硅烷改性氧化石墨烯-聚羧酸复合物的合成及性能
2018-05-02李时雨郭紫薇
王 琴, 李时雨, 潘 硕, 郭紫薇
(北京建筑大学 土木与交通工程学院, 建筑结构与环境修复功能材料北京市重点实验室,北京100044)
1 Introduction
Concrete is a kind of cement-based composite materials, which is composed of cement, sand, stone and some mineral admixtures. Due to its low price, convenient construction and other characteristics, concrete is the most widely used building materials around the world. Nevertheless, with the development of the society, many problems also appear in the applications of concrete in practical engineering. Because of the brittleness and low toughness of the concrete itself, the concrete is prone to produce microcracks under the structure stress, so that the infiltration of the external erosion medium may lead to concrete damage, even results in a serious steel corrosion, further reduces the strength and durability of the structure, reduces the life of the concrete structure and eventually bring about security risks in the course of service[1].
Cement, as the main clinker materials of concrete, will undergo a series of complex chemical reactions as soon as its touch with water, leading to the formation of a series of hydrated products such as calcium silicate gel (CSH), ettringite (AFt), calcium hydroxide crystals (CH) and monosulfur calcium sulphoaluminate crystals (AFm). The main forms of hydration products are lamellar, needle-like and gel-type, which are the fundamental source of concrete strength. However, due to the complex hydration process of cement, the morphology and structure of hydration products will change in varying degrees, resulting in a large number of microscopic defects in the internal microstructure of cement stone, which are both a source of concrete brittleness and low toughness and the reason for poor resistance to environmental erosion. Therefore, the improvement of the microstructure of cement stone is the fundamental solutions of solving the problems of concrete brittleness and low toughness.
Since the middle of the last century, mineral admixtures and organic fibers such as polypropylene fiber and other blends began to be extensively used in the concrete, which formed a more reasonable multi-gel system rather than a single cement gel system, leading to the improvement of the toughness of concrete and other properties at a certain extent. And the use of mineral admixtures has also played a positive role in the durability of concrete[2, 3]. However, adding mineral admixtures and fibers into concrete does not change the nature of the microstructure of the cementitious material. After the study of mineral admixtures and organic fibers, the researchers began to notice the application of nanomaterials in concrete. It was found that the strength and toughness of concrete filled with nanoparticles were significantly improved, and nanomaterial particles could more effectively counteract the microscopic defects, promote the formation of hydration products with more regular crystal and affect the proportion of hydration products through the secondary hydration, so as to effectively improve the microstructure of cement stone. Now the nano-materials which have been applied in the practical engineering are mainly nano-silica, nano-calcium carbonate, rice husk ash and so on. In recent years, some studies have found that the addition of graphene oxide (GO) and other new nano-carbon materials into the cement paste will also have a significant increase in cement toughness[4-6]. The GO has a regulation effect on the hydration of cement, which improve the crystal arrangement of hydration products andthe microstructure of cement stone, and enhances the strength and toughness of cement material.
However, it was found that the addition of GO in the cement paste could obviously increase the viscosity of the paste, and decrease the fluidity, resulting in the decrease of the working performance of the cement paste. In order to ensure the working performance of the paste, it is necessary to further mix polycarboxylate superplasticizer to improve the fluidity of the cement paste. Although GO can be easily dispersed in the aqueous phase, the dispersibility of GO in the cement paste with high alkaline is not easily determined and difficult to control. If the dispersibility is poor, the performance of the cement can also be adversely affected.
The purpose of this study is to use chemical methods to graft the molecules of polycarboxylate on the graphene oxide layer to modify the graphene oxide (S-GO) by polycarboxylate water reducing agent to prepare a polycarboxylate nanoparticle complex(P-S-GO). With the aim of solving the adverse effect of the graphene oxide on the working performance of the cement paste by a combination of the polycarboxylate water reducing agent and the graphene oxide.
2 Experimental
2.1 Materials
The chemicals used in the experiment are highly active graphene oxide powder (GO), and analytic pure vinyltrimethoxysilane (Silane coupling agent KH-171 or A-171, purity>98%), anhydrous ethanol, glacial acetic acid, acrylic acid (AA), HPEG macromonomer (relative molecular mass of about 2400 g/mol), ammonium persulfate (APS), mercaptopropionic acid and ascorbic acid (Vc).
The cement used in the fluidity and rheological tests is the reference cement produced by Qufu Zhonglian Cement Co., Ltd., and its chemical composition, mineral composition and physical properties are shown in Tables 1, 2, and 3, respectively.

Table 1 Chemical composition of the reference cement.
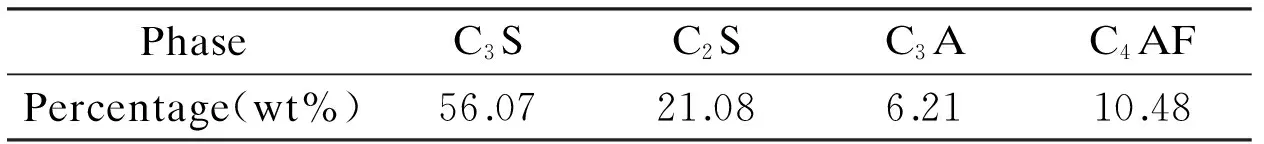
Table 2 Mineral composition of the reference cement.
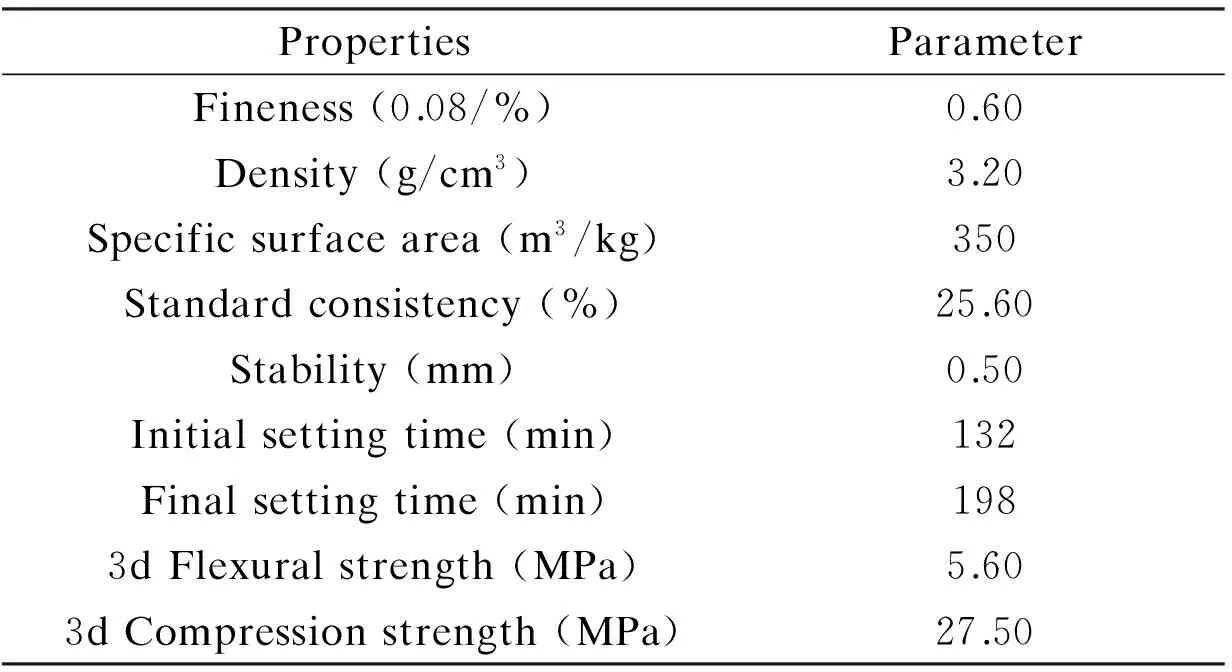
Table 3 Physical properties of the reference cement.
2.2 Silicane modification of graphene oxide (S-GO)
Distilled water and anhydrous ethanol were mixed in a mass ratio of 1∶1 in a conical flask with a stopper, the pH value of the solution was adjusted to 4 to 5 with glacial acetic acid, and then the vinyltrimethoxysilane was added.The mass ratio of GO to vinyltrimethoxysilane was 1∶2. The mixed solution was pre-hydrolyzed in a water bath at 30-40 ℃ for 30 min. After completion of the pre-hydrolysis, a certain amount of GO was then mixed with the pre-hydrolyzed solution. After ultrasonic dispersion for 30 min, the dispersion was transferred into a three-necked flask in a 80 ℃ water bath under stirring for about 5 h for reaction. After completion of the reaction, the reaction solution was cooled to room temperature and then filtered, washed three times with a 1∶1 aqueous ethanol solution and dried at 60 ℃ for 8 h to obtain the products[7- 9]. The reaction scheme is shown in Fig. 1.
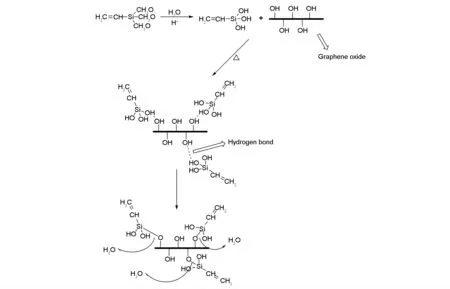
Fig. 1 Silicane modification of graphene oxide (S-GO).
2.3 Synthesis of silane modified graphene oxide-polycarboxylate composites (P-S-GO)
A certain amount of the modified GO was placed in distilled water and pre-ultrasonically dispersed for 0.5-1 h[10-12]. After dispersion, a mixture of the HPEG macromonomer and APS initiator was added to the pre-dispersed liquid in a four-necked flask, which was placed into a 80 ℃ water bath and stirred to fully dissolve the solid. The mass ratio of GO∶ HPEG∶ APS was 1∶17.5∶0.2. The A reaction solution was prepared according to the ratio of AA to mercaptopropionic acid of 4.5∶0.2 with a certain amount of distilled water. The B reaction solution was an ascorbic acid aqueous solution (about 0.2%). The A and B reaction solutions were added dropwise to the flask through a peristaltic pump. The dropping times of the A and B solution were 1.5 and 2.0 h, respectively. After completion of dropping, it was maintained at this temperature for 0.5-1 h. After completion of the reaction, the reaction solution was cooled to room temperature. The product was washed with distilled water several times to ensure that the free polycarboxylate superplasticizer was completely washed to avoid affecting the subsequent tests. The products were dried at about 60 ℃ for 8-10 h, and milled to get the final products[13-15]. The synthesis mechanism is shown in Fig. 2. In this experimental, the self-synthetic polycarboxylate superplasticizer (without GO) with exactly the same constituents with P-S-GO was used as the control sample. Its solid content is about 50% and the water reduction ratio is about 30%.
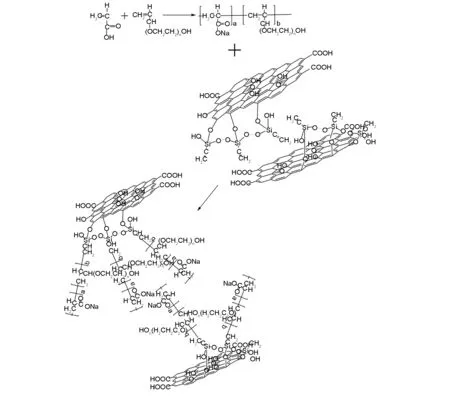
Fig. 2 Synthesis mechanism of the silane modified graphene oxide-polycarboxylate composites (P-S-GO).
2.4 Test on performance of cement paste with P-S-GO
The tests were carried out at (25 ± 3)℃. Cement paste fluidity tests were performed according to the reference GB/T8077-2000 (National Standard of China) concrete admixture homogeneity test method. The water-cement ratio is 0.35 and the specific mixing proportion is shown in Table 4. After preparation of cement paste, it was poured into a mini-cone (top inside diameter: 36 mm, bottom inside diameter: 60 mm, height: 60 mm). The fluidity of cement paste at 0, 30, 60, 90 and 120 min was recorded. Each time the measurement was tested twice and then the fluidity was averaged.
The rheological parameters of cement paste were characterized by a BROOKFIELD RST-CC rheometer. The sample cup FTK-RST and spindle CC3-40 were used. Before testing, the cement paste was prepared according to Chinese standard GB/T8077-2000. The mixing procedure is described as follows. The cement powder was first placed in a beaker, and then the composites, the water reducing agent and water were mixed and added to the powder. The cement mixture was then stirred at 62 rpm for 90 s. After a 15 s interval, the mixture was then stirred at 125 rpm for an additional 90 s to assure the homogeneity of the cement paste. As soon as the mixing stopped, the sample was then poured into the sample cup to carry out the rheological measurement. During the rheological test, the shear rate was increased from 5.6 to 345 rpm and then back to 5.6 rpm with 15 speed intervals. The rheological parameters, apparent viscosity and shear stress of the cement paste with different mixture proportions were measured[16, 17]. The data were analyzed using the software RHE3000. For the GO dispersions and the P-S-GO dispersions, distilled water was used as the dispersion medium with a concentration 2 mg/mL. Mixing proportions of cement pastes are listed in Table 4.
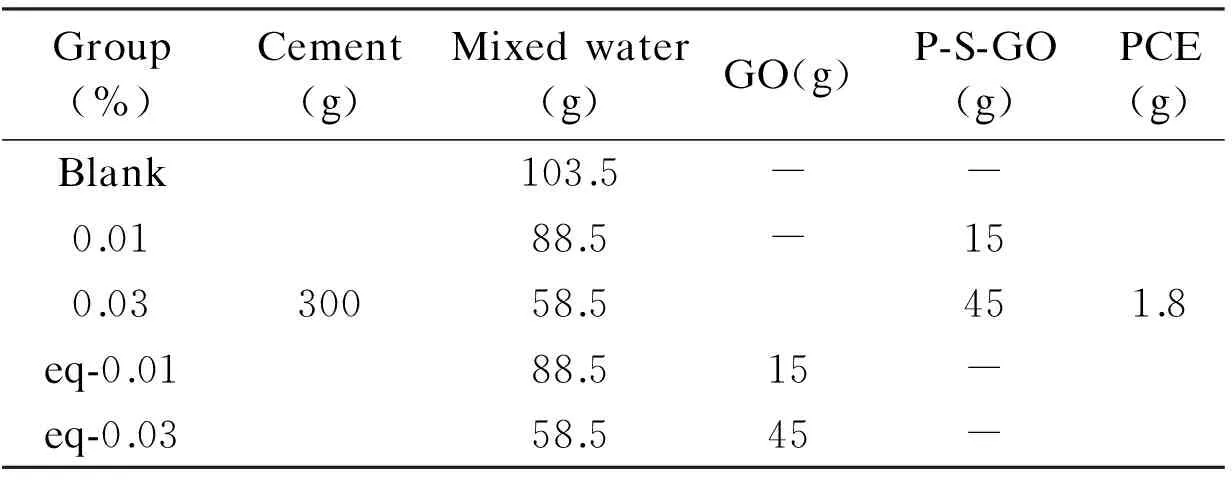
Table 4 Mixing proportions of cement pastes.
3 Results and discussion
3.1 Fourier Transform infrared spectroscopy analysis (FT-IR) of P-S-GO
The chemical structures of GO, S-GO and P-S-GO were characterized by FT-IR. The results are shown in Fig. 3.Fig. 3(a) is a FT-IR spectrum of GO. The spectrum shows a peak in the range of 3 000-3 500 cm-1, which indicates that GO contains hydroxyl groups. The peak at 1 727 cm-1usually corresponds to the carbonyl vibration peak. The peak at 1 224 cm-1is ascribed to the O-H stretching vibration carboxyl group. The peak at 1 053 cm-1is attributed to the C-O bond stretching vibration peak, and there is also a less obvious peak near 1 380 cm-1, corresponding to a C-O-H structure. These indicate that there is hydroxyl, carboxyl and epoxy group in GO.
Fig. 3(b) is the FT-IR spectrum of S-GO. The S-GO also has a hydroxyl peak in the range of 3 000-3 500 cm-1, but its peak intensity is lower than that of the GO, indicating that a part of hydroxyl groups on the surface of the GO is consumed. The peak at 1 740 cm-1usually corresponds to the vibration of the carbonyl group. The peak at 1 620 cm-1may be caused by the presence of double bonds, and the band at 1 000-1 100 cm-1is attributed to the stretching vibration of Si-O. There is a less obvious shoulder at 960 cm-1, which should be associated with Si-OH. The peak at 473 cm-1should be contributed by Si-O-Si. According to the above analysis, it is found that the S-GO has characteristic peaks of GO and the surface of the GO has a silane component containing carbon-carbon double bond, which is similar to the structure of the vinyltrimethoxysilane used. These reveal that GO was successfully modified by the silane coupling agent.
Fig. 1(c) is the FT-IR spectrum of P-S-GO. First, the P-S-GO has an intense absorption peak at 1 869 cm-1compared with the S-GO, indicating that the P-S-GO contains more methyl and methylene. The obvious sharp peak at 2 869 cm-1is contributed by a symmetric stretching of C-H that may be introduced by the acrylic monomer and the long chain of PCE onto the surface of the GO. The Si-O, Si-OH and Si-O-Si peaks are also found, which are similar to Fig. 3(b). These results indicate that the synthesis of the complex does not change the structure of the modified GO.[9,12,14]
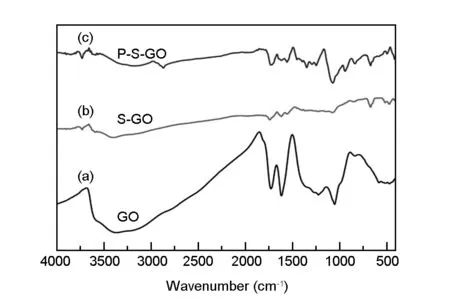
Fig. 3 FT-IR spectra of (a) GO, (b) S-GO and (c) P-S-GO.
3.2 X-ray diffraction analysis (XRD) of P-S-GO
The XRD patterns of the GO, S-GO and P-S-GO are shown in Fig. 4(a-c). It can be seen that the characteristic peak of GO at 2θ= 12° and 2θ= 42° are retained (denoted as the first characteristic peaks and the second characteristic peak) for S-GO and P-S-GO. But there is a gentle convex peak at about 2θ= 30° between the first k and the second characteristic peaks in Fig. 4(b), which is due to the introduction of vinyl trimethoxysilane. In Fig. 4(c), the second characteristic peak remains obvious, but the first characteristic peak intensity is obviously weakened and deviated, and a new peak appears at 2θ= 18°. The amorphous nature of graphene is significantly enhanced due to the introduction of the polycarboxylic acid water reducing agent[12,14].
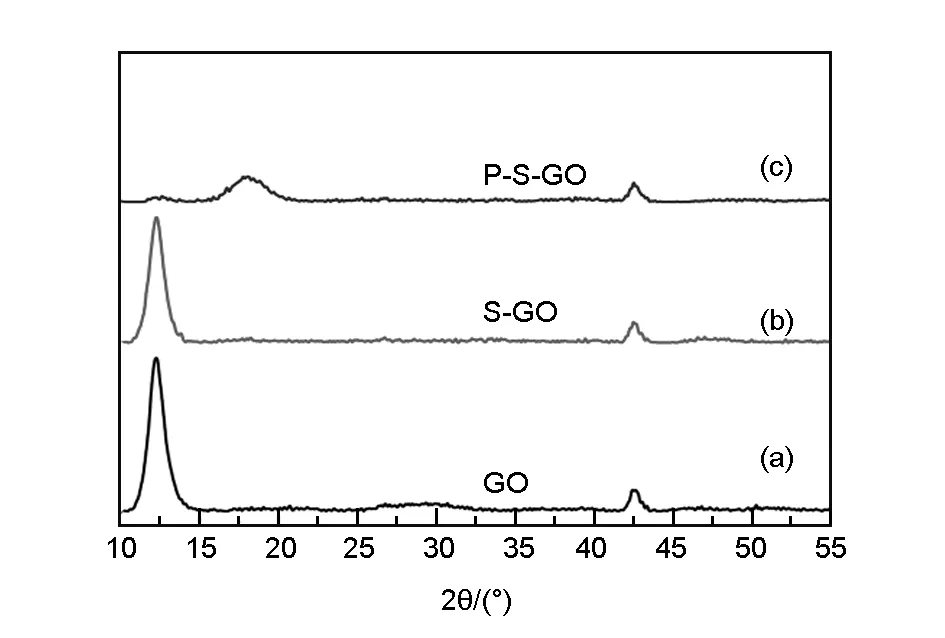
Fig. 4 XRD patterns of (a) GO, (b) S-GO and (c) P-S-GO.
3.3 Particle size analysis of P-S-GO
The particle size analysis results of the GO and P-S-GO are shown in Fig. 5. Fig. 5(a) and 5(b) are the particle size distributions of the GO and PCE-
GO, respectively. It can be seen that the average particle sizes of the GO and P-S-GO are about 546.2 nm and 1 678 nm, respectively. The increase of particle size indicates that the molecular chain of the polycarboxylate is grafted onto the surface of the GO particles to form a wrapping layer, thereby increasing the particle size.
3.4 Dispersibility of P-S-GO
In order to compare the dispersion of GO and P-S-GO in cement paste solution, a saturated lime aqueous solution was used as a simulated solution[9]. The saturated lime water with a pH value of 13 was prepared by dissolving excess calcium hydroxide in distilled water followed by filtration with a 0.22 μm membrane. The experimental results are shown in Fig. 6.
The P-S-GO and GO dispersions were added dropwise to the saturated lime water in the flask (a) and (b) at equivalent volume, respectively. The results show that the common GO dispersion dropped to the saturated lime water forms an agglomerate structure and settles down, while the P-S-GO dispersion still retains the original state in the saturated lime water. This indicates that the P-S-GO has a better dispersibility under high pH values in the cement solution than the GO.
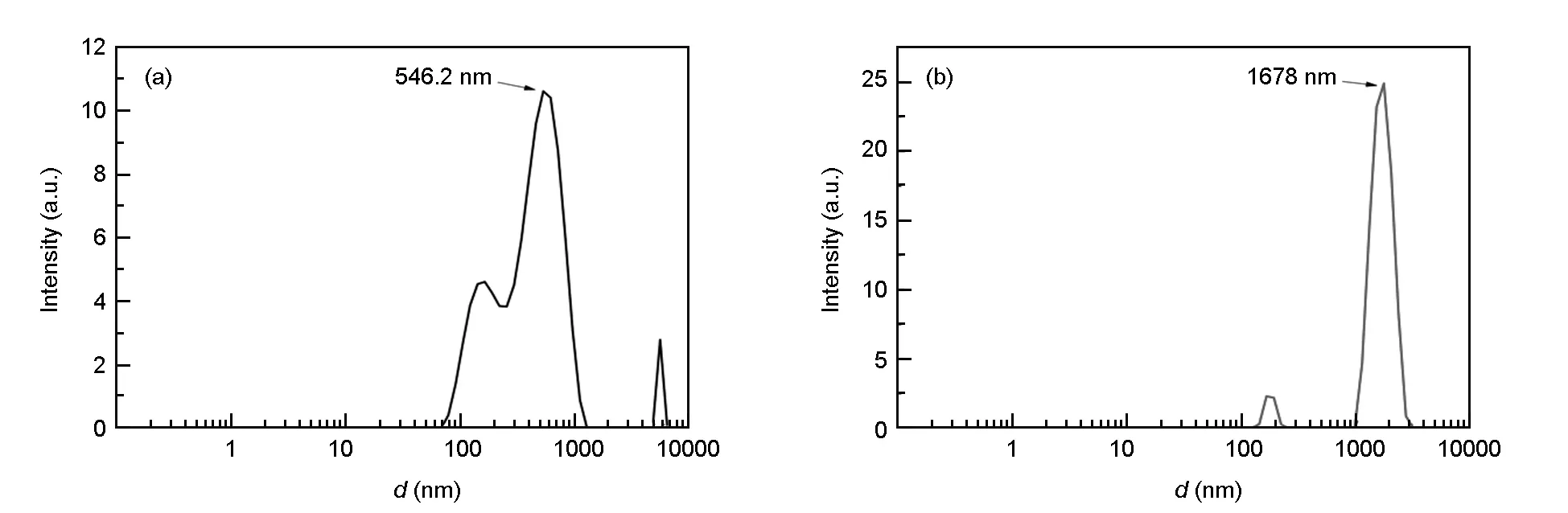
Fig. 5 Particle size distributions of (a) GO and (b) P-S-GO.

Fig. 6 Dispersibility of GO and P-S-GO:
3.5 The fluidity of P-S-GO cement paste
Since the purpose of this study is to solve the unfavorable effect of ordinary GO on the fluidity of cement paste, the cement paste fluidity test is indispensable[17,18]. The effect of GO and P-S-GO on the fluidity of cement pastes was studied at two dosages, 0.01 and 0.03 wt% of cement. The content of the self-made polycarboxylate water reducing agent was 0.6 wt% of cement. The experimental results are shown in Fig. 7.
The results show that the fluidity of the cement pastes decreases with the increase of the GO dosage. Compared with the control group, the fluidity of cement pastes with a GO addition of 0.01 and 0.03 wt% decreases by 10.8% and 36.9 %, respectively. On the contrary, the fluidity of the cement pastes with P-S-GO is similar to that of the control group. Compared with the control group, the fluidity decreases only by 2.0% and 8.3% at 60 min with a P-S-GO addition of 0.01 and 0.03 wt%, respectively. It can be seen that the P-S-GO complex is a good remedy for the negative effect of GO on the fluidity of the cement paste.

Fig. 7 Fluidity curves of cement pastes after dosage of GO and P-S-GO.
The effect of GO on the fluidity of the cement paste is closely related to its existence state in the cement solution. As shown in Fig. 8(a), when GO dispersion is added to the cement paste, a flocculation structure is formed, which is attributed to its high specific surface area and abundant oxygen-containing functional groups. On the one hand, the agglomerated structure wraps a part of the mixed water and reduce the actual amount of mixed water. On the other hand, the large volume of agglomerated particles in the cement paste increases the internal friction. The combination of these two factors leads to the reduction of the paste fluidity[16-18]. On the contrary, when the same amount of the P-S-GO dispersion is added to the paste, as shown in Fig. 8(b), the P-S-GO composite particles do not form significant aggregates in the cement solution and the cement paste maintains a better dispersibility, compared with the GO dispersion. Moreover, the long polymer chains of P-S-GO, which is absorbed on the surface of cement particles, provide electrostatic repulsion as well as steric hindrance between cement grains[13].This phenomenon is similar to that of polycarboxylate[19-21].

Fig. 8 Schematic diagram of dispersion mechanism of P-S-GO in the cement paste.
3.6 Rheological properties of the P-S-GO cement paste
The curves shown in Figs. 9 (a) and (b) show the relationship between the shear stress and shear rate of the cement paste and the change of the apparent viscosity of the cement paste with the shear rate, respectively. In order to characterize the shear deformation of the cement paste, the shear stress and shear rate data are fitted using the Herschel-Bulkley (H-B) model. The model is given by:
τ=τ0+k·γn
(1)
Whereτ0is the yield stress (Pa),kis the consistency,γis shear rate (s-1),nis pseudoplastic index. Whenn<1, the paste is characterized by shear thinning, and the smaller is the n value, the higher is the degree of shear thinning. Whenn> 1, the paste shows shear thickening, and the higher is the n value, the higher is the degree of the shear thickening of the paste[16,17,23]. The fitting results are shown in Table 5.
From Table 5, it can be seen that the yield stress of the experimental groups added with 0.01 and 0.03 wt% of P-S-GO complex is 13.01 and 13.18 Pa, respectively, which are less than 15.44 and 20.74 Pa of the control groups with the same amount of GO, and less than the yield stress of the blank experimental group. This indicate that the P-S-GO complex can effectively reduce the yield stress of the paste. The pseudoplastic indexnof each group is larger than 1, indicating that the pastes exhibit a shear-thickening effect. Thenvalues of experimental groups with the P-S-GO are higher than that of GO control groups and blank group, which suggests that the incorporation of P-S-GO enhances the shear thickening effect of the paste.
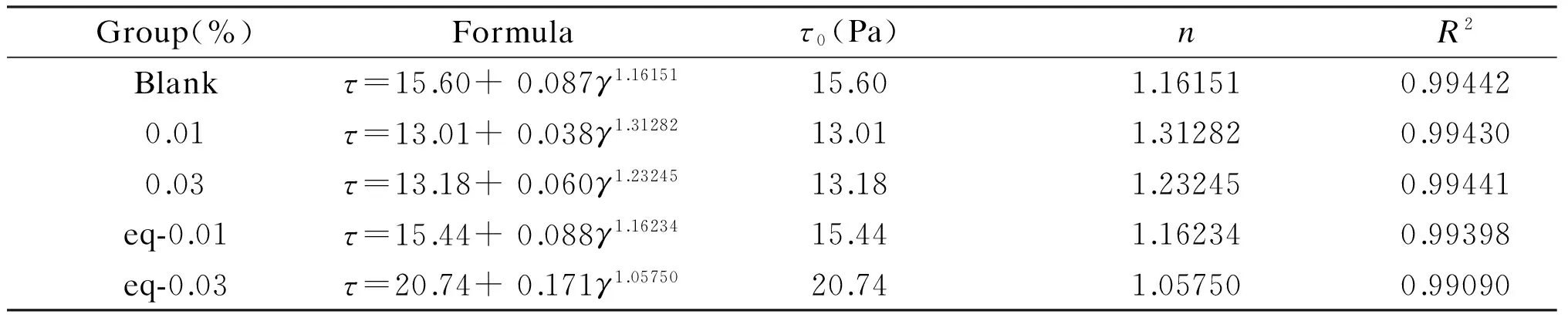
Table 5 Fitting results of rheological parameters of pastes after dosage of GO and P-S-GO.
The curves in Fig. 9 (b) show the relationship between the apparent viscosity of the cement paste and the shear rate. It is found that the viscosity of the paste in each test group decreases with increasing shear rate. The initial viscosities of the cement pastes mixed with the P-S-GO are much lower than that of the cement pastes added with GO under the same dosages. As the shear rate increases, the difference in viscosity between the two samples decreases and tends to be the same viscosity value[17,23].
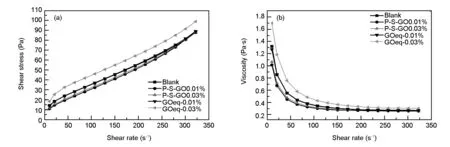
Fig. 9 Rheological property test of cement pastes.
4 Conclusions
A novel P-S-GO complex was successfully synthesized through the copolymerization of the silicane-modified GO and the monomers of polycarboxylate. Vinyltrimethoxysilane can successfully modify GO, the modified GO can react with the monomer of polycarboxylate, so that the polycarboxylate molecular chain can be grafted onto the surface of GO.
Compared with GO, the P-S-GO complex has a better dispersibility and is less liable to form a flocculation structure in an over-based and multi-ion complex environment. The paste fluidity of the P-S-GO cement paste at the same dosage is much better than that of the GO cement paste, which can effectively help counteract the adverse effects of GO on fluidity. The rheological results show that the P-S-GO complex could significantly reduce the yield stress and apparent viscosity of the pastes under the same dosages.
The long polymer chains of the P-S-GO, which is absorbed on the surface of cement particles, provide electrostatic repulsion as well as steric hindrance between cement grains. These two aspects contribute to the better dispersibility and more excellent fluidity of cement pastes under the over-based and multi-ion complex environment.
[1] Alaa M. Rashad. A comprehensive overview about the effect of nano-SiO2on some properties of traditional cementitious materials and alkali-activated fly ash[J]. Construction and Building Materials, 2014, 52: 437-464.
[2] Yang R H, Wen X L, ShuH Y, et al. Performance Influence of composite nano-materials on concrete and cement mortar[J]. Journal of Chongqing Jianzhu University, 2007, 29(5): 144-148.
[3] Arkles B. Tailoring surfaces with silanes[J]. Chemtech, 1977, 7(12): 766-778.
[4] Wang Q, Wang J, Lu C X, et al. Influence of graphene oxide additions on the microstructure and mechanical strength of cement[J]. New Carbon Materials, 2015, 30(4): 349-356.
[5] Peyvandi, Amirpasha. Surface-modified graphite nanomaterials for improved reinforcement efficiency in cementitious paste[J]. Carbon, 2013, 63(2): 175-186.
[6] Lv S H, Deng L J, Yang W Q, et al. Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites[J]. Cement & Concrete Composites, 2016, 66: 1-9.
[7] Yan JL, Chen G J, Cao J, et al. Functionalized graphene oxide with ethylenediamine and 1,6-hexanediamine[J]. New Carbon Materials, 2012, 52(5): 624-624.
[8] 杨永岗, 陈成猛, 温月芳, 等. 氧化石墨烯及其与聚合物的复合[J]. 新型炭材料, 2008, 23(3): 193-200. (YANG Yong-gang, CHE Cheng-meng, WEN Yue-fang, et al. Oxidized graphene and graphene based polymer composites[J]. New Carbon Materials, 2008, 23(3): 193-200.)
[9] 时镜镜, 马文石, 林晓丹. KH-570功能化石墨烯的制备与表征[J]. 无机化学学报, 2012, 28(1): 131-136. (SHI Jing-jing, MA Wen-shi, LINXiao-dan. Synthesis and characterization of functionalized graphene with KH-570[J]. Chinese Journal of Inorganic Chemistry, 2012, 28(1):131-136.)
[10] Kim H M, Lee H S. Water and oxygen permeation through transparent ethylene vinyl alcohol/(graphene oxide) membranes[J]. Carbon Letters, 2014, 15(1): 50-56.
[11] Yang Z, Chen X H, Liu Y Q, et al. Study of carbon nanotubes methylolated and grafted with maleic anhydride[J]. Acta Chimica Sinica, 2006, 64(3): 203-207.
[12] Choi S, Hwang D K, Lee H S. Thermal properties in strong hydrogen bonding systems composed of poly(vinyl alcohol), polyethyleneimine, and graphene oxide[J]. Carbon Letters, 2014, 15(4): 282-289.
[13] Li Y, Guo H, Zhang Y, et al. Synthesis of copolymers with cyclodextrin as pendants and its end group effect as superplasticizer[J]. Carbohydrate Polymers, 2014, 102(1): 278-287.
[14] Zhao L, Guo X, Ge C, et al. Investigation of the effectiveness of PC@GO on the reinforcement for cement composites[J]. Construction & Building Materials, 2016, 113: 470-478.
[15] Kong X M, Shi Z, Lu Z. Synthesis of novel polymer nano-particles and their interaction with cement[J]. Construction & Building Materials, 2014, 68(4): 434-443.
[16] Wang Qin, Cui Xin-you, Wang Jian, et al. Effect of fly ash on rheological properties of graphene oxide cement paste[J]. Construction & Building Materials, 2017, 138: 35-44.
[17] Wang Q, Wang J, Lv C X, et al. Rheological behavior of fresh cement pastes with a graphene oxide additive[J]. New Carbon Materials, 2016, 31(6): 574-584.
[18] Shang Y, Zhang D, Yang C, et al. Effect of graphene oxide on the rheological properties of cement pastes[J]. Construction & Building Materials, 2015, 96: 20-28.
[19] Zhang Y R, Kong X M, Hou SS, et al. Study on the rheological properties of fresh cement asphalt paste[J]. Construction & Building Materials, 2012, 27(1): 534-544.
[20] Zhang YR, Kong X M, Lu ZC, et al. Influence of triethanolamine on the hydration product of portlandite in cement paste and the mechanism[J]. Cement & Concrete Research, 2016, 87: 64-76.
[21] 张力冉, 王栋民, 潘 佳, 等. 新拌水泥浆体絮凝结构与流变行为及有效体积分数的关系[J]. 硅酸盐学报,2014,42 (9): 1209-1218. (ZHANG Li-ran, WANG Dong-min, PAN Jia, et al. Relationship between flocculent structures and rheological behavior/effective volume fraction of fresh cement paste[J]. Journal of the Chinese Ceramic Society, 2014, 42( 9) : 1209-1218.)
[22] Khayat K H,Yahia A, Sayed M. Effect of supplementary cementitious materials on rheological properties, bleeding,and strength of structural grout[J]. Aci Materials Journal, 2008, 105(6): 585-593.
[23] Zhang X, Zhang L. Application of rheological theory in cement-based materials[J]. Fly Ash Comprehensive Utilization, 2013, (4): 9-13.
