炭纤维作为EM生物膜载体优化除污效果的应用研究
2018-05-02安永真王春华王晓旭梁节英
安永真, 王春华, 苗 朋, 王晓旭, 梁节英, 刘 杰
(北京化工大学 常州先进材料研究院 碳纤维工程与技术研究中心, 江苏 常州213000)
1 Introduction
Effective microorganisms (EM), a culture of approximately 80 coexisting beneficial microorganisms predominantly consisting of photosynthetic bacteria, lactic acid bacteria, yeast, fermenting fungi and actinomycetes et al, was developed by Japanese horticulturist Teuro Higa from the University of Ryukyus in Japan[1]. EMs adapt well to the surrounding environment and have a large number of applications including livestock, agriculture, gardening landscaping, composting, cleaning septic tanks, bioremediation, algal control and household uses[2-5]. However, EM (a water-soluble liquid) was commonly used individually and this direct application easily leads to bacterial loss and inferior removal of nitrogen and phosphorus[6-9]. Therefore, the effective use of EM for organic degradation is a challenging issue.
The carrier of the biofilm limits the growth and the reproduction of microbial populations in biofilm systems[10]. Among all the biofilm carriers, carbon fiber (CF) receives extensive attention and has been widely used in water treatment owing to its superior biocompatibility. Activated sludge is typically used as the source of microorganism. But it has the drawbacks of taking a long time to cultivate in the first period and the difficulty of controlling the microbial proportion[11,12]. Previous studies have shown that dosing advantageous or efficient bacteria can increase the processing power of the original treatment systems[13,14]. The aims of this work ate to study the decontamination performance of biofilm systems based on polyester (PET), polyvinyl alcohol (PVA), CF and electrochemically-treated CF as EM biofilm carriers, to explore the system sensitivity to the surrounding environment using the carrier with the best performance and to quantify the optimized system operation parameters to provide a theoretical basis for further practical applications of CF and EM.
2 Experimental
EM used in this study was provided by Cogent Oasis Biotechnology Ltd. (Beijing, China). EM was in a dormant state and activated for 2-3 days at 28±2℃ before application. The EM was considered to be successfully activated as the pH value was achieved 4.0 and it had a sweet sour smell[15]. PET and PVA were supplied by Teijin Ltd. (Osaka, Japan). CF (12 K, tensile strength of 500 kgf/mm2and tensile modulus, of 29×103kgf/mm2) used in this study was produced by a laboratory scale carbon fiber production line (FNS Electric Furnace Ltd, Beijing), which was a PAN-based carbon fiber. The electrochemical treatment of CF was carried out continuously in a laboratory equipment with an anode oxidation method using 10 wt% NH4HCO3aqueous solution as the electrolyte[16].
Cylindrical plexiglass reactors (a diameter of 0.24 m , a height of 0.50 m and a total volume of 0.02 m3) with an aeration tank underneath were used for culturing biofilm. The artificial synthetic wastewater containing glucose, K2HPO4, KH2PO4, (NH4)2SO4, MgSO4, FeSO4·7H2O, ZnSO4·7H2O, CaCl2and MnSO4·H2O were measured by a DR 2800 Spectrophotometer (Hach Company, Germany) to obtain quality indexes of chemical oxygen demand (COD), total nitrogen (TN) and total phosphorus (TP). The composition of influent water is shown in Table 1.

Table 1 Composition of influent water.
All the carriers were used to culture biofilm simultaneously using the rejuvenated EM. The influence of different carriers on EM biofilms during the start-up period was determined by measuring the concentration change of COD. In the stable period, the decontamination performance of EM biofilm systems based on different carriers were studied using the concentrations of COD, TN and TP. The biofilm system with the best decontamination performance was chosen to test its resistivity to the surrounding environment, and its potential for practical application was explored through studying the effects of dissolved oxygen concentration, pH and temperature on its performance.
3 Results and discussion
Influence of EM biofilm systems on wastewater decontamination. The COD of four different carrier systems recorded after 8h of the start-up period is shown in Fig. 1. It is revealed that the removal rate of COD in wastewater systems based on different carriers rise quite quickly from the 10thday to the 14thday. Along with changing the influent wastewater every day, wastewater treatment effects get better by microorganisms of all biofilms. Microorganisms are immobilized on carriers and formed biofilms, which prevent the loss of mobile microorganisms. For EM biofilm based on CFs (with/without electrochemical treatment), 50% removal of COD is achieved on the 10thday and the removal rate can reach 95% (its stable period) in only 14 days. On the other hand, the removal rate of COD with the other two EM biofilm systems tends to be stable after 18-20 days. The interaction of CF and EM can greatly save the start time, which can be attributed to the preferred carrier selection of EM, and the desired biocompatibility and high specific surface area of CF. Meanwhile, the electrochemical treatment can increase the hydrophilicity and the oxygen/nitrogen content of the CF surface, which can further facilitate the microbial attachment and reproduction[16].
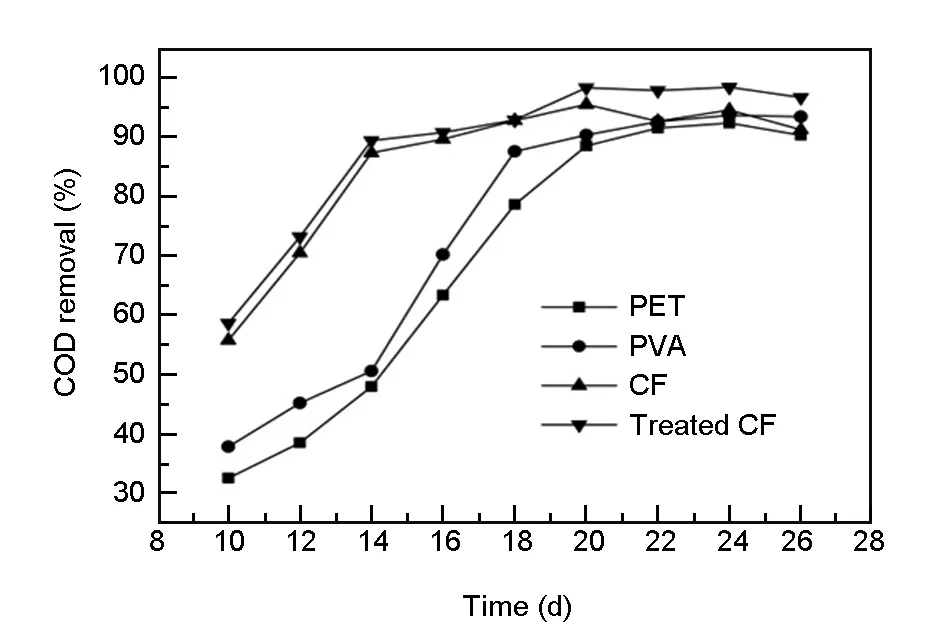
Fig. 1 The influence of different carriers on COD removal rate in the start-up period.
When the bacteria entered their stationary phase and the systems became stable, the concentration of COD, TN and TP of each EM biofilm wastewater system under different hydraulic retention times were measured five times, and the average values of all the parameters including the influent/effluent concentration and the removal rate calculated are shown in Fig. 2.
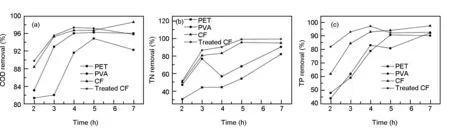
Fig. 2 Variation of the removal rates of (a) COD, (b) TN and (c) TP.
In the first 3 h, the removal rates of COD, TN and TP of EM biofilm systems based on CFs could achieve 95%, 80% and 84%, respectively, which correspond to a 10%-40% increase compared to the other two biofilm systems based on PET and PVA. The results indicate that biofilm systems based on CFs have better performance compared to the ones on PET and PVA in wastewater treatment. In addition, after 4 h hydraulic retention, all the effluent indexes of EM biofilm based on CFs comply with the standard A for urban sewage discharge. Besides, the EM biofilm system based on the electrochemically-treated CF demonstrates relatively better performance than the one based on the untreated CF. This is probably due to the fact that the carrier surface property can affect the decontamination performance of microbial communities[16]. The removal rates of COD, TN and TP of the -EM biofilm system based on the electrochemically-treated CF are 97.1%, 92.5% and 96.0%, respectively after 3 h-5 h. Further prolongation of the hydraulic retention time does not change the wastewater quality indexes, which can be ascribed to the reduced volume load of biofilm reactor. It has been shown that the reduced volume load can result in a lack of microbial metabolic substrates, causing the decrease of microbial growth and endogenous respiration[17].
The system sensitivity to the surrounding environment with the best carrier. Oxygen concentration is an important factor affecting the processing efficiency in the biological treatment process[18]. In order to determine the optimal concentration of dissolved oxygen range to ensure the maximum environmental and economic benefits, we adjust the length of aeration and the aerated period to maintain a constant concentration of dissolved oxygen at different ranges. As shown in Fig. 3a, the COD removal rate could reach more than 80% when the dissolved oxygen concentration is greater than 3 mg/L. The removal rate increases with the dissolved oxygen concentration, and levels off at the oxygen concentration range of 4 -8.5 mg/L. A further increase in the concentration of dissolved oxygen leads to a slightly low COD removal rate. Meanwhile, the removal rate of TN reaches nearly 90% at low dissolved oxygen concentrations and then it decreases at high concentrations. The removal rate of TN can be achieved via transforming nitrite-N and nitrate-N into nitrogen with the aid of denitrifying bacteria under anaerobic conditions[19]. Hence, a high concentration of dissolved oxygen is not in favor of the TN removal. However, the TP removal rate increases significantly as the dissolved oxygen concentration increases to 9.5 mg/L (Fig. 3a). Improvement of the TP removal efficiency is often accompanied with the decrease of TN removal rate. Thus, the dissolved oxygen concentration is the key to an accurate measurement[20]. In this test, the dissolved oxygen concentration is maintained between 4 and 9 mg/L.
It was reported that pH value had a great impact on the rate of denitrification and the microbial absorption capacity to substances[21]. Thus, it is necessary to adjust the pH value of the system to 6.5-7.0 at the beginning of the experiment to provide a suitable environment for the denitrifying bacteria. Fig. 3b shows that the highest decontamination performance is achieved at pH 3-9, which indicates that the EM biofilm system based on electrochemically-treated CF has a strong acid and basic resistance. This may provide the favorable growth and reproduction environment for a wide variety of microorganisms contained in the EM biofilms. Obviously, these microorganisms play a significant role in the wastewater treatment. Although the range of environmental pH value varies significantly, the intracellular pH of microorganisms is generally neutral and this stable intracellular pH can maintain the structural stability of biologically active molecules and provide the optimum environment for the enzymes[22]. The system effluent pH value after treatment is between 6 and 8 with a 3-9 pH value of the influent water (Table 2), indicating that the EM biofilm can regulate the pH value of wastewater. The system has a stronger acid resistance than alkali resistance, which may be ascribed to the isoelectric points of amino acids in microbial cells[23]. As a result, the environment pH value range of the EM biofilm system based on electrochemically-treated CF is broadened, which will greatly simplify the pre-treatment process in practical application and lower the overall operation cost.
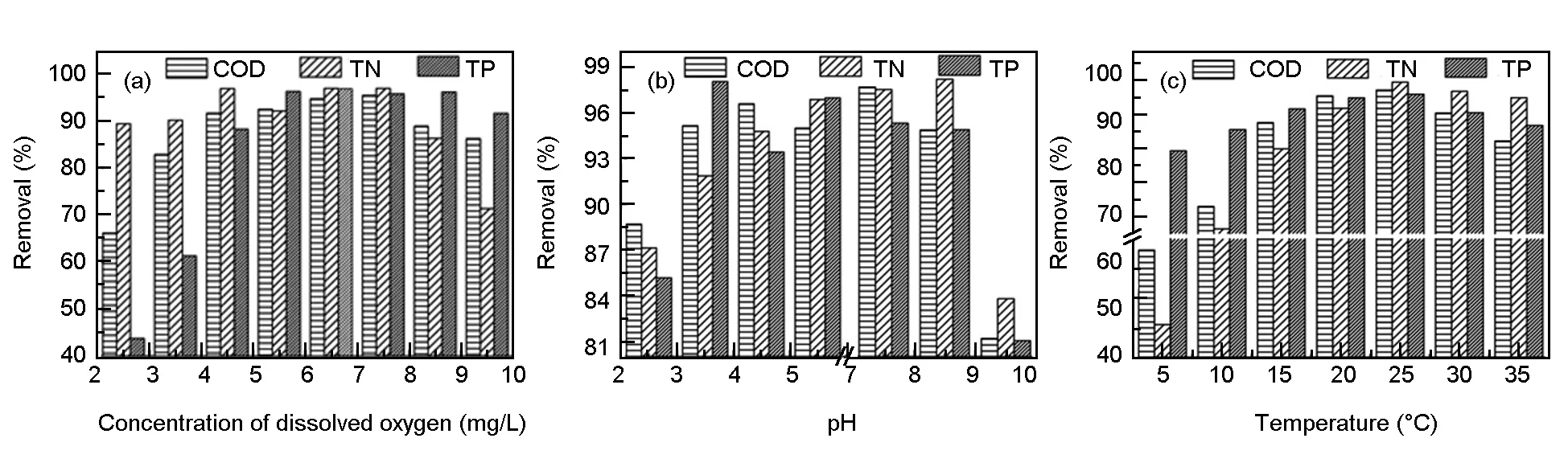
Fig. 3 The removal rates of each index (COD, TN and TP) under different conditions (dissolved oxygen concentration, pH and temperature).

Table 2 The pH values of influent and effluent water.
Temperature is another important parameter affecting the growth of microorganisms. In Fig. 3c, it can be found that the preferred temperature range is 15 -30 ℃, and this temperature range is wider than other general biofilm systems. The treatment efficiency at 5 ℃ is still very poor even after the hydraulic residence time (Fig. 4a) is extended. The main reason may be that low temperature inhibits the activity of a large number of microorganisms and makes microbial metabolism significantly reduced[19]. The treatment effect at 10 and 15 ℃ shows an upward turn (Fig. 4b, 4c).
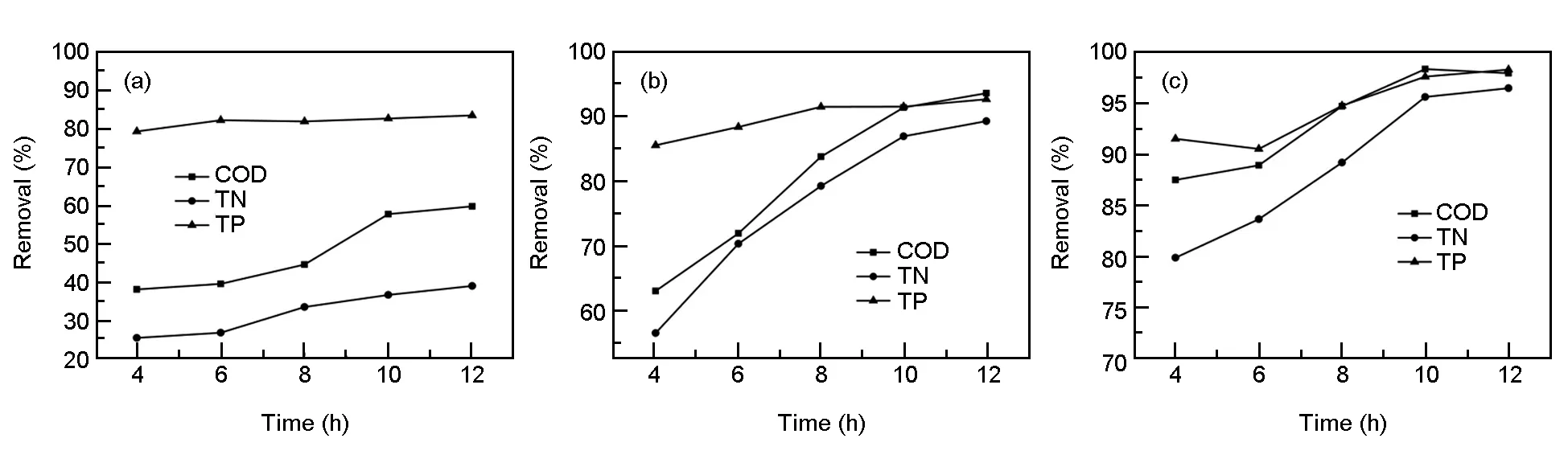
Fig. 4 The removal rates of each index (COD, TN and TP) at (a) 5, (b) 10 and (c) 15 ℃.
It is possible that the activity of some microorganisms is curbed at 10 and 15 ℃ and the EM system requires more time to achieve a better performance. The biological films fall off from the carrier at 35 ℃ with increasing the hydraulic residence time and the desquamated parts may affect the water quality in turn. In practical engineering applications, a relatively prolonged hydraulic residence time can be taken at low temperature conditions (e.g. in winter time). But if it is not possible to avoid too high or too low temperature, probably biofilms used for wastewater treatment will not be the best choice.
4 Conclusions
The use of carrier can efficiently prevent the loss of mobile microorganisms. The biofilm system with CF as EM biofilm carrier can shorten the start period to as few as 2 weeks, which enables the system reach stable fast and increases the total processing efficiency. Electrochemically-treated CF shows an enhanced performance as EM carrier, and the corresponding biofilms can achieve 97.1%, 92.5% and 96.0% removal rates of COD, TN and TP, respectively. This performance is 5%-67% higher than the other two polymer-based carrier systems.The EM biofilm based on the electrochemically-treated CF has a strong resistivity to the changing surrounding environment, which is more beneficial for wastewater treatment applications.
[1] Narendrakumar G, Kumar J A. Evaluation of effective microorganism (EM) for treatment of domestic sewage [J]. Journal of Experimental Sciences, 2011, 2(7): 30-32.
[2] Sigstad E E, Schabes F I, Tejerina F. A calorimetric analysis of soil treated with effective microorganisms [J]. Thermochimica Acta, 2013, 569: 139-143.
[3] Javaid A, Bajwa R. Effect of effective microorganism application on crop growth, yield, and nutrition in vigna radiate (L.) wilczek in different soil amendment systems [J]. Communications in Soil Science and Plant Analysis, 2011, 42(17): 2112-2121.
[4] Jiang H B, Cai J, et al. Mechanism and application of effective microorganisms (EM) in aquaculture [J]. Water Purification Technology, 2014, 33(6): 28-32.
[5] Li C H, Shi Y. The influences of EM microbial organic fertilizer on soil microbial biomass carbon and urease activity in phyllostachys heterocycla plantation [J]. China Forestry Science and Technology, 2013, 27(6): 56-58.
[6] Xu J J, Shao X H. Influence of EM dosage and amount of aeration on purification of aquaculture wastewater [J]. Water Resources Protection, 2013, 29(5): 69-72.
[7] Ehab M R, Mohamed M. The effect of effective microorganisms (EM) on EBPR in modified contact stabilization system [J]. HBRC Journal, 2014, 1-9.
[8] Emad A S. Prospect of effective microorganism technology in wastes treatment in Egypt [J]. Asian Pacific Journal of Tropical Biomedicine, 2011, 1(3): 243-248.
[9] Lv L, Yin C H, Xu Q Q. Cyanobacterial bloom control by environmental effective microorganisms [J]. Environmental Science & Technology, 2010, 8(33): 1-5.
[10] Matsumoto S, Ohtaki A, Hori K. Carbon fiber as an excellent support material for wastewater treatment biofilm [J]. Environmental Science & Technology, 2012, 46: 10175-10181.
[11] Smith K M, Fowler G D, Pullket S, et al. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications [J]. Water Research, 2009, 43: 2569-2594.
[12] Ma Z K, Liu J. Application of carbon fiber as biofilm carriers in denitrification [J]. China Environmental Science, 2003, 23(3): 247-250.
[13] Bouchez T, Patureau D, Dabert P, et al. Ecological study of a bioaugmentation failure [J]. Environmental Microbiology, 2000, 2(2): 179-190.
[14] Alves C F, Melo L F, Vieira M J. Influence of medium composition on the characteristics of a denitrifying biofilm formed by alcaligenes denitrificans in a fluidised bed reactor [J]. Process Biochem, 2002, 37(8): 837-845.
[15] Ting A S Y, Rahman N H A, et al. Investigating metal removal potential by effective microorganisms (EM) in alginate-immobilized and free-cell forms [J]. Bioresource Technology, 2013, 147: 636-639.
[16] Liu J, Bai Y X, Tian Y L. Effect of the process of electrochemical modification on the surface structure and properties of PAN-based carbon fibers [J]. Acta Materiae Compositae Sinica, 2012, 29(2): 16-25.
[17] Castilla C M, Toledo I B, Ferro-Garcia M A. Influence of support surface properties on activity of bacteria immobilised on activated carbons for water denitrification [J]. Carbon, 2003, 41: 1743-1749.
[18] Renner L D, Weibel D B. Physicochemical regulation of biofilm formation [J]. MRS Bulletin, 2011, 36(5): 347-355.
[19] Zhou Q Y, Gao T Y. Microbiology of Environmental Engineering[M]. Beijing: Higher Education Press, 2000: 210-212.
[20] Qi H Y, Wang W B, Zheng Y. Mechanism of biofilm formation and analysis of influencing factors [J]. Microbiology China, 2013, 40(4): 677-685.
[21] Liu Y, Zhao Q L, Zheng X H. Biofilm Wastewater Treatment Technology[M], Beijing: China Architecture & Building Press, 2000: 22-23.
[22] Sun S P, Hatton T A, Chung T S. Hyperbranched polyethyleneimine induced cross-linking of polyamide-imide nanofiltration hollow fiber membranes for effective removal of ciprofloxacin [J]. Environmental Science & Technology, 2011, 45(5): 4003-4009.
[23] Flemming H C, Wingender J. The biofilm matrix [J]. Nature Reviews Microbiology, 2010, 8(9): 623-633.
