基于2,5-噻吩二羧酸的稀土配合物的合成和表征
2018-04-10郑云云黄锐敏苏德森傅建炜
郑云云 黄锐敏 韦 航 黄 彪 苏德森 傅建炜*,
0 Introduction
As one of synthetic approaches,hydro(solv)thermal reaction has played an important role in the construction of inorganic-organic hybrid materials[1-3].Based on this approach,chemists have successfully assembled a large number of topological diversity materials that are difficultly obtained from the general synthetic routine[4-7].Owing to the coordination of organic ligand to metal ion under the hydro(solv)thermal condition resulting in the organic ligand decomposition[8-12]or formation of a new organic compound[13-16],it is difficult for us to design and assemble the inorganic-organic hybrid materials based on the structural information of the organic ligand under the hydro(solv)thermal condition.Hence,investigating the external physical or chemical stimuli influencing on the reformation and decomposition of organic ligand under the hydro(solv)thermal condition is of a key importance for our rational design and assembly of inorganic-organic hybrid materials.
2,5-thiophenedicarboxylic acid (here and after namely H2TDC),when coordinated with metal ion,often exhibit diverse coordination modes,such as monodentate,bidentate,tridentate and tetradentate modes.This coordination feature makes it become a versatile building block in the construction of coordination polymers[17-34].Like many sulfur-containing organic ligands,such as,4,4′-dithiodipyridine[35-41]and(4-pyridylthio)acetic acid[42],however,the relatively weak CS in the H2TDC often break down under the hydro(solv)thermal condition,resulting in a low degree of structure predictability when compared to aromatic polycarboxylate ligands,such as,1,4-benzendicarboxylic acid[43-45]and 1,3,5-trimesic acid[46-49].In the present paper,three polymers,namely, {[La(OH)(SO4)]}n(1),{[La2(TDC)2(SUC)]}n(2)and{[Gd2(TDC)2(ox)(H2O)4]·2H2O}n(3),(H2TDC=2,5-thiophenedicarboxylic acid,SUC=succinate,ox=oxalate)have been successfully synthesized through hydrothermal reaction of H2TDC and Ln(NO3)3(Ln=La,Gd).The single crystal structures of 2 and 3 have been investigated.
1 Experimental
All reagents used were commercially available and were used as received.The hydrothermal syntheses were carried out in polytetrafluoroethylene lined stainless steel containers under autogeneous pressure.The infrared spectra were recorded on a Nicolet AVATAR FT-IR360 Spectrophotometer with pressed KBr pellets.
1.1 Synthesis of{[La(OH)(SO 4)]}n(1)and{[La2(TDC)2(SUC)]}n(2)
2,5-thiophenedicarboxylic acid(0.172 g,1.0 mmol),La(NO3)3·6H2O(0.433 g,1.0 mmol)were mixed in 10.0 mL water with stirring at room temperature.After the pH value of the solution was adjusted to about 4.0 by 1.0 mol·L-1NaOH,the solution was transferred and sealed in a 25 mLTeflon-lined stainlesssteel container.The container was heated to 180℃and held at that temperature for 70 hours,then cooled to 30℃at a rate of 5℃·h-1.Then colorless plate crystals of 1(which was reported by Lu et al.[50])and colorless needle crystals of 2 were manually picked out in 20%yield respectively.Anal.Calcd.(Found)for H2O10S2La2(1)(%):H,0.40(0.52);S,12.73(12.60).IR Spectra for 1(KBr,cm-1):3 407(s),2 975(s),2 926(m),2 891(m),1 628(m),1 543(m),1 455(w),1 384(m),1 272(w),1 090(s),1 049(s),881(m),771(w),645(w),593(w),469(w).Anal.Calcd.(Found)for C16H8O12S2La2(2)(%):C,26.18(26.49);H,1.10(1.02);S,8.74(8.65).IR Spectra for 2(KBr,cm-1):3 489(s),3 432(s),2 926(w),2 852(w),1 609(s),1 550(s),1 525(s),1 462(w),1 385(s),1 152(s),1 078(s),995(w),777(w),727(w),679(w),602(w),532(w),473(w).
1.2 Synthesis of{[Gd 2(TDC)2(ox)(H 2O)4]·2H 2O}n(3)
Complex 3 was prepared in the similar way as described for 1 and 2,except that Gd(NO3)3·6H2O was used toreplace La(NO3)3·6H2O.Colorlessblock crystals of 3 were collected by filtration in 15%yield.Anal.Calcd.(Found)for C14H16O18S2Gd2(3)(%):C,19.76(19.63);H,1.90(1.96);S,7.54(7.46).IR Spectra for 3(KBr,cm-1):3 090(s),2 973(s),2 849(s),2 653(s),2 547(s),2 035(w),1 861(w),1 661(s),1 527(s),1 478(m),1 417(s),1 341(m),1 273(s),1 231(s),1 161(m),1 101(m),1 104(m),1 038(s),931(s),854(s),756(s),672(w),543(m),490(m),463(w).
1.3 X-ray crystallography
Data were collected on a Bruker SMART Apex CCD diffractometer(Mo Kα,λ=0.071 073 nm)at 298 K for 2 and 3,and crystal sizes of crystals for crystallography test were 0.15 mm ×0.02 mm×0.01 mm(2)and 0.20 mm×0.14 mm×0.06 mm(3).Absorption corrections were applied using the multiscan program SADABS[51].The structures were solved by direct methods,and the non-hydrogen atoms were refined anisotropically by the least-squares method on F2using the SHELXTL program[52].The hydrogen atoms of organic ligand were generated geometrically(C-H 0.096 nm,N-H 0.090 nm).Crystal data,as well as details of the data collection and refinement,for the complexes are summarized in Table 1,and selected bond lengths and angles are summarized in Table S1~S3(Supporting information).
CCDC:1818984,2;1818985,3.
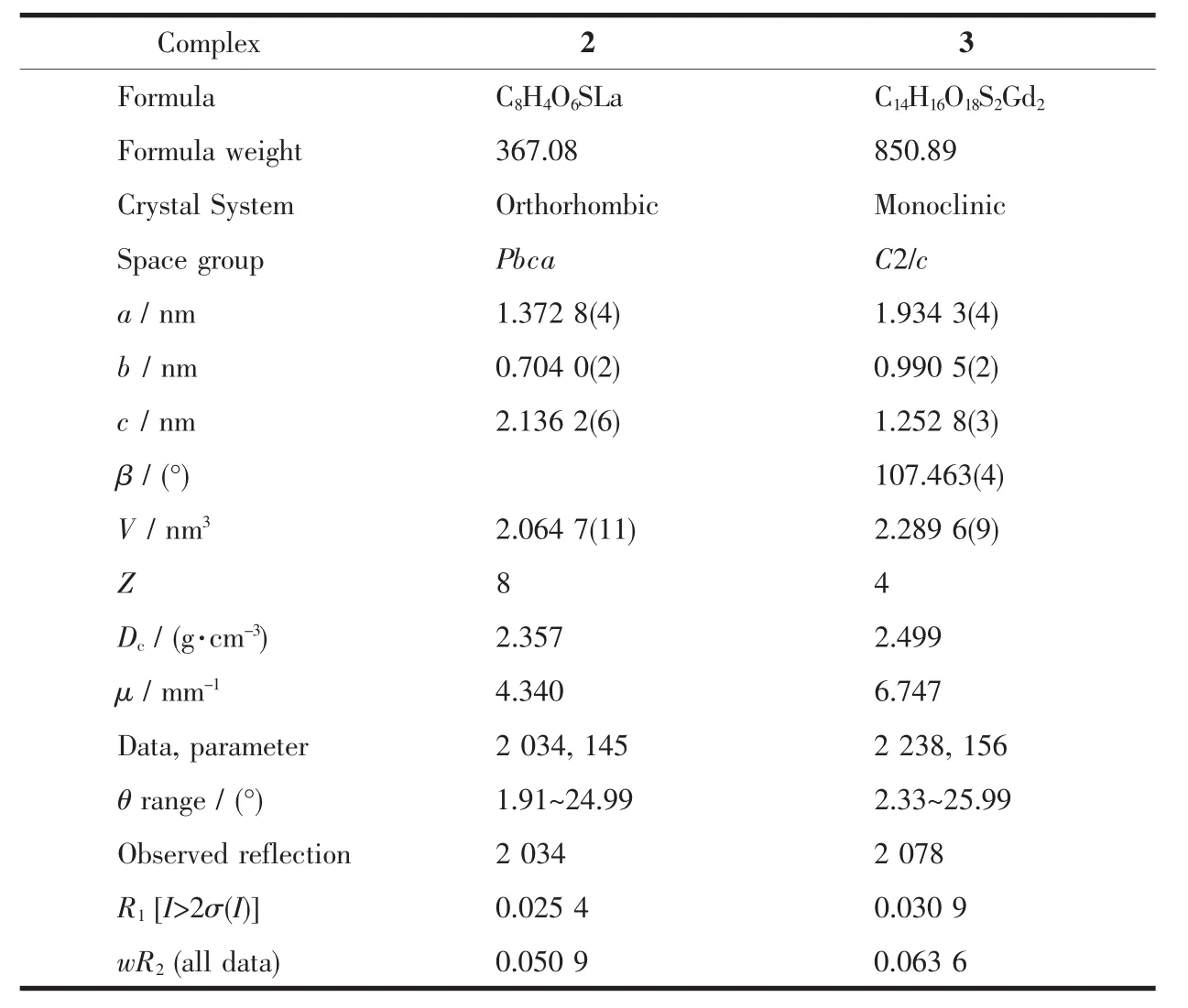
Table 1 Crystal data and details of data collection and refinement for the complexes 2 and 3
2 Results and discussion
Complex 2 consists of two Laバcations,two TDC2-and one SUC2-ligands.Crystal structure analysis reveals that the central Laバcation is nine-coordinated by four carboxylates of four TDC2-ligands with one in bidentate mode and three in monodentate mode,and three carboxylates from three SUC2-ligands with one in bidentate mode and two in monodentate mode in capped square antiprism geometry as shown in Fig.1.The bond lengths of LaO are in the range from 0.238 8(3)to 0.296 5(4)nm,slightly longer than those of[Ln(INO)(H2O)(SO4)]n(INO=isonicotinate-N-oxide)[53].The 2D structure of{[La2(SUC)]}n4n+in 2 can be viewed as each SUC2-ligand with its each oxygen atom bridged with two adjacent Laバ cations(La…La=0.420 1 nm)as shown in Fig.2a,while the 3D structure of 2 can be viewed as adjacent two 2D structures connected through oxygen atoms of the TDC2-ligand with its one carboxylate coordinated to two Laバcations from one 2D layer in syn-syn mode and another coordinated to two Laバfrom adjacent layer in syn-syn and chelate mode as shown in Fig.2b.It was noted that such a unique coordination mode has not observed in TDC-based complex on the survey of Cambridge Data Base[54].
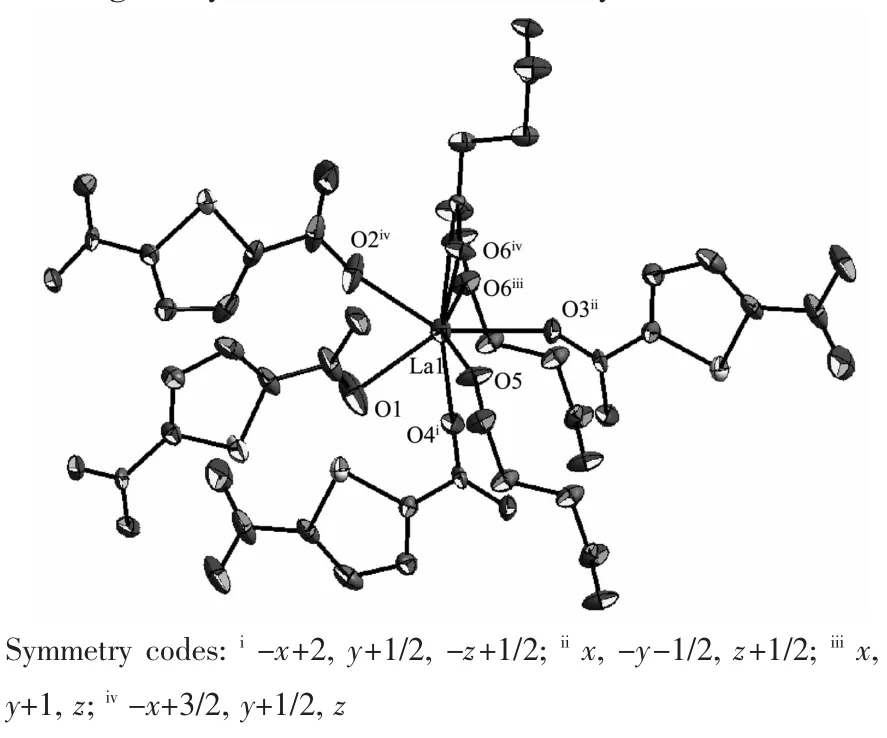
Fig.1 Coordination environment of Laバcenters in 2 with 50%probability ellipsoids
Single-crystal structural analysis reveals that complex 3 consists of two Gdバcations,two TDC2-ligands,one ox2-and six water molecules.There are two independent Gdバcenters in the asymmetry unit in 3(Fig.3).One(Gd1)iseight-coordinated respectively by four TDC2-ligands in monodentate mode and two ox2-in bidentate mode in a di-capped trigonal prism coordination geometry.The other (Gd2)is eightcoordinated by four TDC2-ligands in monodentate mode and four water molecules in a di-capped trigonal prism coordination geometry.The bond lengths of GdO are in the range from 0.230 8(4)to 0.249 2(4)nm,very close to those of[Gd6Cu24(μ3-OH)30(mAla)16(ClO4)(H2O)22](ClO4)17(OH)2·20H2O(mAla=2-methylalanine)[55].The 2D structure of{[Gd(TDC)]}nn+in 3 can be viewed as each TDC2-ligand coordinated to four Gdバcations with its each carboxylate bridged with two Gdバ cation in anti-syn mode and each Gdバcation coordinated with four oxygen atoms respectively from four TDC2-ligands (Fig.4a),while the 3D structure of 3 can be viewed as the adjacent 2D structures pillared by ox2-ligand as shown in Fig.4b.
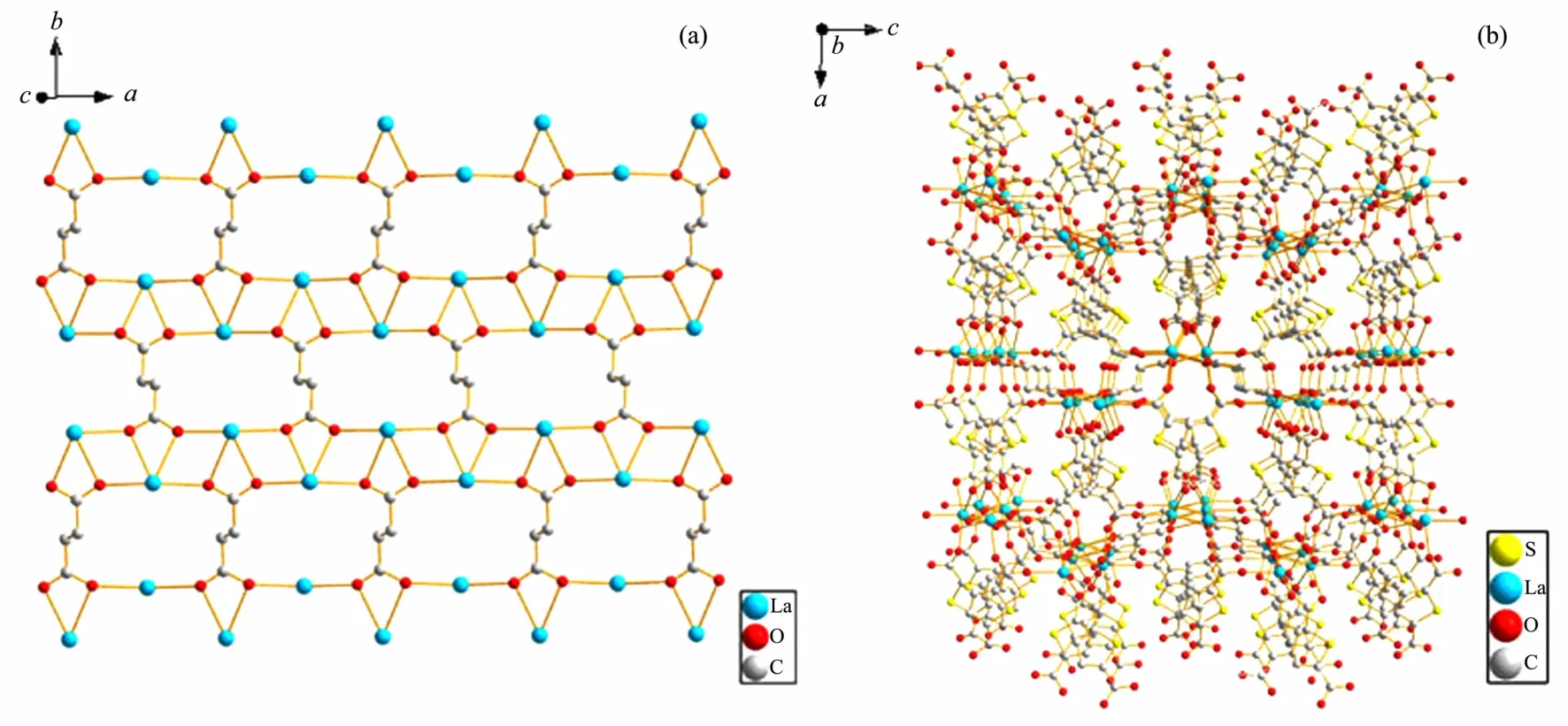
Fig.2 (a)2D structure of{[La2(SUC)]}n4n+in 2;(b)3D structure of 2
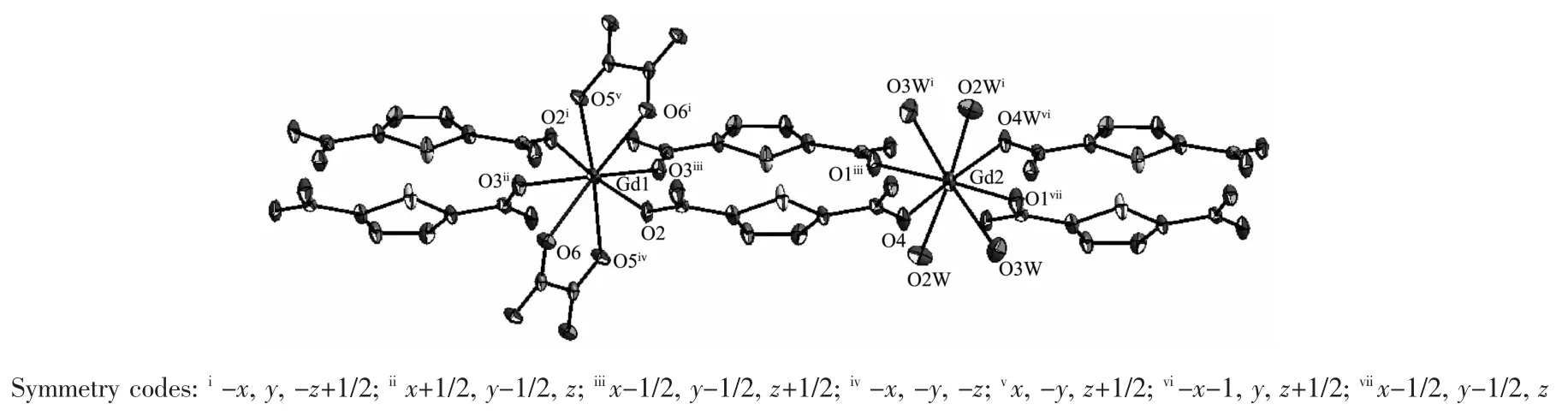
Fig.3 Coordination environment of two independent Gdバcenters in 3 with 50%probability ellipsoids
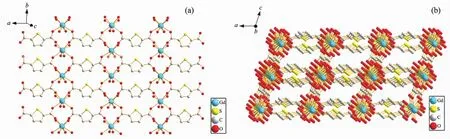
Fig.4 (a)Layer structure of{[Gd(TDC)]}nn+in 3;(b)3D structure of 3
3 Conclusions
In summary,three we have reported syntheses of three lanthanide-based 3D coordination polymers through hydrothermal reaction of Ln(NO3)3(Ln=La,Gd)and H2TDC,and the crystal structure of complexes 2 and 3.H2TDC decomposed into oxalic acid,succinic acid and sulphuric acid in these reactions,respectively.
Supportinginformation is available at http://www.wjhxxb.cn
[1]Feng SH,Xu R R.Acc.Chem.Res.,2001,34(3):239-247
[2]Cundy CS,Cox PA.Chem.Rev.,2003,103(3):663-702
[3]Stock N,Bein T.Angew.Chem.Int.Ed.,2004,43(6):749-752
[4]Chen X M,Tong M L.Acc.Chem.Res.,2007,40(2):162-170
[5]Wei L,Wei Q,Lin Z E,et al.Angew.Chem.,2014,53(28):7188-7191
[6]Zheng ST,Yuan D Q,Zhang J,et al.Inorg.Chem.,2007,46(11):4569-4574
[7]Wei Q,Wang J,He C,et al.Chem.Eur.J.,2016,22(31):10759-10762
[8]Ünalerolu C,Zümreolu-Karan B,Zencir Y,et al.Polyhedron,1997,16(13):2155-2161
[9]Li X,Cao R,Sun D F,et al.Inorg.Chem.Commun.,2003,6(7):815-818
[10]Zhai B,Yi L,Wang H S,et al.Inorg.Chem.,2006,45(21):8471-8473
[11]Zhang L Z,Gu W,Li B,et al.Inorg.Chem.,2007,46(3):622-624
[12]Kong X J,Ren Y P,Long L S,et al.J.Am.Chem.Soc.,2007,129(22):7016-7017
[13]Wu T,Li M,Li D,et al.Cryst.Growth Des.,2016,8(2):568-574
[14]Haloi D J,Singha N K.J.Polym.Sci.Part A:Polym.Chem.,2015,49(7):1564-1571
[15]Wei Q H,Zhang L Y,Yin G Q,et al.J.Am.Chem.Soc.,2004,126(32):9940-9941
[16]Zhang JP,Zheng SL,Huang X C,et al.Angew.Chem.Int.Ed.,2004,43(2):206-209
[17]Chen B L,Mok K F,Ng SC,et al.Polyhedron,1998,17(23/24):4237-4247
[18]Sun X Z,Sun Y F,Ye B H,et al.Inorg.Chem.Commun.,2003,6(11):1412-1414
[19]Jia H P,Li W,Ju ZF,et al.Eur.J.Inorg.Chem.,2010(21):4264-4270
[20]Demessence A,Rogez G,Rabu P.Inorg.Chem.,2007,46(9):3423-3425
[21]Zhang JP,Lin Y Y,Huang X C,et al.Eur.J.Inorg.Chem.,2006(17):3407-3412
[22]Xu J,Cheng J W,Su W P,et al.Cryst.Growth Des.,2011,11(6):2294-2301
[23]Sun Y G,Jiang B,Cui T F,et al.Dalton Trans.,2011,40(43):11581-1190
[24]LIU Jing(刘静),WANG Min(王敏),ZHANG Zhen-Wei(张振伟),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(1):50-54
[25]Tsai C S,Chen W T,Liao J H.J.Chin.Chem.Soc.,2013,60(7):755-761
[26]Xue L P,Chang X H,Li SH,et al.Dalton Trans.,2014,43(19):7219-7226
[27]He Y P,Tan Y X,Zhang J.Cryst.Growth Des.,2014,14(14):3493-3498
[28]Sibille R,Mazet T,Elkam E,et al.Inorg.Chem.,2013,52(2):608
[29]Xue L P,Li Z H,Li SH,et al.Chin.J.Struct.Chem.,2013,32(5):704-708
[30]Zhou L,Wang C,Zheng X,et al.Dalton Trans.,2013,42(46):16375-16386
[31]ZHANG Yan-Hong(张雁红),Adhikari S P,Day C,et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(7):1305-1312
[32]Demessence A,Rogez G,Welter R,et al.Inorg.Chem.,2007,46(9):3423-3425
[33]Guerriero P,Casellato U,Sitran S,et al.Inorg.Chem.,Acta,1987,133(2):337-345
[34]Rosi N L,Kim J,Eddaoudi M,et al.J.Am.Chem.Soc.,2005,127(5):1504-1518
[35]Wang J,Zheng SL,Hu S,et al.Inorg.Chem.,2007,46(3):795-800
[36]Han L,Bu X H,Zhang Q C,et al.Inorg.Chem.,2006,45(15):5736-5738
[37]Diwan K,Singh B,Singh SK,et al.Dalton Trans.,2012,41(2):367-369
[38]Zhu Q,Sheng T,Tan C,et al.Inorg.Chem.,2011,50(16):7618-7624
[39]Zhu H B,Li L,Wang H,et al.Inorg.Chem.Commun.,2010,13(1):30-32
[40]Ma L F,Wang L Y,Du M.CrystEngComm,2009,11(12):2593-2596
[41]Ma L F,Wang Y Y,Wang L Y,et al.Cryst.Growth Des.,2009,9(5):2036-2038
[42]Zhang X M,Fang R Q,Wu H S.J.Am.Chem.Soc.,2005,127(21):7670-7671
[43]Yang SY,Long L S,Jiang Y B,et al.Chem.Mater.,2002,14(8):3229-3231
[44]Sun J,Zhou Y,Fang Q,et al.Inorg.Chem.,2006,45(21):8677-8684
[45]LU Jiu-Fu(卢久富),ZHAO Cai-Bin(赵蔡斌),JIN Ling-Xia(靳玲侠),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(6):961-967
[46]Chui S S Y,Lo S M F,Charmant J P H,et al.Science,1999,283(5405):1148
[47]Chen J X,Yu T,Chen Z X,et al.Chem.Lett.,2003,32(7):590-591
[48]Chen W X,Wu S T,Long L S,et al.Cryst.Growth Des.,2007,7(6):1171-1175
[49]DONG Jiao-Jiao(董娇娇),JIN Jing(金晶),YAN Xin(鄢欣),et al.Journal of Jilin University:Science Edition(吉林大学学报:理学版),2014,52(5):1067-1072
[50]Zhang QZ,Lu CZ,Yang WB,et al.Inorg.Chem.Commun.,2004,7(7):889-892
[51]Sheldrick G M.SADABS,Version 2.05,University of Göttingen,Germany,2000.
[52]Sheldrick GM.SHELXTL-2014,Programfor Crystal Structure Refinement,University of Göttingen,Germany,2014.
[53]He Z,Gao E Q,Wang Z M,et al.Inorg.Chem.,2005,44(4):862-874
[54]Cambridge Structural Database,Version 5.28,Cambridge,UK,2008.
[55]Zhang JJ,Hu SM,Xiang SC,et al.Inorg.Chem.,2006,45(18):7173-7181
