拮抗酵母控制果蔬采后真菌病害研究进展
2018-05-01姜冬梅王刘庆韦迪哲
王 瑶,姜冬梅,王刘庆,韦迪哲,王 蒙
(北京农业质量标准与检测技术研究中心,农业部农产品质量安全风险评估实验室(北京),北京 100097)
新鲜果蔬营养价值很高,是人类平衡膳食的重要组成部分。但果蔬在采后的运输、贮藏、加工等过程中,水分流失、营养物质消耗和抗病能力下降等导致的品质损失十分严重。果蔬中高营养物质、高水分及低pH适宜病原真菌生长,所以,由病原真菌引起的腐烂非常普遍。据统计,我国采后处理过程中由病原真菌引起的果蔬损失率高达30%,即使在发达国家,果蔬损失率也可达20%~25%[1]。除了严重的经济损失外,果蔬采后病害对人类健康造成的安全隐患也不容忽视,青霉菌(Penicillium)、链格孢菌(Alternaria)、镰刀菌(Fusarium)等病原真菌能够在引起果蔬病害的同时在腐烂部位积累真菌毒素,从而危害人类健康。例如能够引起多种果蔬青霉病的扩展青霉(Penicillium expansum),此种病原菌产生的棒曲霉素、桔霉素都是潜在的致癌物质[2]。
目前主要采用化学方法来防治果蔬病害,但长期使用化学杀菌剂会使病原菌产生抗性,化学残留也在威胁着人类健康的同时对环境造成污染,有关部门对其使用的监管日趋严格。另外,随着人们生活水平的提高,对食品安全和品质的要求也越来越高,化学杀菌剂使用也受到局限。过去的二十几年,人们一直在研究能够代替化学杀菌剂的安全无害的果蔬菜后真菌病害防治方法。对环境友好的生物防治方法,近年来受到广泛重视,成为一种发展趋势[3]。拮抗酵母是一类使用较为广泛的生防菌,其主要优点是繁殖能力强,次级代谢产物无毒并且能和与化学杀菌剂共同使用。
本文主要从拮抗酵母的种类及防治效果、作用机制、作用的改良等方面介绍近年来拮抗酵母控制果蔬采后病害研究情况,旨在加深对果蔬菜后真菌病害防控的了解,也可为安全有效地杀菌制剂的开发提供新思路。
1 拮抗酵母的种类及防治效果
目前大部分拮抗酵母取自果实表面、叶片、根部、土壤和海水等。其中,从果实表面分离到的拮抗酵母最多,一些常见果蔬如番茄、柑橘、苹果、葡萄、柠檬、无花果、辣椒等表皮上都能分离到对一些常见病原菌拮抗效果比较好的酵母菌[4-20]。例如,Suzzi等[6]报道了关于葡萄果肉中分离出的若干株天然酿酒酵母的采后防治研究,其中一株酿酒酵母(Saccharomycescerevisiae)和一株接合酵母(Zygosaccharomyces)抑菌谱比较广泛;苹果上分离得到的酒清假丝酵母(Candidasake)CPA-1对苹果采后青霉病、灰霉病及根霉腐病有较好防治效果[8]。除果蔬表皮,叶片、根际、土壤中也存在拮抗酵母菌株[21-24]。例如,从番茄叶片上分离得到的粘红酵母(Rhodotorulaglutinis)Y-44对番茄叶片和果实的灰霉病有防控作用[21];从柑桔根部分离的柠檬形克勒克酵母(Kloeckeraapiculata)34-9对柑桔和葡萄的青霉病和灰霉病有防控作用[22];从南极土壤中分离得到一株白冬孢酵母(Leucosporidiumscottii)At17,是一种噬冷酵母,能够很好地防控由扩展青霉和灰霉引起的苹果青霉病和灰霉病[23];果园土壤中分离到的卡利比克毕赤酵母(Pichiacaribbica)对草莓的根霉腐病和灰霉病有较好防治效果[24]。海洋微生物中也存在拮抗酵母菌株,分离自中国东海的一株海洋红酵母(Rhodosporidiumpaludigenum),能有效控制由扩展青霉导致的梨采后害和由互各交链孢菌导致的冬枣采后病害[25],海洋酵母有较好的抗渗透性,与果实表面分离的酵母相比,更适合应用在环境比较恶劣的条件下。表1列出了部分具有代表性的生防酵母菌株及其防控病害。
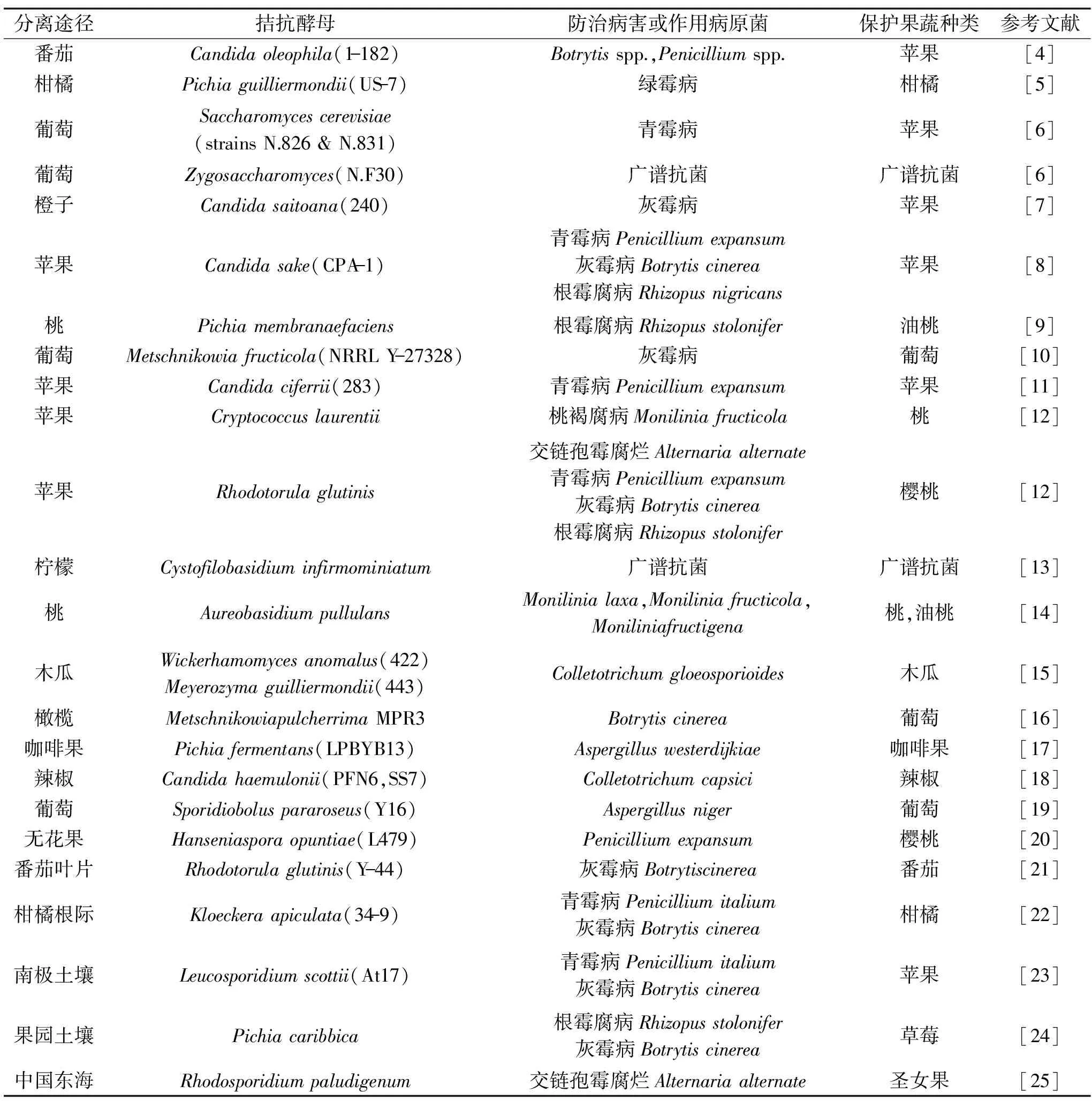
表1 部分具有代表性的生防效果较好的酵母菌株及其防控病害Table 1 Partially representative antagonistic yeasts used for management ofpostharvest diseases
2 拮抗酵母生防作用机制
采后生物防治系统十分复杂并且易受诸多因素影响,温度、pH、水分活度、氧化压力等都能对其产生影响。拮抗酵母通常是通过喷洒或浸涂在果蔬表面起到采后防治作用,若果蔬表面有伤口,拮抗酵母就会在24 h内迅速在伤口上生长,占据伤口空间并消耗营养物质。因此,在第一个24 h内,拮抗酵母的防控机制主要是与病原真菌产生空间与营养上的竞争关系,从而抑制病原菌生长[26-27]。24 h后,其他作用机制逐渐发挥作用,与营养、空间竞争共同防治采后腐烂。伤口处大量繁殖的酵母菌能够诱导寄主的防御机制,通过改变伤口组织pH和一些氧化物的产生来调解酵母菌群密度并改变酵母形态[28-29]。随之拮抗酵母可在果实损伤处形成生物膜,分泌病原菌细胞壁降解酶并消耗病原菌生长所需的铁离子。一些拮抗酵母的作用机理如表2所示。

表2 果蔬采后拮抗酵母生物防控作用机制Table 2 Yeast strains,mechanism of actionagainst postharvest pathogenic molds on different fruits and vegetables
2.1 营养及空间竞争
营养及空间的竞争是拮抗酵母控制真菌病害最主要的作用机理,尤其是针对水果采后真菌病害的防治。采后病害的发生多由病原微生物引起,果实有伤口时,果皮表面的拮抗菌和病原菌孢子同时开始抢占伤口的营养,以营养与空间竞争为拮抗机理的拮抗菌能够在相当短的时间内利用伤口营养大量繁殖,尽可能快的消耗伤口营养、并占领全部空间,使得病原菌得不到合适的营养与空间,不能生栖繁衍,从而抑制病害的发生。拮抗菌和病原菌竞争的营养物质主要是碳水化合物、氮源等[30-36]。Li等[19]研究表明拮抗酵母SporidioboluspararoseusY16能够在葡萄伤口处及表面快速生长繁殖,以抑制病原菌黑曲霉对果实的侵染。将季也蒙假丝酵母(Candidaguilliermondii)接种到桃果实的伤口上,在有病原菌存在的情况下该酵母菌的数量一天内可以增加200多倍,这种高速的繁殖活动反映出拮抗菌与病原菌之间的营养竞争[37]。Filnow[38]用14C标记的果糖、葡萄糖、蔗糖来检验在苹果伤口上的隐球酵母(Candidalaurentii)和掷孢酵母(Sporobolomycesroseus)与Botrytiscinerea病原菌孢子在营养利用能力上的差别,发现拮抗菌比病原菌孢子消耗了更多的糖类物质。
对氮源的竞争作用也是拮抗酵母的作用机理之一,尤其是在碳源充足的果实伤口处。季也蒙假丝酵母(C.guilliermondii)[34]对扩展青霉的拮抗作用就是通过氮源的竞争实现的。
2.2 直接寄生病原菌
许多酵母能够附着在果蔬腐烂部位和病原真菌菌丝上,形成一层生物膜,并且能够分泌一些酶类,如几丁质酶和β-1,3-葡聚糖酶,从而分解病原菌的细胞壁或菌丝体[39-46]。这类拮抗酵母又被称作“killer yeast”。1991年,Wisniewski[47]首次发现了这种防控机制,奥默毕赤酵母(Pichiaguilliermondii)能够紧紧地附着在灰霉的菌丝体上并能够分泌降解病原菌细胞壁的β-1,3-葡聚糖酶。Wan等[48]发现,膜醭毕赤酵母(Pichiamembranaefaciens)对桃软腐病的防控作用也是相同的机理。此外,附着在病原菌上的酵母菌大量消耗病原菌生长所需的铁离子,使病原菌不能生长而起到抑制病害的作用。Saravanakumar等[40]研究表明,拮抗酵母MetschnikowiapulcherrimaMACH1通过消耗病原菌灰霉、交链孢菌、青霉生长所必需的铁离子而抑制了其对苹果的侵染。
2.3 诱导抗病作用
采后果蔬在贮藏过程中,由于自身的后熟和衰老,果蔬的抗病能力逐渐下降,易受到病原菌的侵染,加速腐烂。1994年,Droby和Chalutz首次发现了拮抗酵母的诱导抗病性[49]。张璐等[50]研究膜醭毕赤酵母对草莓采后灰霉病抗病性的诱导作用时发现,膜醭毕赤酵母通过诱导草莓果实β-1,3-葡聚糖酶、过氧化物酶、苯丙氨酸解氨酶等抗性相关酶活性的增强,提高了果实对灰霉病的抵抗能力。随着DNA芯片和高通量测序技术的进步,有关寄主组织和拮抗酵母的基因表达研究也得到了极大发展,这为更好地理解酵母菌诱导抗病机理提供了帮助。Jiang等[51]用微阵列分析的方法分析了罗伦隐球酵母(Cryptococcuslaurentii)诱导圣女果抗病性的机制,研究结果表明,与代谢、信号转导和应激反应相关的基因表达量均上调,与光合作用和能量代谢相关的基因表达量均降低,圣女果抗病性的增强应该与这些相关基因表达量的变化密切关系。Hershkovitz等[52]研究Metschnikowiafructicola诱导葡萄抗病机理时发现,病程相关基因和防御信号相关基因表达量均上调,这些基因与诱导抗性反应相关。蛋白质组学的研究也指出膜醭毕赤酵母诱导了桃果实抗氧化蛋白和病程相关蛋白的表达,验证了这类蛋白在此防控机制中起到的重要作用[53]。卡利比克毕赤酵母[54]和S.pararoseusY16[19]在防治桃和葡萄的采后病害时也是通过诱导果实自身抗病性这一机理来实现的。
2.4 分泌挥发性有机化合物(VOCs)抑菌
挥发性有机化合物是一类低分子量、低极性且饱和蒸气压高的化合物[55],许多拮抗酵母都能产生具有抗真菌性的挥发性有机化合物。Fiori等[56]采用平板对峙法研究了体外实验拮抗酵母产生的VOCs对炭黑曲霉生长的影响,结果表明中间假丝酵母(Candidaintermedia)235和Lachanceathermotolerans751产生的VOCs对炭黑曲霉生长有明显抑制作用,并且对炭黑曲霉引起的葡萄采后腐烂也有较好的抑制作用。Hua等[57]研究表明异常毕赤酵母(Pichiaanomala)WRL-076产生的VOCs能够显著抑制树生坚果上黄曲霉菌孢子的萌发与菌丝的生长,并抑制毒素的合成,经SPME-GC/MS鉴定发现有效物质为2-苯乙醇。Parafati[58]研究了4种酵母Wickerhamomycesanomalus,M.pulcherrima,Aureobasidiumpullulans和酿酒酵母(Saccharomycescerevisiae)在体外实验和体内实验中对灰葡萄孢菌和青霉生长的影响,结果表明,体外实验中,4种酵母都能显著抑制病原菌分生孢子的萌发和菌丝的生长,其中W.anomalus和A.pullulans的效果最为明显;体内实验中,W.anomalus产生的VOCs能够完全抑制人为接种引起的草莓灰霉病,显著抑制柑橘的青霉病。
3 拮抗酵母生防作用的改良
一般生物防治中,都使用单一的生物控制剂。但经实验研究,诸多手段联用能够提高一些生防酵母的拮抗作用,并使其效果更佳稳定。
3.1 与其他拮抗微生物结合
单一生防酵母的防控作用有时并不理想,但与其他拮抗微生物结合使用时往往可以提高其生防效力。Etebarian等[59]的研究表明,使用2种拮抗菌枝顶孢属(Acremoniumbreve)和假单孢菌属(Pseudomonassp.)的混合物可完全抑制苹果灰霉病和青霉病的发生。Janisiewicz[60]的研究表明美极梅奇酵母和罗伦隐球酵母混合使用比单独使用控制采后苹果青霉菌腐烂效果更好。
3.2 与化学物质结合
在商业菌制剂产品的大规模使用过程中,酵母暴露于诸如高温、冷冻干燥、喷雾干燥、氧化压力等各种各样的环境压力下。因此,在商品化制备中,增强环境耐受能力是提高拮抗的酵母的生存能力和性能的一种有效策略。Teixidó等[61]研究表明,通过向培养基中添加甘油、葡萄糖或海藻糖,能够增加清酒假丝酵母胞内总多元醇和糖的含量,并且提高其对水分胁迫的耐受性,从而提高其在扩展青霉对苹果侵害时的防治效果。同样,将罗伦隐球酵母在含海藻糖的培养基中培养可以增加其胞内海藻糖含量,该菌经冷冻干燥,应用于低温和控制大气环境条件下后,发现其适应性和生防能力有所增强[62]。一个菌制剂产品的商业化需要足够的保质期,干制剂或液体制剂已被广泛用于制备酵母生物防治产品[40]。干制剂的优点是能够在非冷藏条件下长期保存,产品在存储过程中免受污染,便于产品的运输、配送和存储[63]。液体制剂优点是制造该产品的成本较低,脱水和复水过程往往导致拮抗菌部分死亡,液体制剂不需要干燥,使用时也可免去复水过程[64]。两种类型的菌制剂往往需要外源保护剂来改善环境压力对细胞适应性和防效的影响。5%或10%的外源海藻糖的添加显著提高了冻干后假丝酵母和粘红酵母的生存能力[26]。在液体制剂中抗氧化剂L-抗坏血酸能够增强糖保护剂(海藻糖和半乳糖)对罗伦隐球酵母和膜醭毕赤酵母的活力保护作用[65]。
酵母和其他抗菌化合物结合也能够有效提升生防效果。Qin等[66]报道称,水杨酸能够增强黏红酵母防治扩展青霉和链格孢对甜樱桃的侵害。低浓度的水杨酸不影响酵母和致病菌的生长,但是能够诱导诸如多酚氧化酶、苯丙氨酸脱氨酶和β-1,3-葡聚糖酶等与防控致病菌有关的酶的活性,推测水杨酸是通过诱导宿主果蔬的生化防御反应而非对致病菌产生毒害效应来增强拮抗酵母的生防效果,诸多研究能够证明此结论[67-69]。
在多种水果,如苹果[70]、梨[71]、桃[72]和枇杷[73]中,植物生长调节和防御剂——茉莉酸甲酯也能够用来增强拮抗酵母的生防效果。壳聚糖和其衍生物,以其抗真菌和诱导宿主防御反应的特点,能够防治采后病害。基于这些特性,壳聚糖可作为有效添加剂用以提高诸如罗伦隐球酵母的生防效果[74]。几丁质能够提高胶红酵母的抗真菌作用[75]。植酸能够提高粘红酵母对草莓采后灰霉病和自然腐病的生防效率[76]。
另外一类与生防制剂结合使用防治采后病害的化合物是无机盐和矿物质,如氯化钙[77]、钼酸铵[78]、碳酸氢钠[79]、硅胶[80]和硼酸盐[81]。在一定浓度下,这些物质能够直接抑制病原菌生长,但是对拮抗酵母的活性没有影响。一些可增强拮抗酵母生防效力的化学物质如表3所示。

表3 提高拮抗酵母生防效果的添加物Table 3 Effect of additive for improving biocontrol on antagonistic yeasts
3.3 与物理方法结合
拮抗酵母与一些物理方法结合使用也可提高其生防效力。Zhang等[82]研究表明,季也蒙毕赤酵母与热处理结合可以更好控制采后樱桃番茄灰霉腐烂黑点腐烂及根霉腐烂。微波处理可以增强罗伦隐球酵母对匍枝根霉引起的梨采后病害的防治效果[83]。Chun等[84]的研究表明,紫外线照射处理(UV-C)与热带假丝酵母(Candidatropicalis)结合使用对菠萝采后黑腐病的防治效果优于单独使用热带假丝酵母。
4 应用前景
一个能够成功应用的拮抗菌需要具备在果蔬加工贮藏条件下遗传稳定性高、低浓度有效、适应性强、抗菌谱广、生产成本低、便于售卖以及安全无害等特点。但由于果蔬种类、加工贮藏条件不尽相同,结抗酵母生防效果可能会有很大差异[3]。因此在推广一种生防菌制剂之前需要对其进行试点研究和大规模的商业测试并且需要选择不同地点的多个试点进行测试,以获得大量数据对结抗菌剂的防控效果进行评估。尽管目前全球范围的研究中,已有不少拮抗酵母对果蔬采后病害防控有较好效果,但能够将其制成拮抗菌剂并成功投入市场的仍然有限,比较常见的有三种:ShemerTM、CandifruitTM和Boni-ProtectTM。这几种菌制剂能够对不同种类果蔬的多种采后病害进行防控,这也是为什么这几种生防菌剂具有商业可行性的原因,其中基于梅奇酵母NRRL Y-27328研制而成的Shemer,能够对葡萄孢属真菌、青霉菌、根霉、曲霉属真菌引起的草莓、葡萄、甘薯、胡萝卜和柑橘等果蔬的采后腐烂有较好的防控作用[85]。
为能研发出更好的生防酵母菌剂,今后还需要加强以下几方面的研究:a. 继续加强酵母菌生防机理的研究,从分子水平揭示采后病原菌与酵母菌相互作用的机制;b. 进一步分离和筛选能有效抑制采后病害的拮抗酵母菌;c. 加强酵母菌作为保鲜剂在果蔬上使用方式的研究,特别是研究酵母菌在采前使用对采后病害控制的效果;d. 构建拮抗微生物工程菌株,将拮抗性能强的拮抗菌基因转移到另一种在果蔬表面更具适应性的酵母菌中,从而提高生防效果。
5 展望
新鲜果蔬中的农药残留将持续成为监管部门和消费者十分关心的问题之一。拮抗酵母防治相对化学合成杀菌剂更为健康环保,符合现代消费者的消费理念和需求,具有广阔的发展前景。拮抗酵母防治有望成为取代化学杀菌剂防治的有效方法,但大规模的商业化使用是一个漫长且成本高的过程。能够实现商业应用的拮抗酵母需具备遗传稳定性高、适应力强、成本低等诸多特性,虽然目前人们已经研究出很多有防治效果的菌株,但能够真正投入使用的仍然很有限,需要克服的问题有:a. 提高商业条件下的生防效果;b. 保证防效的基础上,降低成本;c. 保持拮抗产品的有效期;d. 产品应具有广谱性;e. 应具备良好的营销策略。总之,拮抗酵母抗菌剂的研发还需大量探索,现代生物技术,如基因组学、蛋白组学等的快速发展和先进仪器设备的应用都会为拮抗酵母抗菌剂的研发带来机遇。
[1] Sharma RR,Dinesh S,Rajbir S. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists:A review[J]. Biological Control,2009,50(3):205-221.
[2]Andersen B,Smedsgaard J,Frisvad JC. Penicillium expansum:consistent production of patulin,chaetoglobosins,and other secondary metabolites in culture and their natural occurrence in fruit products[J]. Journal of Agriculture and Food Chemistry,2004,52(8):2421-8.
[3]Droby S,Wisniewski M,Macarisin D,et al. Twenty years of postharvest biocontrol research:is it time for a new paradigm?[J]. Postharvest Biology and Technology,2009,52(2):137-145.
[4] Wilson CL,Wisniewski ME,Droby S,et al. A selection strategy for microbial antagonists to control postharvest diseases of fruits and vegetables[J]. Scientia Horticulturae,1993,53(3):183-189.
[5] Droby S,Hofstein R,Wilson CL,et al. Pilot testing of Pichia guilliermondii:a biocontrol agenat of postharvest diseases of citrus fruit[J]. Biological Control,1993,3(1):47-52.
[6] Suzzi G,Romano P,Ponti I,et al. Natural wine yeasts as biocontrol agents[J]. Journal of Applied Microbiology,1995,78(78):304-308.
[7] Elghaouth A,Wilson CL,Wisniewski M. Ultrastructural and cytochemical aspects of the biological control ofBotrytiscinereabyCandidasaitoanain apple fruit[J]. Phytopathology,1998,88(4):282-291.
[8] Vinas I,Usall J,Teixidó N,et al. Biological control of major postharvest pathogens on apple withCandidasake[J]. International Journal of Food Microbiology,1998,40(1-2):9-16.
[9] Qing F,Shiping T. Postharvest biological control of rhizopus rot of nectarine fruits byPichiamembranefaciens[J]. Plant Disease,2000,84(11):1212-1216.
[10]Kurtzman CP,Droby S. Metschnikowia fructicola,a new ascosporic yeast with potential for biocontrol of postharvest fruit rots[J]. Systematic and Applied Microbiology,2001,24(3):395.
[11]Vero S,Mondino P,Burgueno J,et al. Characterization of biocontrol activity of two yeast strains from Uruguay against blue mold of apple[J]. Postharvest Biology and Technology,2002,26(1):91-98.
[12]Qin G,Tian S,Xu Y. Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions[J]. Postharvest Biology and Technology,2004,31(1):51-58.
[13]Vero S,Garmendia G,Garat MF,et al. Cystofilobasidium infirmominiatum as a biocontrol agent of postharvest diseases on apples and citrus[J]. Acta Horticulturae,2011,905(3):169-180.
[14]Mari M,Martini C,Guidarelli M,et al. Postharvest biocontrol ofMonilinialaxa,MoniliniafructicolaandMoniliniafructigenaon stone fruit by twoAureobasidiumpullulansstrains[J]. Biological Control,2012,60(2):132-140.
[15]Lima J R,Gondim D M F,Oliveira J T A,et al. Use of killer yeast in the management of postharvest papaya anthracnose[J]. Postharvest Biology and Technology,2013,83:58-64.
[16]Parafati L,Vitale A,Restuccia C,et al. Biocontrol ability and action mechanism of food-isolated yeast strains againstBotrytiscinerea,causing post-harvest bunch rot of table grape[J]. Food Microbiology,2015,47:85-92.
[17]Gv D M P,Beux M,Pagnoncelli M G,et al. Isolation,selection and evaluation of antagonistic yeasts and lactic acid bacteria against ochratoxigenic fungusAspergilluswesterdijkiaeon coffee beans[J]. Letters in Applied Microbiology,2015,62(1):96-101.
[18]Punika N,Chaisemsaeng P. Application of antagonistic yeasts for postharvest disease control on chili fruits[C].International Conference on Biological,Chemical and Environmental Sciences,2016.
[19]Li Q,Li C,Li P,et al. The biocontrol effect ofSporidioboluspararoseus,Y16 against postharvest diseases in table grapes caused byAspergillusniger,and the possible mechanisms involved[J]. Biological Control,2017,113:18-25.
[20]De P E,Serradilla M J,Ruizmoyano S,et al. Combined effect of antagonistic yeast and modified atmosphere to controlPenicilliumexpansuminfection in sweet cherries cv.Ambrunés[J].International Journal of Food Microbiology,2017,241:276-282.
[21]Kalogiannis S,Tjamos SE,Stergiou A,et al. Selection and evaluation of phyllosphere yeasts as biocontrol agents against grey mould of tomato[J].European Journal of Plant Pathology,2006,116(1):69-76.
[22]Long CA,Wu Z,Deng BX. Biological control ofPenicilliumitalicumofCitrusandBotrytiscinereaofGrapeby Strain 34-9 of Kloeckera apiculata[J].European Food Research and Technology,2005,221(1):197-201.
[23]Vero S,Garmendia G,González MB,et al. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple(Malus×domestica)[J]. Fems Yeast Research,2013,13(2):189-199.
[24]Liu J,Wisniewski M,Droby S,et al. Increase in antioxidant gene transcripts,stress tolerance and biocontrol efficacy ofCandidaoleophilafollowing sublethal oxidative stress exposure[J].Fems Microbiology Ecology,2012,80(3):578-590.
[25]Wang Y,Wang P,Xia J,et al. Effect of water activity on stress tolerance and biocontrol activity in antagonistic yeast Rhodosporidium paludigenum[J]. International Journal of Food Microbiology,2010,143(3):103.
[26]Li BQ,Zhou ZW,Tian SP. Combined effects of endo-and exogenous trehalose on stress tolerance and biocontrol efficacy of two antagonistic yeasts[J]. Biological Control,2008,46(2):187-193.
[27]Liu J,Wisniewski M,Droby S,et al. Increase in antioxidant gene transcripts,stress tolerance and biocontrol efficacy ofCandidaoleophilafollowing sublethal oxidative stress exposure[J].Fems Microbiology Ecology,2012,80(3):578-590.
[28]Hershkovitz V,Ben-Dayan C,Raphael G,et al. Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agentMetschnikowiafructicola[J]. Molecular Plant Pathol,2012,13(4):338-349.
[29]Fiori S,Scherm B,Liu J,et al. Identification of differentially expressed genes associated with changes in the morphology of Pichia fermentans on apple and peach fruit[J]. Fems Yeast Research,2012,12(7):785-795.
[30]Zhang D,Davide S,Angelo G,et al. Efficacy of the antagonistAureobasidiumpullulans PL5 against postharvest pathogens of peach,apple and plum and its modes of action[J]. Biological Control,2010,54(3):172-180.
[31]Zhang D,Spadaro D,Valente S,et al. Cloning,characterization,expression and antifungal activity of an alkaline serine protease ofAureobasidiumpullulans PL5 involved in the biological control of postharvest pathogens[J]. Food Microbiology. 2012,153,453-464.
[32]Castoria R,Curtis F D,Lima G,et al. Aureobasidium pullulans,(LS-30)an antagonist of postharvest pathogens of fruits:study on its modes of action[J]. Postharvest Biology and Technology,2001,22(1):7-17.
[33]Ippolito A,Ghaouth A E,Wilson C L,et al. Control of postharvest decay of apple fruit byAureobasidiumpullulans,and induction of defense responses[J]. Postharvest Biology and Technology,2000,19(3):265-272.
[34]Scherm B,Ortu G,Muzzu A,et al. Biocontrol activity of antagonistic yeasts againstPenicilliumexpansumon apple[J]. Journal of Plant Pathology,2003,85(3):205-213.
[35]Spadaro D,Vola R,Piano S,et al. Mechanisms of action and efficacy of four isolates of the yeastMetschnikowiapulcherrima,active against postharvest pathogens on apples[J]. Postharvest Biology and Technology,2002,24(2):123-134.
[36]Reyes M E Q,Rohrbach K G,Paull R E. Microbial antagonists control postharvest black rot of pineapple fruit[J]. Postharvest Biology and Technology,2004,33(2):193-203.
[37]范青,田世平. 季也蒙假丝酵母对采后桃果实软腐病的抑制效果[J]. Journal of Integrative Plant Biology,2000,42(10):1033-1038.
[38]Filonow AB. Role of competition for sugars by yeasts in the biocontrol of gray mold of Apple[J]. Biocontrol Science Technology,1998,8(2):243-256.
[39]Björnberg A,Schnürer J. Inhibition of the growth of grain-storage moldsinvitroby the yeast Pichia anomala(Hansen)Kurtzman[J]. Canadian Journal of Microbiology,1993,39(6):623-628.
[40]Saravanakumar,Duraisamy,Ciavorella,et al. Metschnikowia pulcherrima strain MACH1 outcompetesBotrytiscinerea,AlternariaalternataandPenicilliumexpansumin apples through iron depletion[J]. Postharvest Biology and Technology,2008,49(1):121-128.
[41]Zhang D,Spadaro D,Garibaldi A,et al. Potential biocontrol activity of a strain ofPichiaguilliermondii,against grey mold of apples and its possible modes of action[J]. Biological Control,2011,57(3):193-201.
[42]Magallon-Andalon C G,Luna-Solano G,Ragazzo-Sanchez J A,et al. Parasitism and substrate competitions effect of antagonistic yeasts for biocontrol ofColletotrichumgloeosporioidesin papaya(CaricapapayaL.)var Maradol[J]. Sanchez,2012.
[43]Haïssam J M. Pichia anomala in biocontrol for apples:20 years of fundamental research and practical applications[J]. Antonie Van Leeuwenhoek,2011,99(1):93-105.
[44]Chan Z,Tian S. Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action[J]. Postharvest Biology and Technology,2005,36(2):215-223.
[45]Platania C,Restuccia C,Muccilli S,et al. Efficacy of killer yeasts in the biological control ofPenicilliumdigitatumon Tarocco orange fruits(Citrussinensis)[J]. Food Microbiology,2012,30(1):219.
[46]Aloui H,Licciardello F,Khwaldia K,et al. Physical properties and antifungal activity of bioactive films containingWickerhamomycesanomalus,killer yeast and their application for preservation of oranges and control of postharvest green mold caused byPenicilliumdigitatum[J]. International Journal of Food Microbiology,2015,200(2015):22-30.
[47]Wisniewski M,Biles C,Droby S,et al. Mode of action of the postharvest biocontrol yeast,Pichia guilliermondii,Characterization of attachment toBotrytiscinerea[J].Journal of Molecular Signaling,1991,39(4):245-258.
[48]Wan YK,Tian SP. Antagonistical mode of Pichia membranefaciens toRhizopusStoloniferin wounds of peach fruit[J]. 植物学报(英文版),2002,44(11):1384-1386.
[49]Droby S,ChalutzE.Mode of action of biocontrol agents of postharvest diseases[J].Biological Control of Postharvest Diseases,1994,3:63-76.
[50]张璐,张瑶,刘丽丹,等. 膜醭毕赤酵母对草莓采后灰霉病抗病性的诱导[J]. 食品科学,2013,34(22):286-291.
[51]Feng J,Zheng XD,Chen JS. Microarray analysis of gene expression profile induced by the biocontrol yeastCryptococcuslaurentiiin cherry tomato fruit[J]. Gene,2009,430(1-2):12-16.
[52]Hershkovitz V,Ben-Dayan C,Raphael G,et al. Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agentMetschnikowiafructicola[J]. Molecular Plant Pathology,2012,13(4):338-349.
[53]Chan Z,Qin G,Xu X,et al. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit[J]. Journal of Proteome Research,2007,6(5):1677-1688.
[54]Xu B,Zhang H,Chen K,et al. Biocontrol of postharvestRhizopusdecay of peaches withPichiacaribbica[J]. Current Microbiology,2013,67(2):255-261.
[55]Vespermann A,Kai M,Piechulla A B. Rhizobacterial volatiles affect the growth of fungi andArabidopsisthaliana[J]. Applied and Environmental Microbiology,2007,73(17):5639-5641.
[56]Fiori S,Urgeghe PP,Hammami W,et al. Biocontrol activity of four non-and low-fermenting yeast strains againstAspergilluscarbonarius,and their ability to remove ochratoxin A from grape juice[J]. International Journal of Food Microbiology,2014,189(7):45-50.
[57]Hua S S T,Beck J J,Sarreal S B L,et al. The major volatile compound 2-phenylethanol from the biocontrol yeast,Pichia anomala,inhibits growth and expression of aflatoxin biosynthetic genes ofAspergillusflavus[J]. Mycotoxin Research,2014,30(2):71-78.
[58]Parafati L,Vitale A,Restuccia C,et al. Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray,green and blue postharvest decays[J]. Food Microbiology,2017,63:191-198.
[59]Etebarian H R,Sholberg P L,Eastwell K C,et al. Biological control of apple blue mold withPseudomonasfluorescens[J]. Canadian Journal of Microbiology,2005,51(7):591.
[60]Janisiewicz W J,Saftner R A,Conway W S,et al. Control of blue mold decay of apple during commercial controlled atmosphere storage with yeast antagonists and sodium bicarbonate[J]. Postharvest Biology and Technology,2008,49(3):374-378.
[61]Teixidó,Vin,Usall,et al. Improving ecological fitness and environmental stress,tolerance of the biocontrol yeastCandidasakeby manipulation of intracellular sugar alcohol and sugar content[J]. Mycological Research,1998,102(11):1409-1417.
[62]Li BQ,Tian SP. Effects of trehalose on stress tolerance and biocontrol efficacy ofCryptococcuslaurentii[J]. Journal of Appllied Microbiology,2006,100(4):854-861.
[63]Melin P,Hakansson S,Eberhard T H,et al. Survival of the biocontrol yeast Pichia anomala,after long-term storage in liquid formulations at different temperatures,assessed by flow cytometry[J]. Journal of Applied Microbiology,2006,100(2):264-271.
[64]Abadias M,Usall J,Teixidó N,et al. Liquid formulation of the postharvest biocontrol agentCandidasakeCPA-1 in isotonic solutions[J]. Phytopathology,2003,93(4):436-442.
[65]Liu J,Tian SP,Li BQ,et al. Enhancing viability of two biocontrol yeasts in liquid formulation by applying sugar protectant combined with antioxidant[J]. BioControl,2009,54(6):817-824.
[66]Qin GZ,Tian SP,Xu Y,et al. Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit[J]. Physiological and Molecular Plant Pathology,2003,62(3):147-154.
[67]Farahani L,Etebarian HR. Enhancement of the efficacy of two antagonistic yeasts with salicylic acid againstPenicilliumexpansum[J]. Archives of Phytopathol and Plant Protection,2012,45(3):260-267.
[68]Yu T,Zheng XD. Salicylic acid enhances biocontrol efficacy of the antagonistCryptococcuslaurentii,in apple fruit[J]. Journal of Plant Growth Regulation,2006,25(2):166-174.
[69]Zhang H,Ma L,Mark T,et al. Salicylic acid enhances biocontrol efficacy of Rhodotorula glutinis against postharvestRhizopusrot of strawberries and the possible mechanisms involved[J]. Food Chemistry,2010,122(3):577-583.
[70]Leila E,Hassan RE,Heshmatolah A,et al. Enhancement of biocontrol activity ofTorulasporadelbrueckiiwith methyl jasmonate against apple blue mould disease[J]. Archives of Phytopathology and Plant Protection,2012,45(19):2355-2363.
[71]Zhang H,Ma L,Turner M,et al. Methyl jasmonate enhances biocontrol efficacy ofRhodotorulaglutinis,to postharvest blue mold decay of pears[J]. Food Chemistry,2009,117(4):621-626.
[72]Yao H,Tian S. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved[J]. Journal of Appllied Microbiology,2005,98(4):941-950.
[73]Cao S,Zheng Y,Wang K,et al. Effect of yeast antagonist in combination with methyl jasmonate treatment on postharvest anthracnose rot of loquat fruit[J]. Biological Control,2009,50(1):73-77.
[74]Meng XH,Qin GZ,Tian SP. Influences of preharvest sprayingCryptococcuslaurentiicombined with postharvest chitosan coating on postharvest diseases and quality of table grapes in storage[J]. LWT-Food Science and Technology,2010,43(4):596-601.
[75]Zhang X,Sun Y,Yang Q,et al. Control of postharvest black rot caused byAlternariaalternata,in strawberries by the combination ofCryptococcuslaurentii,andBenzo-(1,2,3)-thiadiazole-7-carbothioic acidS-methylester[J]. Biological Control,2015,90:96-101.
[76]Zhang H,Yang Q,Lin H,et al. Phytic acid enhances biocontrol efficacy of Rhodotorula mucilaginosa,against postharvest gray mold spoilage and natural spoilage of strawberries[J]. LWT-Food Science and Technology,2013,52(2):110-115.
[77]Gholamnejad J,Etebarian HR. Retractedarticle:Effect of calcium chloride on the biocontrol efficacy of two antagonistic yeasts againstPenicilliumexpansumonapple fruit[J]. Phytoparasitica,2009,37(3):255-261.
[78]Wan Y K,Tian S P,Qin G Z. Enhancement of biocontrol activity of yeasts by adding sodium bicarbonate or ammonium molybdate to control postharvest disease of jujube fruits[J]. Letters in Appllied Microbiology,2003,37(3):249.
[79]Qin X,Xiao H,Liu L,et al. Effects ofHanseniasporauvarumintegrated with salicylic acid or sodium bicarbonate on postharvest decay of grapes[C]. International Conference on Chemical Engineering and Advanced. 2013:1780-1785.
[80]Qin GZ,Tian SP. Enhancement of biocontrol activity ofCryptococcuslaurentiiby silicon and the possible mechanisms involved[J]. Phytopathology,2005,95(1):69.
[81]Cao B,Li H,Tian S,et al. Boron improves the biocontrol activity ofCryptococcuslaurentiiagainstPenicilliumexpansumin jujube fruit[J]. Postharvest Biology Technology,2012,68(8):16-21.
[82]Zhang H,Wang L,Zheng X,et al. Effect of yeast antagonist in combination with heat treatment on postharvest blue mold decay andRhizopusdecay of peaches[J]. International Journal of Food Microbiology,2007,115(1):53-8.
[83]Zhang H,Fu C,Zheng X,et al. Control of postharvest,Rhizopus,rot of peach by microwave treatment and yeast antagonist[J]. European Food Research and Technology,2004,218(6):568-572.
[84]Ou C,Liu Y,Wang W,et al. Integration of UV-C with antagonistic yeast treatment for controlling post-harvest disease and maintaining fruit quality ofAnanascomosus[J]. Biocontrol,2016,61(5):591-603.
[85]Karabulut OA,Tezcan H,Daus A,et al. Control of preharvest and postharvest fruit rot in strawberry byMetschnikowiafructicola[J]. Biocontrol Science and Technology,2004,14(14):513-521.
