配合物(BenzMeIm)2[PtCl4]的合成与表征
2018-04-10MohamadAliHikmatMahmoudJamalAzheenGerberThomasHostenEric
Mohamad Ali Hikmat Mahmoud Jamal Azheen Gerber Thomas Hosten Eric
(1Department of Chemistry,College of Education,University of Salahaddin,Erbil,Iraq)(2Department of Chemistry,Nelson Mandela Metropolitan University,Port Elizabeth,South Africa)
配合物(BenzMeIm)2[PtCl4]的合成与表征
Mohamad Ali Hikmat*,1Mahmoud Jamal Azheen1Gerber Thomas2Hosten Eric2
(1Department of Chemistry,College of Education,University of Salahaddin,Erbil,Iraq)(2Department of Chemistry,Nelson Mandela Metropolitan University,Port Elizabeth,South Africa)
通过离子液体氯化1-苄基-3-甲基咪唑(BenzMeIm-Cl)与PtCl2的反应,合成了配合物(BenzMeIm)2[PtCl4],并用元素分析、红外光谱、紫外-可见光谱、1H NMR、13CNMR和单晶X射线衍射对其进行了表征。单晶X射线分析表明,配合物结构属于P21/c空间群,晶胞参数和结构解析参数为:a=0.981 80(5)nm,b=0.861 47(3)nm,c=0.144 332(7)nm,β=92.480(2)°,V=121.96(1)nm3,R1=0.014 4,wR2=0.038 8。
氯化1-苄基-3-甲基咪唑;铂;配合物;晶体结构
In this account we report the synthesis,characterization,and X-ray crystal structure of the complex(BenzMeIm)2[PtCl4]from the reaction of 1-benzyl-3-methylimidazolium chloride(BenzMeIm-Cl)with PtCl2(Scheme 1).

Scheme 1 Synthesis of the complex(BenzMeIm)2[PtCl4]
1 Experimental
1.1 Materials and instrument
1-Benzyl-3-methylimidazolium chloride(BenzMeIm-Cl)is commercially available(Shanghai Biochemical,China),and PtCl2was obtained from Yuri Chemicals,China.
The1H and13C NMR spectra were recorded at 300 K using a Bruker Ultra-Shield 300 MHz spectrometer.Deuterated dimethyl sulfoxide was used as the solvent and the peak positions were obtained relative to SiMe4.Infrared spectra were recorded on a Shimadzu FTIR spectrometer using KBr discs in the 4 000~400 cm-1range,and a Pye-Unicam 300 spectrometer using CsIdiscs in the range 4 000~200 cm-1.UV-Vis spectra were obtained using an AE-UV 1690 (UK)330 spectrophotometer with the samples in DMSO solution(1 mmol·L-1).Melting points were determined with a MPD-100 melting point Pixel technology. The elemental analyses for carbon,hydrogen and nitrogen were performed by a Vario Elementary EL ⅢMicrocube CHNSelemental analyzer.The molar conductivity measurements were carried out with 1 mmol·L-1solutions at 298 K with a Jenway 4200 (0.93 cell constant)conductometer.
1.2 Synthesis of(BenzMeIm)2[PtCl4]
A solution of PtCl2(2.66 g,100 mmol)in 10 mL of ethanol was added to a solution of 1-benzyl-3-methylimidazolium chloride (BenzMeIm-Cl,4.16 g,200 mmol)in 20 mL of ethanol.The mixture washeated to reflux for 2 h,and then cooled to room temperature.A yellow precipitate was isolated by filtration.A small amount of the precipitate was dissolved in acetonitrile and left to evaporate slowly at room temperature.After one week yellow crystals were obtained.Yield:68%.m.p.(dec)163℃.Anal.Calcd.for C22H26N4Cl4Pt(%):C 38.63,H 3.80,N 8.19.Found(%):C 38.60,H 3.78,N 8.18.IR(cm-1): ν(C-H aromatic)3 025, ν(C-H aliphatic)2 980,ν(C=N)1 603,ν(C=C)1 567,ν(C-N)1 492,ν(Pt-Cl)321.1H NMR: δ9.28(s,1H,H(2));7.80(d,1H,H(4));7.72(d,1H,H(3));7.40~7.64(m,5H,H(12)~H(16));5.43(d,2H,C(1)H2);3.86(s,3H,C(5)H3).13C NMR:δ136.61~136.67 for two N=CH-H(C(1),C(5))totomerizems,134.87 for C(7)~C(11),128.71~128.90 for CH=CH aromatic ring,51.79 for(CH2C(5))and 38.79 for(C(1)).UV-Vis(DMSO,λmax/nm(ε/(dm3·mol-1·cm-1))):400(2 500),320(8 000).A molar conductivity measurement of 1 mmol·L-1DMSO solution of the compound (72 S·mol-1·cm2)indicates that it is a 1∶2 electrolyte.
1.3 X-ray crystallography
Yellow crystals of(BenzMeIm)2[PtCl4]suitable for X-ray crystallographic measurements were obtained by the slow evaporation of its dichloromethane solutions.The crystal data of(BenzMeIm)2[PtCl4]was collected at 200 K using a Bruker Kappa ApexⅡdiffractometer with graphite-monochromated Mo Kαradiation(λ=0.071 073 nm).APEX Ⅱ[10]was used for data collection and SAINT[10]for cell refinement and data reduction.The structure was solved and refined by least-squares procedures using SHELXL-2014[11]with SHELXLE[12]as a graphical interface.All non-hydrogen atoms were refined anisotropically,and the hydrogen atoms were added in idealized geometrical positions in a riding model.Data were corrected for absorption effects using the numerical method implemented in SADABS[10].Table 1 gives the crystallographic data and collection parameters.All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were included in the models by calculating the positions (riding model)and refined with calculated isotropic displacement parameters.Selected bond distances and angles are summarized in Table 2.
CCDC:1532135.
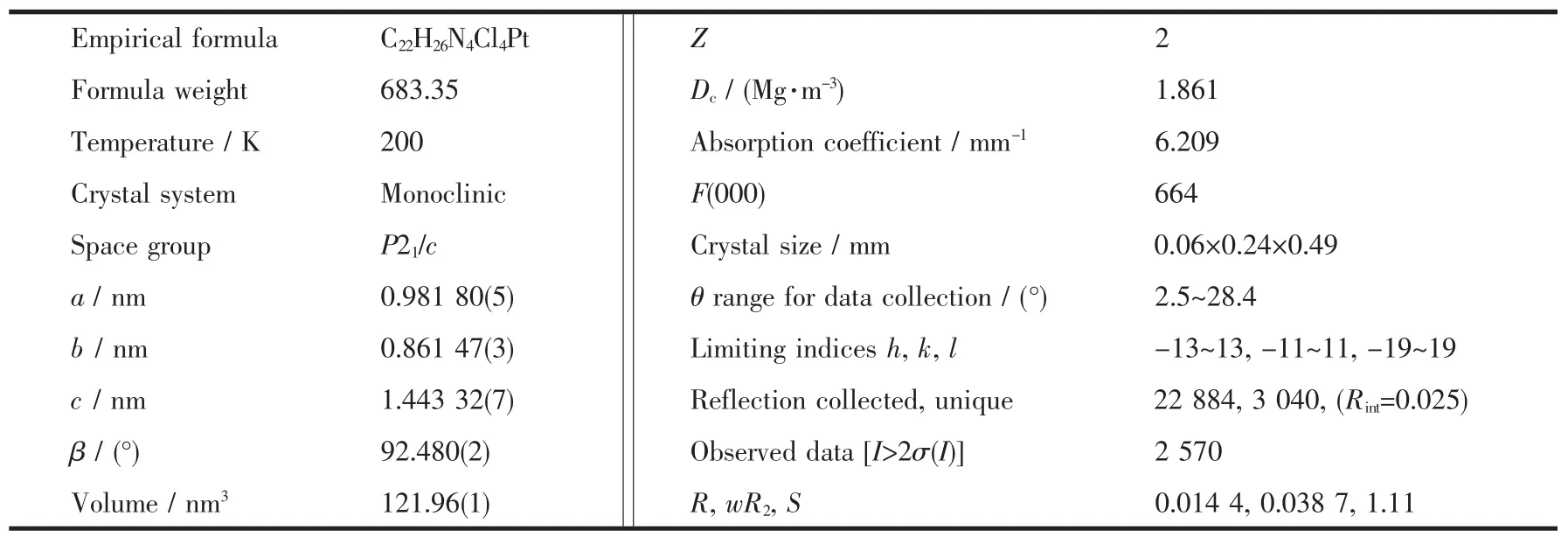
Table 1 Crystal data and structure refinement of the complex

Table 2 Selected bond distances(nm)and angles(°)of the complex
2 Results and discussion
2.1 IR and UV-Vis spectrum analysis
The IR spectrum of the compound (Fig.S1)is characterized by absorption bands at 3 025 and 2 980 cm-1,which are assigned to ν(C-H aromatic)and ν(CH aliphatic)respectively, at 1 603 cm-1forν(C=N),1 560 cm-1forν(C=C),1 456 cm-1forν(C-N)and at 321 cm-1forν(Pt-Cl)[13].

Fig.1 Electronic spectra of(BenzMeim)2[PtCl4]
The UV-Vis spectrum of the salt in DMSO(Fig.1)shows three absorption bands at 400 nm(25 000 cm-1),320 nm(31 250 cm-1)and 270 nm(37 037 cm-1)which are assigned to the transitions1A1g→1B1g,1A1g→1Egand Cl-→Pt2+(charge transfer),typical of a square planar geometry[14].
2.2 NMR spectra
1H NMR spectrum of the complex is shown in Fig.2.The1H NMR spectrum shows the singlet band for N-CH=N at 9.27,doublets in the range of 7.82~7.73 for the CH=CH protons,and triplets in the range of 4.22~4.19 for(CH2Et),and at 3.87~3.82 for(-NCH3),triplets at 1.44~1.38 for 3H(CH3Et)groups.

Fig.2 1H NMR for[BenzMeim]2[PtCl4]
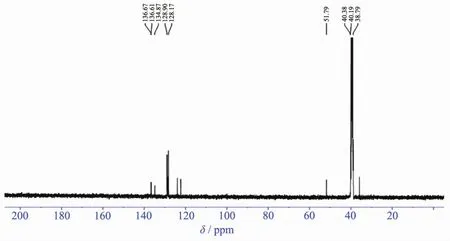
Fig.3 13CNMR for[BenzMeim]2[PtCl4]
The13C NMR spectral data(Fig.3)show peaks at 136.24 for N=CH-N,123.51~121.93 for CH=CH,44.08~40.11 for H2Et,35.69 for CH3-N,the band at 15.11 for CH3Et.The13C-DEPT-135 spectrum shows one upward singlet at 136.87 for N=CH-N,two upward singlets at 124.14~122.55 for CH=CH,an downward singlet at-44.71 for CH2Et,another upward singlet at 36.33 for N-CH3and an upward singlet at 15.76 for CH3Et[15-16].
2.4 Crystal structure of the complex

Fig.4 Crystal structure of(BenzMeIm)2[PtCl4]
The molecular structure of the complex is shown in Fig.4.The structure is centrosymmetrical with the Ptギon the inversion point.The two BenzMeIm+cations are identical and symmetrical,and only one is shown in Fig.1.The[PtCl4]2-anion assumesthedistorted square planar geometry D4h,and the metal is coordinated to four chlorides.Both trans-angles in the anion are ideal at 180.00°and the cis-angles Cl(1)-Pt(1)-Cl(2)iand Cl(1)i-Pt(1)-Cl(2)are both 90.09(2)°,with Cl(1)-Pt(1)-Cl(2)and Cl(1)i-Pt(1)-Cl(2)iequal to 89.91(2)°.The lengths of the Pt(1)-Cl(1)and Pt(1)-Cl(2)bonds are 0.230 74(6)and 0.230 95(6)nm,respectively.
The bond lengths in the imidazolium ring of the(BenzMeIm)+cations show the delocalization of the double bond over N(2)-C(2)-N(1),with both the C(2)-N bond lengths equal to 0.132 6(3)nm.Both the C(3)-N(2)(0.137 8(3)nm)and C(4)-N(1)(0.137 5(3)nm)bonds are single,with the C(3)-C(4)bond double at 0.134 9(4)nm.The N(1)-C(1)-C(11)bond angle is 112.8(2)°,typical of a sp3-hybridized carbon atom.
3 Conclusions
The Ptギcomplex(BenzMeIm)2[PtCl4]has been synthesized from the reaction of PtCl2with 1-benzyl-3-methylimidazolium chloride.The characterization of the structure of the compound shows a square planar geometry around the metal.
Acknowledgements:The authors are thankful to the Chemistry Department,College of Education,at Salahaddin University for accomplishing this work.
Supporting information is available at http://www.wjhxxb.cn
[1]Hassan M,Kozhevnikov I V,Siddiqui H R,et al.Inorg.Chem.,2001,40:795-800
[2]Chorbani M H.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2012,E68:o2605
[3]Herbert J M,Woodgate P D,Denny W A.J.Med.Chem.,1987,30:2081-2086
[4]Lui K C,Chen H H.J.Heterocycl.Chem.,1984,21:911-912
[5]Chauvin Y,Olivier H,Wyrvalski CN,et al.J.Catal.,1997,165:275
[6]Appleby D,Hussey C L,Seddon K R,et al.Nature,1986,323:614-616
[7]Sun I W,Ward E H,Hussey CL,et al.Inorg.Chem.,1987,26:2140-2143
[8]Schreiter ER,Stevens JE,Ortwerth MF,et al.Inorg.Chem.,1999,38:3935-3937
[9]Buttrus N H,Saeed I A,Al-Joburi SA.Raf.J.Sci.,2007,18:84-90
[10]APEX 2,SAINT,SADABS,Bruker AXS Inc.,Madison,Wisconsin,USA,1996.
[11]Sheldrick G M.Acta Crystallogr.Sect.C:Cryst.Struct.Commun.,2015,C71:3-8
[12]Hübschle CB,Sheldrick GM,Dittrich B.J.Appl.Crystallogr.,2011,44:1281-1284
[13]Mai K,Takayoshi S.Inorg.Chim.Acta,2010,363:3602-3605
[14]Kriza A,Parnau C,Popa N,et al.J.Inorg.Chem.,2004,1:179-183
[15]Venkatesan P,Maruthavanan T.Bull.Chem.Soc.Ethiop.,2011,25:419-425
[16]Field L D,Sternhell S,Kalman JR.Organic Structures from Spectra.3rd Ed.Chichester:John Wiley&Sons,2005:64
Synthesis and Characterization of Complex(BenzMeIm)2[PtCl4]
Mohamad Ali Hikmat*,1Mahmoud Jamal Azheen1Gerber Thomas2Hosten Eric2
(1Department of Chemistry,College of Education,University of Salahaddin,Erbil,Iraq)(2Department of Chemistry,Nelson Mandela Metropolitan University,Port Elizabeth,South Africa)
A complex of(BenzMeIm)2[PtCl4]was prepared by the reaction of 1-benzyl-3-methylimidazolium chloride(BenzMeIm-Cl)with PtCl2.The structure of the complex was characterized by single crystal X-ray analysis,and it crystallizes in the monoclinic P21/c space group,with a=0.981 80(5)nm,b=0.861 47(3)nm,c=0.144 332(7)nm,β=92.480(2)°,V=121.96(1)nm3,R1=0.014 4,wR2=0.038 8.CCDC:1532135.
1-benzyl-3-methylimidazolium chloride;Ptギ;complex;crystal structure
0 Introduction
Ionic liquid salts have
an increasing interest due to their applications in many fields such as medicine,organic and inorganic synthesis,electrochemistry,separation processes and as novel materials[1].For example,2-methylsulfanyl-1H-pyrimidin-3-ium iodide is a potential antitumor agent[2-4].The complexes containing the platinum group metals have also attracted great interest,mainly because of their application as catalysts[5],and several reports have described spectrochemical and electrochemical studies in ionic liquid media[6-8].New complexes of palladiumギand platinumギcontaining the carbazolium,indolium and pyrrolium benzyl derivatives have been synthesized and characterized and the results exhibit the square planar geometry for both of Pdギ and Ptギcomplexes[9].
O614.82+6
A
1001-4861(2018)04-0728-05
10.11862/CJIC.2018.096
2017-08-24。收修改稿日期:2018-01-25。
*
。 E-mail:hikmatmohamad@yahoo.com,Tel:+964 770 131 9328
