061355. 2014-10-21.
Pacini E. 1996. Types and meaning of pollen carbohydrate reserves.Sexual Plant Reproduction, 9, 362–366.
Parish R W, Phan H A, Iacuone S, Li S F. 2012. Tapetal development and abiotic stress: A centre of vulnerability.Functional Plant Biology, 39, 553–559.
Pierik R, Sasidharan R, Voesenek L A. 2007. Growth control by ethylene: Adjusting phenotypes to the environment.Journal of Plant Growth Regulation, 26, 188–200.
Pimentel D. 1991. Diversi fication of biological control strategies in agriculture.Crop Protection, 10, 243–253.
Prabha K, Sood R, Gupta S C. 1982. High temperature-induced inactivation of sporophytic self-incompatibility in ipomoea fistulosa.New Phytologist, 92, 115–122.
Prasad P V V, Boote K J, Allen L H, Sheehy J E, Thomas J M G. 2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Field Crops Research, 95, 398–411.
Roitsch T, González M C. 2004. Function and regulation of plant invertases: sweet sensations.Trends in Plant Science, 9,606–613.
Rosegrant M W, Cline S A. 2003. Global food security:Challenges and policies.Science, 302, 1917–1919.
Rossini M A, Maddonni G A, Otegui M E. 2011. Inter-plant competition for resources in maize crops grown under contrasting nitrogen supply and density: Variability in plant and ear growth.Field Crops Research, 121, 373–380.
Ruan Y L, Jin Y, Yang Y J, Li G J, John B. 2010. Sugar input,metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat.Molecular Plant, 3, 942–955.
Ruan Y L, Patrick J W, Bouzayen M, Osorio S, Fernie A R.2012. Molecular regulation of seed and fruit set.Trends in Plant Science, 17, 656–665.
Saini H S, Aspinall D. 1982. Abnormal sporogenesis in wheat(Triticum aestivumL.) induced by short periods of high temperature.Annals of Botany, 49, 835–846.
Salse J, Piégu B, Cooke R, Delseny M. 2004. New in silico insight into the synteny between rice (Oryza sativaL.) and maize (Zea maysL.) highlights reshuffling and identifies new duplications in the rice genome.The Plant Journal,38, 396–409.
Sangoi L, Gracietti M A, Rampazzo C, Bianchetti P. 2002.Response of Brazilian maize hybrids from different eras to changes in plant density.Field Crops Research, 79, 39–51.
Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H,Jackson D. 2006. A trehalose metabolic enzyme controls in florescence architecture in maize.Nature, 441, 227–230.
Schoper J B, Lambert R J, Vasilas B L, Westgate M E. 1987.Plant factors controlling seed set in maize - The in fluence of silk, pollen, and ear-leaf water status and tassel heattreatment at pollination.Plant Physiology, 83, 121–125.
Schussler J R, Westgate M E. 1991. Maize kernel set at low water potential: I. Sensitivity to reduced assimilates during early kernel growth.Crop Science, 31, 1189–1195.
Smith H. 1995. Physiological and ecological function within the phytochrome family.Annual Review of Plant Biology,46, 289–315.
Smith H M, Samach A. 2013. Constraints to obtaining consistent annual yields in perennial tree crops. I: Heavy fruit load dominates over vegetative growth.Plant Science, 207,158–167.
Song Y, Ito S, Imaizumi T. 2010. Similarities in the circadian clock and photoperiodism in plants.Current Opinion in Plant Biology, 13, 594–603.
Sreenivasulu N, Schnurbusch T. 2012. A genetic playground for enhancing grain number in cereals.Trends in Plant Science, 17, 91–101.
Suwa R, Hakata H, Hara H, El-Shemy H A, Adu-GyamfiJ J, Nguyen N T, Kanai S, Lightfoot D A, Mohapatra P K,Fujita K. 2010. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea maysL.) genotypes.Plant Physiology and Biochemistry, 48, 124–130.
Suzuki N, Rivero R M, Shulaev V, Blumwald E, Mittler R. 2014.Abiotic and biotic stress combinations.New Phytologist,203, 32–43.
Szitty G, Silhavy D, Molnár A, Havelda Z, Lovas Á, Lakatos L, Bánfalvi Z, Burgyá J. 2003. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation.The EMBO Journal, 22, 633–640.
Tester M, Bacic A. 2005. Abiotic stress tolerance in grasses.From model plants to crop plants.Plant Physiology, 137,791–793.
Tian D, Traw M B, Chen J Q, Kreitman M, Bergelson J. 2003.Fitness costs of R-gene-mediated resistance inArabidopsis thaliana.Nature, 423, 74–77.
Tokatlidis I S, Koutroubas S D. 2004. A review of maize hybrids’dependence on high plant populations and its implications for crop yield stability.Field Crops Research, 88, 103–114.
Tollenaar M, Lee E A. 2002. Yield potential, yield stability and stress tolerance in maize.Field Crops Research, 75,161–169.
Vollbrecht E, Springer P S, Goh L, Buckler I V E S, Martienssen R. 2005. Architecture of floral branch systems in maize and related grasses.Nature, 436, 1119–1126.
Weber H, Borisjuk L, Wobus U. 2005. Molecular physiology of legume seed development.Annual Review of Plant Biology,56, 253–279.
Wellmer F, Riechmann J L. 2010. Gene networks controlling the initiation of flower development.Trends in Genetics,26, 519–527.
Westgate M E. 1994. Water status and development of the maize endosperm and embryo during drought.Crop Science, 34, 76–83.
Westgate M E, Passioura J B, Munns R. 1996. Water status and ABA content of floral organs in drought-stressed wheat.Functional Plant Biology, 23, 763–772.
Wilkins P W, Thorogood D. 1992. Breakdown of self-incompatibility in perennial ryegrass at high temperature and its uses in breeding.Euphytica, 64, 65–69.
Winkel T, Renno J F, Payne W A. 1997. Effect of the timing of water deficit on growth, phenology and yield of pearl millet (Pennisetum glaucum(L.) R. Br.) grown in Sahelian conditions.Journal of Experimental Botany, 48, 1001–1009.
Wopereis M C S, Kropff M J, Maligaya A R, Tuong T P. 1996.Drought-stress responses of two lowland rice cultivars to soil water status.Field Crops Research, 46, 21–39.
Yang J, Zhang J, Wang Z, Xu G, Zhu Q. 2004. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling.Plant Physiology, 135, 1621–1629.
Yano M, Kojima S, Takahashi Y, Lin H, Sasaki T. 2001. Genetic control of flowering time in rice, a short-day plant.Plant Physiology, 127, 1425–1429.
Zalabák D, Pospíšilová H, Šmehilová M, Mrízová K, Frébort I, Galuszka P. 2013. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants.Biotechnology Advances, 31, 97–117.
Zinn K E, Tunc-Ozdemir M, Harper J F. 2010. Temperature stress and plant sexual reproduction: Uncovering the weakest links.Journal of Experimental Botany, 61,1959–1968.
Zou J, Rodriguez-Zas S, Aldea M, Li M, Zhu J, Gonzalez D O, Vodkin L O, DeLucia E, Clough S J. 2005. Expression pro filing soybean response toPseudomonas syringaereveals new defense-related genes and rapid HR-specific downregulation of photosynthesis.Molecular Plant-Microbe Interactions, 18, 1161–1174.
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China
- Characterization of two novel heat shock protein 70s and their transcriptional expression patterns in response to thermal stress in adult of Frankliniella occidentalis (Thysanoptera: Thripidae)
- Rapid semi-quantification of triacylglycerols, phosphatidylcholines,and free fatty acids in the rice bran of one grain
- lnfluence of different nitrogen application on flour properties,gluten properties by HPLC and end-use quality of Korean wheat
- Evaluation indices of sour flavor for apple fruit and grading standards
- Relationship of chemical properties of different peanut varieties to peanut butter storage stability
