Characterization of T-complex polypeptide 1 (TCP-1) from the Chilo suppressalis HSP60 family and its expression in response to temperature stress
2018-05-08YUTongyingLUMingxingCUIYadong
YU Tong-ying , LU Ming-xing, CUI Ya-dong
1 Biology and Food Engineering School, Fuyang Normal University, Fuyang 236000, P.R.China
2 School of Horticulture and Plant Protection & Institute of Applied Entomology, Yangzhou University, Yangzhou 225009, P.R.China
1. Introduction
Molecular chaperones are an evolutionarily-conserved family of proteins that are responsible for the folding and assembly of newly-synthesized proteins and the refolding of denatured proteins (Yamet al.2008). Furthermore,chaperones can prevent the unfolding of denatured proteins and promote the aggregation of dissolved protein (Sonnaet al.2002). Heat shock protein, HSP60, is a ubiquitous,highly-abundant molecular chaperone that is induced in response to high temperature, hypoxia, infection, and trauma (Ohashiet al.2000; Borges and Ramos 2005).HSP60 plays an important role in enhancing the ability of cells to tolerate stress and maintaining protein stability and functionality (Wanget al.2012). HSP60 is further classified into types I and II (Chenet al.2011); type II occurs in the cytosol of eukaryotes and includes T-complex polypeptide 1(TCP-1), which is conserved in eukaryotes, archaebacteria and eubacteria (Kubotaet al.1995). TCP-1 assists in the folding of newly synthesized proteins, such as tubulins and actins (Sternlichtet al.1993), cyclin E (Wonet al.1998),and α-transducin (Farret al.1997).
The rice stem borer,Chilo suppressalis(Walker)(Lepidoptera: Pyralidae) is a major pest of rice in Asia, North Africa and southern Europe (Luoet al.2014).C.suppressalisoccurs in both southern and northern China, which indicates a high tolerance to extreme temperatures (Guoet al.2002).The mechanistic basis of thermal tolerance inC.suppressalisis unclear; genes encoding four large and seven small HSPs have been identified inC.suppressalisand were proposed to have roles in temperature stress (Sonodaet al.2006; Cuiet al.2010a, b, c; Luet al.2014; Panet al.2017).
HSP60 has been previously characterized inC.suppressalis(Cuiet al.2010c), but its role in this insect pest is not entirely clear. In this study, we isolated and characterizedTcp-1inC.suppressalis. The expression ofTcp-1in various insect tissues and in response to thermal stress were also investigated.
2. Materials and methods
2.1. Insects
Populations ofC.suppressaliswere collected from a suburb of Yangzhou (32°39´N, 119°42´E), which was located in the Jiangsu Province, China.C.suppressaliswas reared for three or more generations in an indoor breeding room maintained at (27±1)°C with a 16 h L:8 h D photoperiod and approximately 75% humidity as described previously(Shanget al.1979).
2.2. RNA extraction and cDNA synthesis
Total RNA was isolated using the SV Total RNA Isolation System (Promega, USA) and combined with DNase digestion to eliminate DNA contamination. cDNA was synthesized from 1 μL of RNA using oligo(dT)18primers(Fermentas, Canada) by rapid ampli fication of cDNA ends(3´- and 5´-RACE); these experiments were conducted using the TaKaRa RACE cDNA Ampli fication Kit (TaKaRa, Dalian,China) according to the manufacturer.
2.3. Cloning and characterization of Tcp-1
Degenerate primers were used to amplify the partial segments ofTcp-1(Table 1). Full-length cDNAs ofTcp-1were obtained using 5´- and 3´-RACE (SMARTer™ RACE,Clontech, USA) and the primers were shown in Table 1. The sequence of theTcp-1ORF was con firmed by 5´-RACE.Genomic DNA ofC.suppressaliswas extracted using the AxyprepTMMultisource Genomic DNA Kit (Axygen, USA).Based on the sequence of the full-lengthTcp-1gene, pairs of specific primers (Table 1) were designed to amplifyTcp-1genomic fragments. These products were purified using a gel extraction kit (Axygen, USA), cloned into pGEM-T Easy vector (Promega, USA), and transformed into competentEscherichia coliDH5α cells for sequencing.
2.4. Sample preparation
In experiments focusing on differential expression in seven tissue types, fifth-instar larvae were selected with similar body sizes; each group contained ten larvae and experiments were repeated four times. Larvae were anesthetized on ice before dissection. Heads, epidermis,fat body, foregut, midgut, hindgut, and malpighian tubules were collected from larvae and rinsed with a 0.9% sodium chloride solution. The samples were frozen immediately in liquid nitrogen and stored at –70°C until needed for analysis.
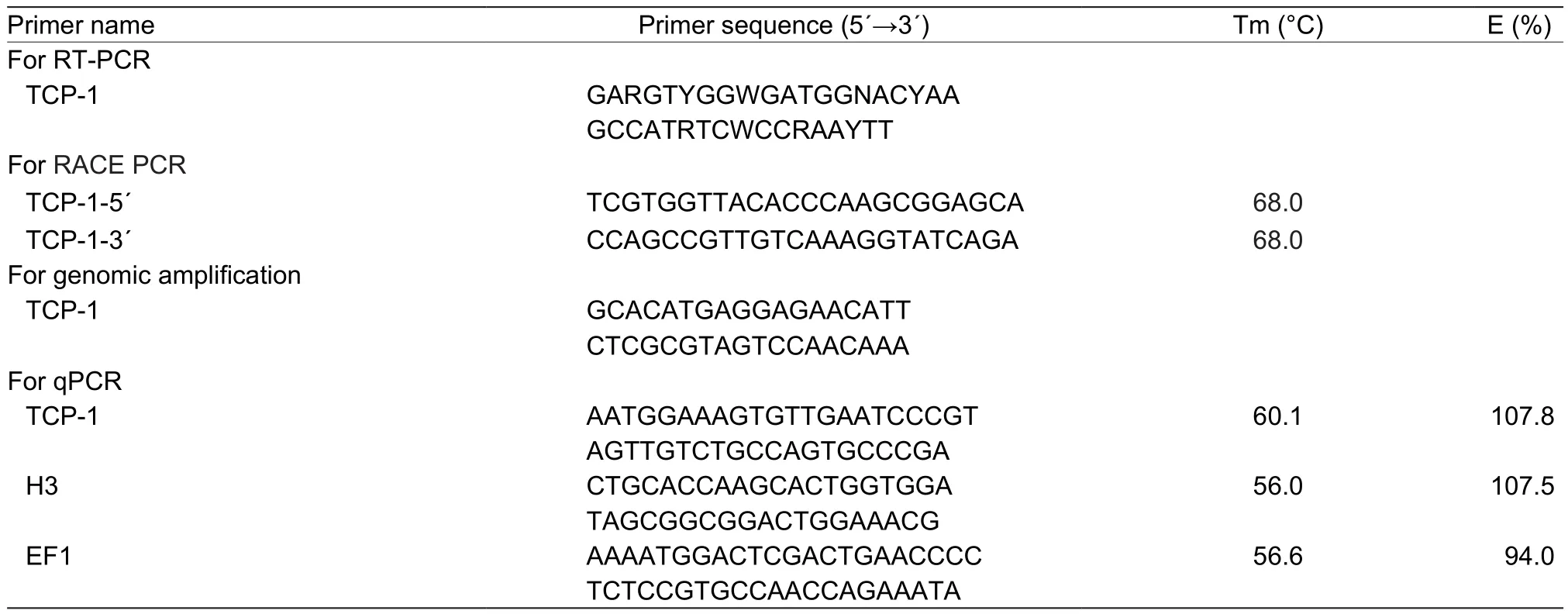
Table 1 Primers used in this study1)
2.5. Temperature treatments
Larvae (n=30) were con fined individually in glass tubes, and experimental groups were exposed to selected temperatures(–11, –9, –8, –6, –3, 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30,33, 36, 39, 42, and 43°C) for 2 h in a constant temperature subzero incubator (DC-3010, Jiangnan Equipment,Changzhou, China). Larvae were then allowed to recover at(27±1)°C for 2 h, after which surviving larvae were frozen in liquid nitrogen and stored at –70°C. The larvae exposed to 27°C were regarded as the control group. Each treatment included four or more surviving larvae.
2.6. Quantitative real-time PCR analysis
Total RNA was extracted using the SV Total RNA Isolation System (Z3100, Promega, USA), followed by DNase treatment to eliminate DNA contamination. The integrity of RNA in all samples was verified by comparing the ribosomal RNA bands in ethidium bromide-stained gels and purity was evaluated using spectrophotometric measurements(BioPhotometer plus; Eppendorf, Germany) at 260 and 280 nm. Reactions were performed in a 20 μL volume containing 10 μL of iTaq™ Universal SYBR®Green Supermix (Bio-Rad, USA), 6 μL ddH2O, 1 μL of each genespecific primer (Table 1) and 2 μL of cDNA templates.Reactions were carried out using a CFX-96 Real-time PCR System (Bio-Rad, USA). The efficiencies of the target and reference genes were similar (Nolanet al.2006; Bustinet al.2009). The quantity ofTcp-1mRNA was calculated using the 2–ΔΔCTmethod (Pfaffl 2001) and normalized to the abundance of histone 3 (H3) and elongation factor 1 (EF1)genes for tissues and thermal stress, respectively (Xuet al.2017). Expression in larvae maintained at (27±1)°C and in insect heads were used as controls as described previously(Schmittgen and Livak 2008). The homogeneity of PCR products was con firmed by melting curve analysis, which was read every 5 s per 0.5°C increment from 65 to 95°C.Treatments included four replicates, and each reaction was run in triplicate.
2.7. Phylogenetic analysis
ORF Finder Software (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) was used to identify ORFs in theTcp-1sequence.Sequence analysis tools of the ExPASy Molecular Biology Server, including Translate, Compute pI/MW and Blast(Swiss Institute of Bioinformatics). ScanProsite (http://prosite.expasy.org/scanprosite/) was used to detect motifs in TCP-1. Amino acid sequences were used to estimate phylogeny using neighbor-joining, minimum evolution, maximum likelihood, and maximum parsimony methods. Phylogenetic trees were constructed with 1 000 bootstrap replicates using MEGA version 7.0 (Kumaret al.2016).
2.8. Statistical analysis
Homogeneity of variances among different groups was evaluated by Levene’s test. One-way ANOVA and a LSD test were used to detect significant differences among treatments, followed by Tukey’s tests and SPSS16.0 (Pallant 2007).
3. Results
3.1. Sequence analysis
In this study, degenerative primers based on a conserved region ofTcp-1were used to amplify cDNAs derived fromC.suppressalislarvae. The 5´ and 3´ flanking sequences were obtained by 5´- and 3´-RACE. After assembly, a 2 144 bp full-length cDNA sequence was obtained (Fig. 1)that contained start and stop codons at 99–101 bp (ATG)and 1 733–1 735 bp (TAA), respectively. The sequence contained a 98 bp 5´-UTR with a polyadenylation signal sequence (AATAAA), a 1 635-bp ORF, a 408-bp 3´-UTR,and a poly(A) tail at the 3´ end (GenBank accession number: MF471349) (Fig. 1). The deduced translational product contained 545 amino acids with a predicted mass of 59.42 kDa and isoelectric point of 5.29. TCP-1 had two signature sequences homologous to the TCP-1 family of proteins, namely RSAYGPNGMNKMI (residues 44–56) and QDAEVGDGT (residues 93–101). Genomic analyses indicated there were lacked introns in theTcp-1gene.
3.2. Phylogenetic analysis
The deduced amino acid sequence of TCP-1 inC.suppressaliswas highly similar to orthologous proteins in other insects. We used CLUSTALX and MEGA 7.0 to compare selected TCP-1 proteins using neighbor-joining, minimum evolution, maximum likelihood and maximum parsimony methods. The four phylogenetic trees were highly similar and each contained two clusters (Fig. 2). TCP-1 fromC.suppressalis,Bombyx mori,Danaus plexippus,Papilio xuthusandOperophtera brumatafell into a single well-supported cluster with a high level of similarity (60–99%),indicating that TCP-1 inC.suppressalisis closely related to other Lepidopteran forms of the protein.

Fig. 1 The complete cDNA sequence and predicted amino acid sequence of the gene encoding T-complex polypeptide 1(TCP-1) in Chilo suppressalis. Two highly-conserved typical motifs of TCP-1 family were selected in the boxes. The asterisk indicates the translational termination codon.
3.3. Expression of Tcp-1 in different tissues of C. suppressalis larvae
Quantitative real-time PCR indicated thatTcp-1was expressed differentially in the seven tissues/organs ofC.suppressalislarvae (F6,17=7.093;P=0.001) (Fig. 3).Expression ofTcp-1was the highest in the hindgut and the lowest in malpighian tubules. Expression in larval heads was 4.30 times higher than expression in malpighian tubules.Tcp-1expression was similar in the foregut, midgut and epidermis (Fig. 3).
3.4. Expression of Tcp-1 in C. suppressalis exposed to temperature stress
Tcp-1expression was different from the control (27°C) inC.suppressalislarvae exposed to reduced temperatures(F14,38=4.158;P<0.001) (Fig. 4).Tcp-1expression was 2.01-fold higher at 6°C than that of the control (27°C) (Fig. 4).Tcp-1expression at –6, –3, 9, 12, 15, 18, and 21°C was nearly the same as those of the controls (27°C).Tcp-1expression was 1.70-fold higher than that of the control(27°C) after a 2-h exposure to 24°C.
The expression ofTcp-1was induced when temperature increased from 27 to 43°C (F6,18=4.970,P=0.002)(Fig. 5).Tcp-1expression was 1.35- and 1.29-fold higher than the control (27°C) after a 2-h exposure to 30 and 36°C, respectively. Expression ofTcp-1declined when temperature exceeded 36°C, and the expression level at 43°C was significantly lower than that at 27°C (P=0.002;Fig. 5).
4. Discussion
In this study,Tcp-1was cloned and characterized from the rice stem borer,C.suppressalis. The cDNA sequence encoded a 1 635-bp ORF, and the predicted translational product contained 545 amino acids. TCP-1 had an estimated mass of 59.42 kDa and an isoelectric point of 5.29.Tcp-1contained no introns, which is also the case forhsp60in humans and Chinese hamster (Venner 1990).However,hsp60had five introns inFrankliniella occidentalis(Luet al.2016), indicating heterogeneity in this gene. An inverse correlation between intron size and gene expression has been suggested, and genes lacking introns or containing shorter introns showed higher levels of expression than genes containing multiple or longer introns (Comeron 2004).
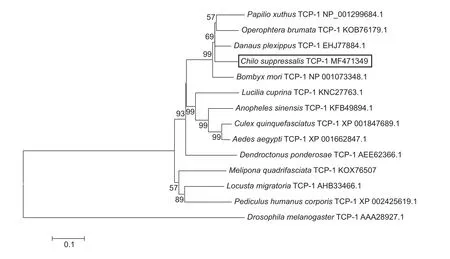
Fig. 2 Phylogenetic tree of T-complex polypeptide 1 (TCP-1) proteins determined by the neighbor-joining method. Chilo suppressalis TCP-1 is labeled with black rectangle.
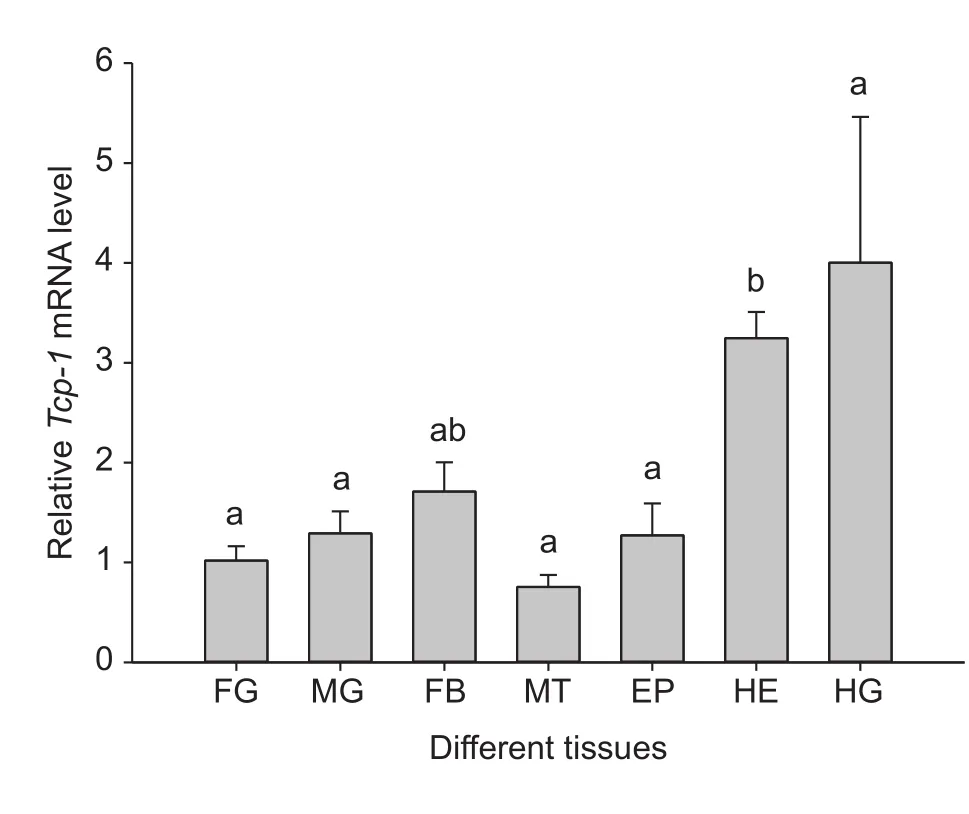
Fig. 3 Relative expression of Tcp-1 in different tissues (organs)of Chilo suppressalis as determined by quantitative real-time PCR. FG, foregut; MG, midgut; FB, fat body; MT, malpighian tubules; EP, epidermis; HE, head; HG, hindgut. Expression in insect heads was regarded as a control. Columns labeled with different letters indicate significantly different (P<0.05) by one-way ANOVA and Tukey’s test. Error bars indicate SE.
The role of molecular chaperones in response to various stressors has been widely studied in eukaryotes.Our results demonstrated thatTcp-1expression was the highest in the hindgut of fifth-instar larvae and the lowest inC.suppressalisheads. However, Kumaret al.(2015)reported that the expression ofhsp60inLucilia cuprinawas significantly increased in the malpighian tubules and fat body. One explanation is that hindgut and malpighian tubules reabsorb water, salts and other substances prior to excretion by the insect, and HSPs protect these tissues or organs from toxic injury. This may be also related to the function of TCP-1 in the folding of newly synthesized proteins(Yamet al.2008), including tubulins and actins (Sternlichtet al.1993), cyclin E (Wonet al.1998), α-transducin (Farret al.1997) and von Hippel-Lindau protein (Hansenet al.2002). Álvarez-Fernándezet al.(2015) demonstrated that TCP-1 chaperonin complex as a key regulator of the actin cytoskeleton essential for the wound healing response inDrosophila.
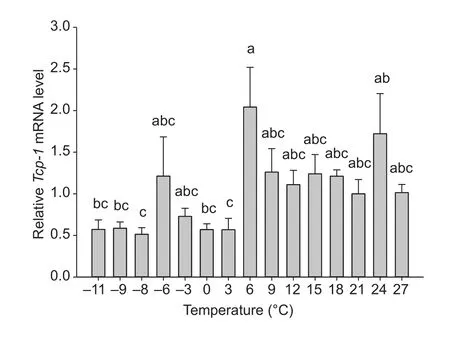
Fig. 4 Relative expression of Tcp-1 in Chilo suppressalis larvae exposed to low temperatures. Expression at 27°C was regarded as a control. Columns labeled with different letters indicate significantly different (P<0.05) by one-way ANOVA and Tukey’s test. Error bars indicate SE.
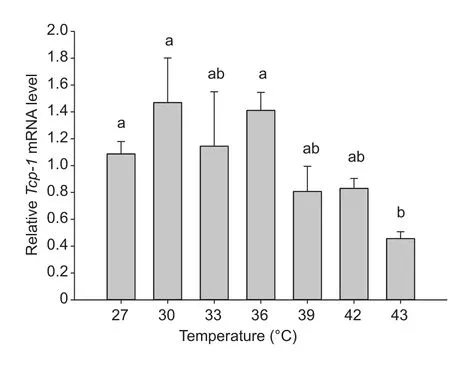
Fig. 5 Relative expression of Tcp-1 in Chilo suppressalis larvae exposed to elevated temperature (27–43°C). Expression at 27°C was regarded as a control. Columns labeled with different letters indicate significantly different (P<0.05) by one-way ANOVA and Tukey’s test. Error bars indicate SE.
Heat shock proteins play an important role in environmental stress tolerance and heat adaptation (Frydenberget al.2003; Hoffmannet al.2003). The production of HSPs can affect insect growth and development and negatively impact many physiological processes (Krebs and Feder 1997;Feder and Hofmann 1999; Huang and Kang 2007). TCP-1, a member of the HSP60 family, was first shown to be induced by heat shock in human cells (Schenaet al.1996). In this study, expression ofTcp-1inC.suppressaliswas inhibited at 39 and 43°C, indicating that transcription had decreased.However, Cuiet al.(2010c) previously demonstrated thathsp60expression inC.suppressaliswas the highest after a 2-h exposure to 36°C and declined at 39°C. Huang and Kang (2007) observed a similar induction pattern forhsp60inLiriomyza sativaewhere expression was induced in response to heat shock and inhibited when temperatures exceeded 42.5°C. It has also been reported that heat shock can suppress activation of the mitogen-activated protein kinase (MAPK) pathway (Gaoet al.2014). Inhibition of the MAPK pathway can reduce the synthesis of ternary complex factors (TCFs), which further inhibit expression of TCP-1(Gendronet al.2003).
Surprisingly, exposure to low temperatures did changeTcp-1expression inC.suppressalis.Tcp-1expression was the highest, which was 2.01-fold higher than the control after a 2-h exposure to 6°C. However, Huang and Kang (2007)observed thathsp60expression at low temperatures (–20,–17.5, –15, –12.5, –10, –7.5, –5, –2.5, 0, 2.5, and 25°C)were nearly the same as the control (25°C). Difference inTcp-1expression inC.suppressalisat low temperature may be related to the structure and function of TCP-1 (Yamet al.2008). Factors that contribute to cold hardiness in insects have been described, including cryoprotectants,supercooling points, antifreeze proteins, homeoviscous adaptation, and heat shock proteins (Storey and Storey 1991; Graetheret al.2000; Kostálet al.2003). Kayukawaet al.(2005) clearly demonstrated that expression levels ofTcp-1are highly correlated with the cold hardiness inDelia antiquapupae. Since the production of HSPs may impact the survival and viability of offspring, it is important to understand the cost/benefit ratio balance in terms ofhspgene expression (Luet al.2014).
In summary, TCP-1 helps protect insects from cellular damage. The role of TCP-1 in adjusting actin and tubulin levels is worthy of further study and will advance our understanding of TCP-1 function in insect behavior and development.
5. Conclusion
We obtainedTcp-1encoding heat shock protein forC.suppressalis, and its predicted amino acid sequence showed high similarities with published TCP-1s of other insects in Lepidoptera. When exposed to heat stress, the expression ofTcp-1was induced. Our results indicated thatTcp-1expression was differentially expressed inC.suppressalistissues, and was impacted by temperature stress, and provided useful information in understanding the thermotolerance ofC.suppressalisat the molecular basis.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (31401733) and the Incubation Study Project of Science and Technology of Fuyang Normal University, China (2014KJFH02). We sincerely thank Dr.Carol L. Bender from Consulting Limited Liability Company,USA for editing and providing comments on the manuscript.We express our deep gratitude to the Testing Center of Yangzhou University, China.
Álvarez-Fernández C, Tamirisa S, Prada F, Chernomoretz A,Podhajcer O, Blanco E, Martín-Blanco E. 2015. Identification and functional analysis of healing regulators inDrosophila.PLoS Genetics, 11, e1004965.
Borges J C, Ramos C H I. 2005. Protein folding assisted by chaperones.Protein and Peptide Letters, 12, 257–261.
Bustin S A, Benes V, Garson J A, Hellemans J, Huggett J,Kubista M, Mueller R, Nolan T, Pfaf fl M W, Shipley G L,Vandesompele J, Wittwer C T. 2009. The MIQE guidelines:Minimum information for publication of quantitative real-time PCR experiments.Clinical Chemistry, 55, 611–622.
Chen J S, Chang L C, Wu C C, Yeung L K, Lin Y F. 2011.Involvement of F-actin in chaperonin-containing t-complex 1 beta regulating mouse mesangial cell functions in a glucoseinduction cell model.Experimental Diabetes Research,2011, 645–647.
Comeron J M. 2004. Selective and mutational patterns associated with gene expression in humans: Influences on synonymous composition and intron presence.Genetics,167, 1293–1304.
Cui Y D, Du Y Z, Lu M X. 2010a. Cloning of the heat shock protein 70 gene fromChilo suppressalisand the analysis of its expression characteristics under heat stress.Acta Entomologica Sinica, 53, 841–848. (in Chinese)
Cui Y D, Du Y Z, Lu M X, Hu M Z. 2010b. Effect of thermal stress on the generation of ROS, HSP90 and apoptosis in haemocytes ofChilo suppressalis(Lepidoptera: Pyralidae)larvae.Acta Entomologica Sinica,53, 721–726. (in Chinese)
Cui Y D, Du Y Z, Lu M X, Qiang C K. 2010c. Cloning of the heat shock protein 60 gene from the stem borer,Chilo suppressalis, and analysis of expression characteristics under heat stress.Journal of Insect Science, 10, 1–13.
Farr G W, Scharl E C, Schumacher R J, Sondek S, Horwich A L. 1997. Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms.Cell, 89, 927–937.
Feder M E, Hofmann G E. 1999. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology.Annual Review of Physiology, 61,243–282.
Frydenberg J, Hoffmann A A, Loeschcke V. 2003. DNA sequence variation and latitudinal associations in hsp23,hsp26 and hsp27 from natural populations ofDrosophila melanogaster.Molecular Ecology, 12, 2025–2032.
Gao S C, Yin H B, Liu H X, Sui Y H. 2014. Research progress on mapk signal pathway in the pathogenesis of osteoarthritis.China Journal of Orthopaedics and Traumatology, 27,441–444. (in Chinese)
Gendron F P, Neary J T, Theiss P M, Sun G Y, Gonzalez F A, Weisman G A. 2003. Mechanisms of P2X7 receptormediated ERK1/2 phosphorylation in human astrocytoma cells.American Journal of Physiology(Cell Physiology),284, 571–581.
Graether S P, Kuiper M J, Gagne S M, Walker V K, Jia Z, Sykes B S, Davies P L. 2000. Beta-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect.Nature, 406, 325–328.
Guo H F, Li Q, Fang J C, Zhang H. 2002. Comparison of cold hardiness in three species of overwintering rice stem borers in Nanjing area.Jiangsu Journal of Agricultural Sciences,18, 85–88. (in Chinese)
Hansen W J, Ohh M, Moslehi J, Kondo K, Kaelin W G, Welch W J. 2002. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity.Molecular and Cellular Biology, 22, 1947–1960.
Hoffman A A, Sorensen J G, Loeschcke V. 2003. Adaptation ofDrosophilato temperature extremes: Bringing together quantitative and molecular approaches.Journal of Thermal Biology, 28, 175–216.
Huang L H, Kang L. 2007. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress.Insect Molecular Biology, 16, 491–500.
Kayukawa T, Chen B, Miyazaki S, Itoyama K, Shinoda T,Ishikawa Y. 2005. Expression of mRNA for the t-ccomplex polypeptide-1, a subunit of chaperonin CCT, is upregulated in association with increased cold hardiness inDelia antiqua.Cell Stress and Chaperones, 10, 204–210.
Kostál V, Berková P, Simek P. 2003. Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae ofChymomyza costata(Drosophilidae).Comparative Biochemistry and Physiology(Part B), 135, 407–419.
Krebs R A, Feder M E. 1997. Deleterious consequences of Hsp70 over expression inDrosophila melanogasterlarvae.Cell Stress and Chaperones, 2, 60–71.
Kubota H, Hynes G, Willison K. 1995. The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol.European Journal of Biochemistry, 230, 3–16.
Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets.Molecular Biology and Evolution, 33, 1870–1874.
Kumar S M, Janardhan Reddy P V, Sreedhar A S, Tiwari P K.2015. Molecular characterization and expression analysis ofhsp60gene homologue of sheep blow fly,Lucilia cuprina.Journal of Thermal Biology, 52, 24–37.
Lu M X, Hua J, Cui Y D, Du Y Z. 2014. Five small heat shock protein genes fromChilo suppressalis: Characteristics of gene, genomic organization, structural analysis, and transcription pro files.Cell Stress and Chaperones, 19,91–104.
Lu M X, Li H B, Zheng Y T, Shi L, Du Y Z. 2016. Identification,genomic organization and expression pro files of four heat shock protein genes in the western flower thrips,Frankliniella occidentalis.Journal of Thermal Biology, 57,110–118.
Luo G H, Li X H, Han Z J, Guo H F, Yang Q, Wu M, Zhang Z C,Liu B S, Qian L, Fang J C. 2014. Molecular characterization of thepiggyBac-likeelement, a candidate marker for phylogenetic research ofChilo suppressalis(Walker) in China.Biomedcentral Molecular Biology, 15, 28.
Nolan T, Hands R E, Bustin S A. 2006. Quanti fication of mRNA using real-time RT-PCR.Nature Protocols, 1, 1559–1582.
Ohashi K, Burkart V, Flohé S, Kolb H. 2000. Cutting edge:heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex.Journal of Immunology,164, 558–561.
Pallant J. 2007.SPSS Survival Manual:A Step by Step Guide to Data Analysis Using SPSS for Windows Version 15. Open University Press, Maidenhead, England.
Pan D D, Lu M X, Li Q Y, Du Y Z. 2017. Characteristics and expression of genes encoding two small heat shock protein genes lacking introns fromChilo suppressalis.Cell Stress and Chaperones, 3, 1–10.
PfafflM W. 2001. A new mathematical model for relative quantification in real-time RT-PCR.NucleicAcids Research,29, e45.
Schena M, Shalon D, Heller R, Chai A, Brown P O, Davis R W.1996. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes.Proceedings of the National Academy of Sciences of the United States of America, 93, 10614–10619.
Schmittgen T D, Livak K J. 2008. Analyzing real-time PCR data by the comparative CTmethod.Nature Protocols, 3,1101–1108.
Shang Z Z, Wang Y S, Zou Y H. 1979. Study on rearing method of rice stem borerChilo suppressalisWalker.Acta Entomologica Sinica, 2, 164–167. (in Chinese)
Sonna L A, Fujita J, Gaf fin S L, Lilly C M. 2002. Molecular biology of thermoregulation: Effects of heat and cold stress on mammalian gene expression.Journal of Applied Physiology, 92, 1725–1742.
Sonoda S, Fukumoto K, Izumi Y, Yoshida H, Tsumuki H. 2006.Cloning of heat shock protein genes (hsp90andhsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer,Chilo suppressalisWalker.Archives of Insect Biochemistry and Physiology, 63, 36–47.
Sternlicht H, Farr G W, Sternlicht M L, Driscoll J K, Williso K,Yaffe M B. 1993. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actinin vivo.Proceedings of the National Academy of Sciences of the United States of America, 90, 9422–9426.
Storey K B, Storey J M. 1991. Biochemistry of cryoprotectants.Insects at Low Temperature, 4, 64–93.
Venner T J, Singh B, Gupta R S. 1990. Nucleotide sequences and novel structural features of human and chinese hamster hsp60 (Chaperonin) gene families.DNA and Cell Biology,9, 545–552.
Wang F, Chen J, Shi Y H, Lu X J, Li M Y. 2012. Molecular cloning, sequence analysis and stress-related changes of the heat shock protein 60 gene inNeobenedenia melleni.Zoological Research, 33, 603–608.
Won K A, Schumacher R J, Farr G W, Horwich A L, Reed S I.1998. Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT.Molecular and Cellular Biology,18, 7584–7589.
Xu J, Lu M X, Cui Y D, Du Y Z. 2017. Selection and evaluation of reference genes for expression analysis using qRT-PCR inChilo suppressalis(Lepidoptera: Pyralidae).Journal of Economic Entomology, 110, 683–691.
Yam A Y, Xia Y, Lin H T J, Burlingame A, Gerstein M,Frydman J. 2008. De fining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies.Nature Structural and Molecular Biology, 15, 1255–1262.
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China
- Genes encoding heat shock proteins in the endoparasitoid wasp,Cotesia chilonis, and their expression in response to temperatures
- Molecular mechanisms controlling seed set in cereal crop species under stress and non-stress conditions
- Rapid semi-quantification of triacylglycerols, phosphatidylcholines,and free fatty acids in the rice bran of one grain
- lnfluence of different nitrogen application on flour properties,gluten properties by HPLC and end-use quality of Korean wheat
- Evaluation indices of sour flavor for apple fruit and grading standards
