Synthesis and Luminescence Properties of RedPhosphor BaZn2(BO3)2∶Eu3+ for Light-emitting Diode
2018-04-19WUChengxiaoDENGDegangRUANFengpingYUHuaXUShiqing
WU Cheng-xiao, DENG De-gang*, RUAN Feng-ping, YU Hua, XU Shi-qing
(1. College of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China; 2. College of Materials and Environmental Engineering, Hangzhou Dianzi University, Hangzhou 310018, China)
1 Introduction
WLED (White light-emitting diode) has broad application prospects because of the advantages such as low power consumption, long life and no pollution. At present, the research on luminescent materials, the society is still in the field of rare earth elements. The commercial white LED production is a combination of YAG∶Ce3+yellow phosphor and blue LED chip[1-3]. Part of the blue light emitted by the chip from the gaps in the phosphor powder, the rest of the phosphor converted to broadband emission of yellow light, and both of them are superimposed to get white light. However, the yellow light emitted by YAG∶Ce3+is lack of red light radiation, which leads to the low color rendering of white LED. There are two ways to solve the above problems: adding the red light phosphor emitted by blue light into the YAG∶Ce3+phosphor powder to improve the color rendering index of white light; exciting three primary phosphor by near ultraviolet or UV radiation InGaN chip to obtain high quantum efficiency white light[4]. In these two kinds of white LED, it is necessary to study the efficient red phosphors.
In recent years, rare earth ions have been widely studied as activators of fluorescent powder. Also, the Eu3+ion is an ideal activator of red phosphor. The Eu3+ion doped materials of LED has a very good absorption on the blue emission, but also a very high quantum efficiency under the excitation of UV light, especially Eu3+ion occupys anti-inversion symmetry in the host, which has strong emission in red because of the5D0→7F2transition[5-6].
For the advantages such as cheap raw materials, high stability and luminescence efficiency, optical properties of Eu3+ion doped borate phosphor were widely studied over the past years[7-10]. The two-peak adjustable phosphor doped with a single rare earth Eu3+ion in the host BaZn2(BO3)2has not yet been published. The 4f transition of Eu3+ion is mainly due to the interaction of electric dipole and magnetic dipole[11-12]. The lattice symmetry is stronger, and the intensity of electric dipole transition is greater. However, the lattice symmetry has little effect on the magnetic dipole transition. Therefore, the Eu3+ion is an ideal activator in lattice symmetry[13-15]. In this paper, the two-peak adjustable BaZn2(BO3)2∶xEu3+phosphor was prepared by high temperature solid state method, and then we studied the luminescent properties.
2 Experiments
The BaZn2(BO3)2∶xEu3+phosphors were prepared by high temperature solid state method, according to the chemical formula of weighing the materials BaCO3(A.R.), ZnO (99.99%), H3BO3(A.R.) and Eu2O3(99.99%), then thoroughly homogenized by grinding, and presintered in air at 850 ℃ for 4 h in muffle furnace. Then we reground the samples into powders.
A Bruker Axs D2 PHASER diffractometer with graphite monochromatized Cu Kα radiation (λ=0.154 06 nm) was used to record the powder X-ray diffraction (XRD) data with 2θranging from 10° to 80°, operating at 10 mA and 30 kV. A PL3-211-P spectrometer (HORIBA JOBIN YVON, America) with a 450 W xenon lamp as the excitation source was employed to detect the excitation and emission spectra. The decay times were also measured using this equipment, and a pulsed spectral LED at 370 nm was excited, operating in the multichannel scaling mode. And we used the white powder BaSO4as a reference to text the diffuse reflectance spectra with a UV-3600 UV-Vis spectrometer (Shimadzu, Japan). The photoluminescence was measured by an exciting spectra and thermal quenching analyzer for phosphors (Everfine, China).
3 Results and Discussion
Fig.1(a) shows that the phosphor phase can be compared with the standard card in good agreement, which indicates the substitution of Eu3+for Ba2+, but no change of crystal structure. Also the peak position, with variation of concentration, the overall move to large angle direction, indicates that the small radius of Eu3+ions replace the large radius of Ba2+ions, which can be seen in Fig.1(b).
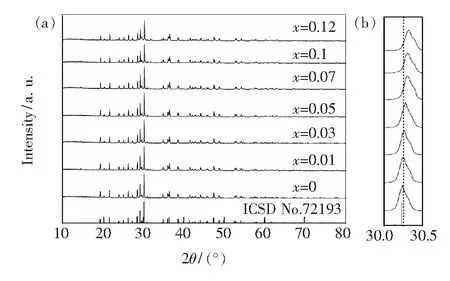
Fig.1 (a) XRD patterns of BaZn2(BO3)2∶xEu3+(x=0, 0.01, 0.03, 0.05, 0.07, 0.1, 0.12) and the standard date card of ICSD-72193 as a reference; (b) Magnified XRD patterns in the region between 30.0° and 30.5°.
Fig.2 show the refinement XRD patterns of BaZn2(BO3)2phosphor. The space group isP212121, which are showed in Tab.1. It is an orthorhombic cell with dimensionsa=0.932 7(7) nm,b=1.211 9(6) nm, andc=0. 492 4(3) nm. Fig.3 show the cell structure of BaZn2(BO3)2. It exhibits a framework of that Ba atom is coordinated by six oxygen atoms, BO3triangles and ZnO4tetrahedra that share vertices. There are two types of Zn atoms. A tetrahedral with the Zn atom as the center shares an O atom with the Ba atom, and the other shares two O atoms[12]. Final atomic coordinates and temperature factors for BaZn2(BO3)2host are shown in Tab.2.

Fig.2 Observed, calculated and difference synchrotron XRD profiles for the Rietveld refinement of BaZn2-(BO3)2. Bragg reflections are indicated with tick marks.
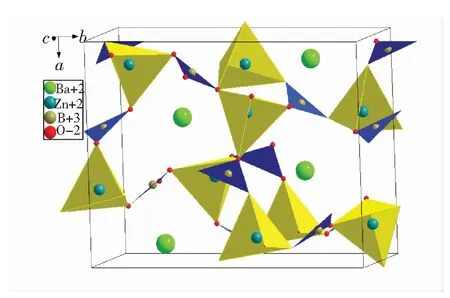
Fig.3 Cell structure of BaZn2(BO3)2(black solid lines denote the unit cell)
Tab.1RietveldrefinementandcrystaldataofBaZn2-(BO3)2∶0.1Eu3+phosphors
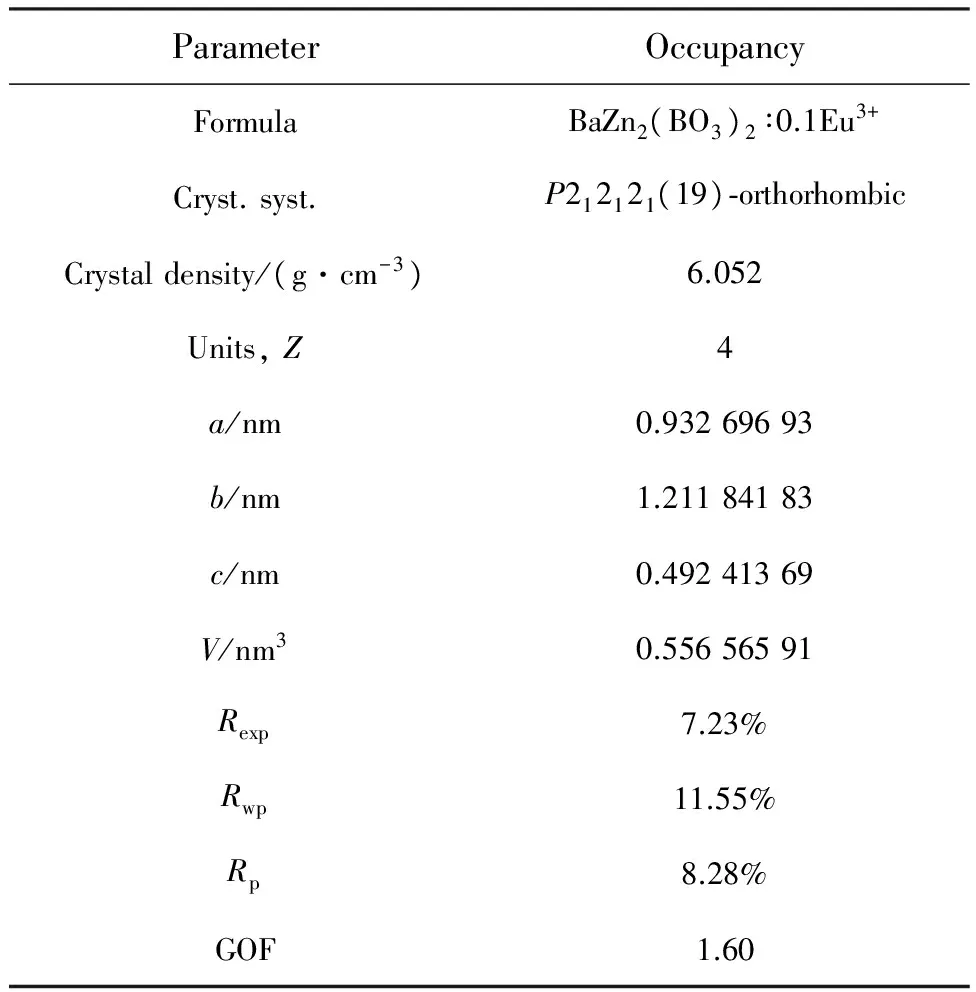
ParameterOccupancyFormulaBaZn2(BO3)2∶0.1Eu3+Cryst.syst.P212121(19)⁃orthorhombicCrystaldensity/(g·cm-3)6.052Units,Z4a/nm0.93269693b/nm1.21184183c/nm0.49241369V/nm30.55656591Rexp7.23%Rwp11.55%Rp8.28%GOF1.60
Tab.2FinalatomiccoordinatesandtemperaturefactorsforBaZn2(BO3)2host
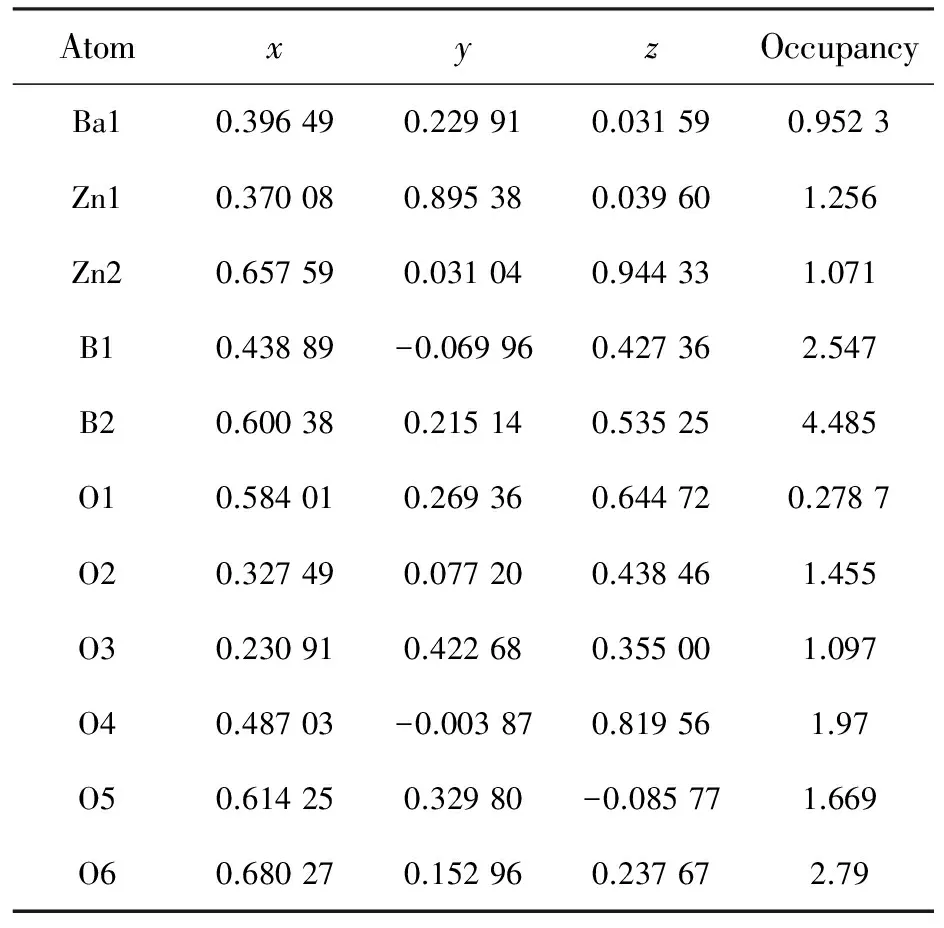
AtomxyzOccupancyBa10.396490.229910.031590.9523Zn10.370080.895380.039601.256Zn20.657590.031040.944331.071B10.43889-0.069960.427362.547B20.600380.215140.535254.485O10.584010.269360.644720.2787O20.327490.077200.438461.455O30.230910.422680.355001.097O40.48703-0.003870.819561.97O50.614250.32980-0.085771.669O60.680270.152960.237672.79
There is a strong reflection from 400 to 800 nm in host BaZn2(BO3)2and an obvious absorption at the range of 200-400 nm because of the energy transition between conduction and valence band, which can be seen clearly in Fig.4(a). As BaZn2-(BO3)2∶xEu3+shows that a narrowband absorption near 465 nm at the band, which is due to the absorption of Eu3+on the7F0→5D2transition. In fact, there should be a Eu3+on the7F0→5L6transition absorption in the vicinity of 396 nm, but for the strong host absorption in the ultraviolet band, the absorption of Eu3+is not obvious. The absorption spectrum of Eu3+ions is caused by the internal f-f transition, which is influenced by the external electrons. But the host of the crystal field has a small effect on the absorption. The absorption spectra of Eu3+in BaZn2(BO3)2∶xEu3+samples are the same shape as those of the narrow band absorption spectra of the f-f transition, and the absorption peak position does not appear a obvious shift. According to the spectrum[16-17], the electric dipole transitionL=0 is forbidden, but due to the deviation of the inversion center and the configuration of 4f, the parity opposite configuration G or configuration D mixed, such f-f transitions become allowed.
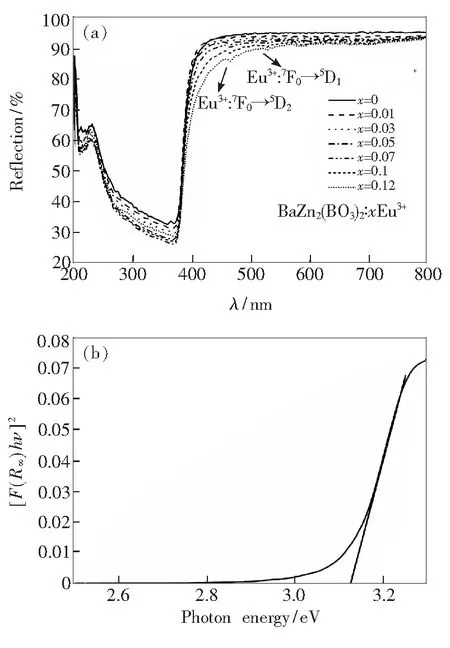
Fig.4 (a) Diffuse reflection spectra of BaZn2(BO3)2∶xEu3+(x=0, 0.01, 0.03, 0.05, 0.07, 0.1, 0.12). (b) Absorption spectra of BaZn2(BO3)2as calculated by the Kubelka-Munk formula.
The band-gap of BaZn2(BO3)2host can be calculated according to formula (1)[18]:
[F(R)hν]n=A(hν-Eg),
(1)
whereF(R∞) is the Kubelka-Munk function,n=2 for an direct transition or 1/2 for an indirect transition,hνrepresents the photon energy,Ais constant, andEgis the value of the band gap. BaZn2-(BO3)2is a direct band-gap semiconductor, so the value ofnis 2. The absorption spectra of BaZn2-(BO3)2host can be got from the diffuse reflection by using the Kubelka-Munk function[19]:
F(R)=(1-R)2/2R=K/S,
(2)
whereRis the reflectance,KandSare the absorption and the scattering coefficients, respectively. Fig.4(b) presents the absorption spectra of BaZn2-(BO3)2derived with the Kubelka-Munk function. For BaZn2(BO3)2from the linear extrapolation ofK/S=0,Egvalue was calculated to be about 3.12 eV. According to the band-gap width of semiconductors, they are divided into narrow band-gap semiconductor (Eg<2 eV), wide band-gap semiconductor (Eg>2 eV) and gapless semiconductor (Eg~0 eV). It is obvious that BaZn2(BO3)2belongs to wide band-gap semiconductor like ZnO (3.37 eV) and SiC (2.2 eV).

Fig.5 (a) Photoluminescence excitation spectra of BaZn2-(BO3)2∶xEu3+phosphors. (b) Photoluminescence spectra of BaZn2(BO3)2∶xEu3+phosphors under the excitation of 375 nm. (c) The change trend of emission intensity of two peaks.
As it shown in Fig.5(a), when the emission peak 550 nm is used to monitor the excitation spectrum, there is a strong excitation peak at 375 nm in the host. And with the Eu3+ion concentration increasing, the excitation peak appears at 393 nm, which is due to the7F0→5L6(393 nm) transition of Eu3+ion. However, when the mole fraction of Eu3+is 10%, the intensity of the peak begins to decline due to the concentration quenching. In Fig.5(b), we find that when we increase the doped concentration of Eu3+ions, the emission of host at the 550 nm is gradually weakened, and the emission spectrum in the vicinity of 615 nm belongs to the Eu3+ions5D0→7F1or7F2significantly enhanced, which further shows that the energy transfer from the host to the Eu3+promotes the luminescence of Eu3+in the host. When the mole fraction of Eu3+reaches 10%, the concentration quenching occurs, which are shown in Fig.5(c).
The excitation spectra of the BaZn2(BO3)2∶xEu3+samples are recorded between 350 and 450 nm by monitoring the5D0→7F2emission at 615 nm. It can be seen from Fig. 6(a), The sharp intense excitation peaks of Eu3+ions from 300 to 450 nm include7F0→5L7(385 nm),7F0→5L6(392-400 nm), and7F0→5D3(415 nm) emissions[10,20]. And the strong excitation at 393 nm is used to monitor the excitation for emission spectra of the phosphor. The wavelength band of Eu3+is relatively narrow, and the wavelength is mainly located in the orange and red range. Eu3+emission spectrum is the energy of the electron from the state5D0to state7FJ(J=0, 1, 2, 3, 4). When Eu3+is in a different lattice position, the intensity of the transition is different. When the5D0→7F1radiative transition occurs, Eu3+is the center of inversion in the lattice; when the5D0→7F2and5D0→7F4radiative transitions occur, Eu3+is the center of non inversion in the lattice. There are many strong emission peaks in the emission spectra of 500-700 nm, and the emission peaks in this range are composed of the5D0→7FJ(J=0, 1, 2, 3, 4) energy transition of Eu3+. The characteristic emission peaks of five Eu3+ions are shown in Fig.6(b): the emission of5D0→7F0and5D0→7F3corresponding to the emission bands near 580 nm and 653 nm is smaller, while the emission peaks in the corresponding emission band 590 nm, near 615 nm and 683 nm appeared in5D0→7F1,5D0→7F2and5D0→7F4transition are obvious. The 590 nm transition is the magnetic dipole transition in the five transition bands,and the5D0→7F2transition in the vicinity of 615 nm is an electric dipole transition. The intensity ratio of the characteristic diffraction peaks produced by5D0→7F2and5D0→7F1transitions can be used to determine the coordination environment of Eu3+ions. The5D0→7F2electric dipole transition occurs and the red light (about 615 nm) is dominated by5D0→7F2electric dipole transition[21]. It can be seen from the figure, at the beginning, the emission intensity of5D0→7F2was significantly higher than that of other emission peaks, but with the increase of ion concentration in BaZn2(BO3)2∶xEu3+, the two peaks began to increase and sharpening, and split, and the asymmetry ratio is smaller than the orange(R/O). It is found that BaZn2(BO3)2∶Eu3+phosphor is a kind of potential red phosphor for white LED with excitation and emission spectra.
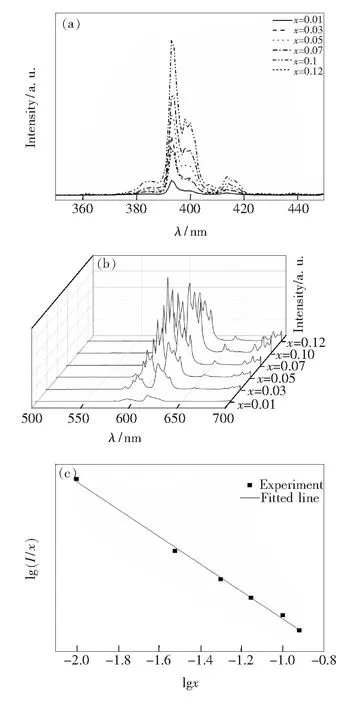
Fig.6 (a) Photoluminescence excitation spectra of BaZn2-(BO3)2∶xEu3+phosphors. (b) Photoluminescence spectra of BaZn2(BO3)2∶xEu3+phosphors under the excitation of 393 nm. (c) Relationship between lgxand lg(I/x).
With the formula given by Blasse and Grabmaier[22], we have calculated the value of critical distance between the Eu3+ions:
Rc≈2[3V/4πxcZ]1/3,
(3)
whereVis the unit cell volume,xcandZare the quenching concentration of activator and the value of formula units per unit cell. For BaZn2(BO3)2host, usingZ=4,xc=0.1 andV=0.556 565 91 nm3,then the obtainedRcis 0.673 nm.
From Dexter’s theory, we get the energy transfer between Eu3+ions is due to the electric multipole-multipole interactions[23]. When it occurs among the same types of activators, the extent of the interactions can be revealed from the change intensity of emission. The emission intensity per activator ion can be calculated by the formula[24-25]:
I/x=k[1+β(x)Q/3]-1,
(4)
whereIrepresents the intensity of emission,xis the concentration of activator,kandβare constants; whileQ=3, 6 or 8 is for the energy transfer between the nearest-neighbor, dipole-dipole or dipole-quadrupole ions respectively, andQ=10 is for the quadrupole-quadrupole interactions. Fig.6(c) shows that the relationship between lg(I/x) and lgxis linear and the value of slope is -0.74. From the above formula (4), the value ofQwas calculated to be 2.32, which is closed to 3. It indicates that the main mechanism of concentration quenching of the Eu3+emission in BaZn2(BO3)2∶xEu3+phosphors is the energy transfer among the nearest neighbor ions.

Fig.7 Energy level diagram of the BaZn2(BO3)2host, Eu3+and schematic of the energy transfer mechanism from the BaZn2(BO3)2host to Eu3+.

Fig.8(a) and (b) illustrate the fluorescence decay curves of BaZn2(BO3)2and BaZn2(BO3)2∶xEu3+phosphors at room temperature monitored at 550 nm, the BaZn2(BO3)2∶xEu3+phosphors monitored at 615 nm respectively. In general way, with the concentration of activators increasing, the distance between them decreases and thus energy transfer among them occurs. With various concentrations of activators, the decay times are different[27-30]. There are two luminescence centers in BaZn2(BO3)2∶xEu3+, so that we can fit the decay curves with the double-exponential equation[31]:
I(t)=A1exp(-t/τ1)+A2exp(-t/τ2), (5)
whereI(t) represents the intensity of emission at timet,A1andA2are constants,tis time,τ1andτ2are the exponential components of decay times. The following equation can be used to calculate the average decay times (τ)[32]:
(6)
when monitored at 550 nm, the lifetime of the BaZn2(BO3)2host is calculated to be 0.15 ms. There are no obvious difference among the lifetimes of BaZn2-(BO3)2∶xEu3+(0.01≤x≤0.12) phosphors. We calculate the average value of their lifetimes is about 0.14 ms. While monitored at 615 nm, the lifetimes of BaZn2(BO3)2∶xEu3+(0.01≤x≤0.12) are determined from 1.20 ms to 2.20 ms. With the doping of Eu3+, the lifetime of 550 nm becomes shorter, furthermore the lifetime of 615 nm increases gradually with the increasing doped concentrations of Eu3+. It indicates that the energy transfer occurs from BaZn2(BO3)2host to Eu3+ions.
Fig.9 shows the CIE chromaticity coordinates
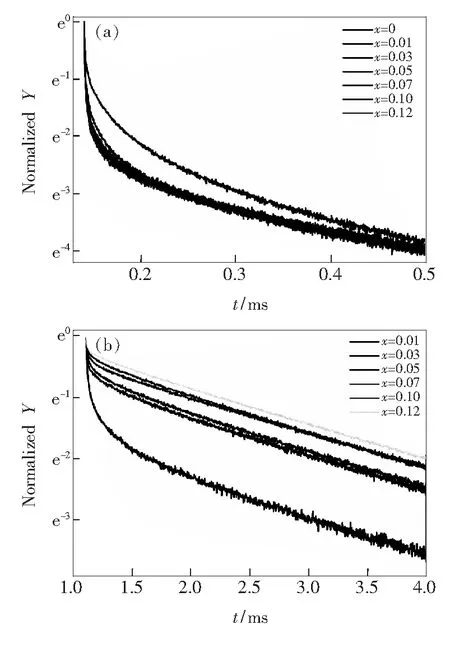
Fig.8 (a) Decay profiles of BaZn2(BO3)2∶xEu3+phosphor monitored at 550 nm. (b) Decay profiles of BaZn2(BO3)2∶xEu3+phosphor at 615 nm.
for the BaZn2(BO3)2∶xEu3+phosphors with differentxvalues. The color hue can be tuned from yellow to red, and the corresponding chromaticity coordinates (x,y) varying from (0.458 0, 0.338 7) to (0.616 7, 0.346 1) summarized in the inset table. It indicates the BaZn2(BO3)2∶xEu3+phosphors would be a potential color coordinate adjustable phosphor for using in white light-emitting diode.

Fig.9 CIE chromaticity diagram of BaZn2(BO3)2∶xEu3+phosphors excited at 393 nm(x= 0.01, 0.03, 0.05, 0.07, 0.1, 0.12), the inset shows the phosphors together with their photos taken under 393 nm excitation.
4 Conclusion
BaZn2(BO3)2∶xEu3+phosphor was prepared by high temperature solid state method. The excitation peak position of the host is located at 375 nm, the excitation peak of Eu3+is located at 392 nm, and the peak of the emission spectrum is located near 550 nm and 615 nm. The emission of wavelengths at 550 nm is due to the semiconductor luminescence of the host itself, and emission at 615 nm is due to the5D0→7F2transition of Eu3+. And with the addition of Eu3+ions, the energy of the host transfers to the Eu3+. When the doping concentration is 10%, BaZn2(BO3)2∶0.1Eu3+phosphor with excellent performance in various aspects has a strong red emission from580 to 630 nm, and also a potential application in white LED.
[1] YAO G Q, DUAN J F, REN M,etal.. Preparation and luminescence of blue light conversion material YAG∶Ce [J].J.Lumin., 2001, 22:22-26.
[2] XU Y, CHEN L H, LI Y Z. Phosphor-conversion white light using InGaN ultraviolet laser diode [J].Appl.Phys.Lett., 2008, 92:021129.
[3] LIN C C, LIU R S. Advances in phosphors for light-emitting diodes [J].Phys.Chem.Lett., 2011, 2:1268.
[4] YAN B, LIN L, WU J,etal.. Photoluminescence of rare earth phosphors Na0.5Gd0.5WO4∶RE3+and Na0.5Gd0.5(Mo0.75-W0.25)O4∶RE3+(RE=Eu, Sm, Dy) [J].J.Fluoresc., 2011, 21:203-211.
[5] ZENG X Q, SEOUNG-JAE I M, JANG S H,etal.. Luminescent properties of (Y, Gd)BO3∶Bi3+,RE3+(RE=Eu, Tb) phosphor under VUV/UV excitation [J].J.Lumin., 2006, 121(1):126.
[6] TIAN L H, KIM S J, PARK H L,etal.. Variation of the photoluminescence and vacuum ultraviolet excitation characteristics of BaZr(BO3)2∶Eu3+by the incorporation of Al3+, La3+, or Y3+into the lattice [J].Mater.Res.Bull., 2006, 41(1):29-37.
[7] SUN L D, QIAN C, LIAO C S,etal.. Luminescence phosphors of Li+doped nanosized Y2O3∶Eu3+[J].SolidStateCommun., 2001, 119(6):393-396.
[8] WANG Z L, LIANG H B, GONG M L,etal.. Luminescence investigation of Eu3+activated double molybdates red phosphors with scheelite structure [J].J.AlloysCompd., 2007, 432:308-312.
[9] FENG G, JIANG W H, CHEN Y B,etal.. A novel red phosphor NaLa4(SiO4)3F∶Eu3+[J].Mater.Lett., 2011, 65(1):110-112.
[10] DENG D G, YU H, HUA Y J,etal.. Ca4(PO4)2O∶Eu2+red-emitting phosphor for solid-state lighting: structure, luminescent properties and white light emitting diode application [J].J.Mater.Chem. C, 2013, 1:3194-3199.
[11] CHEN C, WU B, JIANG A,etal.. A new-type ultraviolet SHG crystal—β-BaB2O4[J].ChinaSer. B, 1985, 28:235-243.
[12] SMITH R W, KESZLER D A. The noncentrosymmetric orthoborate BaZn2(BO3)2[J].SolidStateChem., 1992, 100:325-330.
[13] CHEN F, YUAN X, ZHANG F,etal.. Photoluminescence properties of Sr3(PO4)2∶Eu2+, Dy3+double-emitting blue phosphor for white LEDs [J].Opt.Mater., 2014, 37:65-69.
[14] DORENBOS P. Energy of the first 4f7→4f65d transition of Eu2+in inorganic compounds [J].J.Lumin., 2003, 104:239-260.
[15] XIE R J, HIROSAKI N, MITOMO M,atal.. Optical properties of Eu2+in-SiAlON [J].Phys.Chem. B, 2004, 108:12027.
[16] HARANATH D, SHANKER V, CHANDER H,etal.. Tuning of emission colours in strontium aluminate long persisting phosphor [J].J.Appl.Phys., 2003, 36:2244-2248.
[17] JIE L, LIAN H Z, SHI C S. Improved optical photoluminescence by charge compensation in the phosphor system CaMoO4∶Eu3+[J].Opt.Mater., 2007, 29(12):1591-1594.
[18] CHEN F, YUAN X, ZHANG F,etal.. Photoluminescence properties of Sr3(PO4)2∶Eu2+, Dy3+double-emitting blue phosphor for white LEDs [J].Opt.Mater., 2014, 37:65-69.
[19] WANG Z, LIANG H, GONG M,etal.. Luminescence investigation of Eu3+activated double molybdates red phosphors with scheelite structure [J].J.AlloysCompd., 2007, 432(1-2):308-312.
[20] CHEN X Y, LIU G K. The standard and anomalous crystal-field spectra of Eu3+[J].SolidStateChem., 2005,178:419-428.
[21] GUO N, HUANG Y J, YOU H P,etal.. Ca9Lu(PO4)7∶Eu3+, Mn2+: a potential single-phased white-light-emitting phosphor suitable for white-light-emitting diodes [J].Inorg.Chem., 2010, 49(23):10907-10913
[22] BLASSE G. Energy transfer in oxidic phosphors [J].PhilipsRes.Rep., 1969, 24:131-144.
[23] YU H, DENG D G, LI Y Q,etal.. Luminescent properties of red-emitting LiSr4B3O(9-3x/2)Nx∶Eu2+phosphor for white-LEDs [J].Opt.Commun., 2013, 289:103-108.
[24] LIU H, ZHANG Y, LIA L,etal.. Synthesis, broad-band absorption and luminescence properties of blue-emitting phosphor Sr8La2(PO4)6O2∶Eu2+for n-UV white-light-emitting diodes [J].Ceram.Int., 2014, 40:13709-13713.
[25] LI K, GENG D, SHANG M,etal.. Color-tunable luminescence and energy transfer properties of Ca9Mg(PO4)6F2∶Eu2+, Mn2+phosphors for UV-LEDs [J].J.Phys.Chem. C, 2014, 118:11026-11034.
[26] WANG Z L, LIN C K, LIU X M,etal.. Tunable photoluminescent and cathodoluminescent properties of ZnO and ZnO∶Zn phosphors [J].J.Lin.Phys.Chem. B, 2006, 110:9469-9476.
[27] LI H, ZHAO R, JIA Y,etal.. Sr1.7Zn0.3CeO4∶Eu3+novel red-emitting phosphors: synthesis and photoluminescence properties [J].ACSAppl.Mater.Interf., 2014, 6:3163-3169.
[28] SOHN K S, CHOI Y Y, PARK H D. Photoluminescence behavior of Tb3+-activated YBO3phosphors [J].J.Electrochem.Soc., 2000, 147:1988-1992.
[29] HUANG C H, KUO T W, CHEN T M. Novel red-emitting phosphor Ca9Y(PO4)7∶Ce3+, Mn2+with energy transfer for fluorescent lamp application [J].ACSAppl.Mater.Interf., 2010, 2:1395-1399.
[30] LV W, JIA Y, ZHAO Q,etal.. Synthesis. structure. and luminescence properties of K2Ba7Si16O40∶Eu2+for white light emitting diodes [J].J.Phys.Chem. C, 2014, 118:4649-4655.
[31] YU H, DENG D G, XU S Q,etal.. Luminescent properties of novel Ca2.89Mg0.11(PO4)2∶Eu2+single-phase white light-emitting phosphor for white LEDs [J].Ceram.Int., 2015, 41:3800-3805.
[32] DEXTER D, SCHULMAN J H. Theory of concentration quenching in inorganic phosphors [J].J.Chem.Phys., 1954, 22:1063-1070.

吴程潇(1992-),男,安徽太湖人,硕士研究生,2015年于安徽科技学院获得学士学位,主要从事稀土发光方面的研究。

E-mail: 18867141292@163.com邓德刚(1975-),男,湖北仙桃人,博士,教授,硕士生导师,2007年于中科院上海光机所获得博士学位,浙江省151第二层次资助人员,主要从事稀土掺杂玻璃和光纤、半导体照明用发光材料、特种玻璃等光电功能材料及器件的研究。
E-mail: dengdegang@cjlu.edu.cn
