两个由醋酸根和含吡啶基配体构筑的Znギ配聚物的合成、晶体结构及其荧光性质
2018-04-10蒋正静殷竟洲李荣清张载超陆路德
蒋正静 殷竟洲 李荣清 张载超 陆路德
0 Introduction
In recent years,coordination polymers have increasingly attracted considerable attention for their structural and topological diversities as well as their potential applications in the fields including luminescence[1-7],gas storage/separation[8-10],magnetic properties[11],catalysis[12-13],drug delivery[14]and precursors for nanoparticles[15],etc.The assembly of coordination polymers can be influenced by some external factors,such as temperature,solvent,reactant concentration,and pH value,etc.,and some internal factors,such as coordination geometries of the central metals,configurations,and nature of the organic ligands[16-17].Among these factors,the choice of the organic ligands is the greatest influence in determining the type and topology of the product.Properties of organic ligands,such as solubility,coordination activity,length,geometry,and relative orientation of the donor groups,play a very important role in dictating polymer framework topology[17].So,it is very important to design the organic ligands or regulate the synthesis conditions.To realize the infinite extension of structure,organic ligands with two or more coordination atoms,such as N,O,and Satoms,are often employed[5,18].Therefore,most coordination polymers are constructed from metal ions and carboxylic acid-or nitrogen-containing ligands.Lewis base coordinated zincギcarboxylate polymers are an important class of coordination polymers,which have been known for many years[19-21].The spherical d10configuration of the Znギcation has associated with it a highly flexible coordination sphere which allows it to adopt multiple geometries to suit the demands of its environment.As a result,Zn is an ideal metal for use in constructing extended coordination networks with ligands that can bridge the metal centers in one or multiple directions.On the other hand,the properties and structures of such materials are easily variable with changes in the counterion present.Anionic carboxylates are highly flexible and versatile O-donor ligands in that a range of substituents may be introduced on the carbonyl carbon to modulate its reactivity and coordination propensity to result in a variety of coordination modes such as monodentate,chelating,bidentate bridging,monoatomic bridging,and chelating bridging[4].Moreover,the choice of the organic ligands is the greatest influence in determining the type and topology of the product.So,the properties and structures of such materials are easily variable with changes in ligand ratio,ligand type and the counterion present.Pyridylcontaining ligand,such as INH or 2-APy,being an interesting N-type of heterocyclic system,has attracted much attention of many researchers[21-23].In earlier efforts,we have used INH ligand with Cuガions to form metal-organic coordination polymers[22-23].As part of our ongoing research in this area,we herein report the synthesis,structures and luminescent properties of two Zincギcoordination polymers constructed by acetate and pyridyl-containing ligands:[Zn(CH3COO)2(INH)]n(1)and[Zn(CH3COO)2(2-APy)]n(2).
1 Experimental
1.1 Materials and measurements
INH(Isonicotinic acid hydrazide;Isoniazid)and 2-APy(2-Aminopyridine)were purchased from Alfa Aesar,UK.All solvents and other chemicals were purchased and use received.Element analyses were performed on a Perkin-Elmer 2400LS elemental analyzer.Powder X-ray diffraction (PXRD)patterns were measured on an ARL/X′PRA powder diffractometer at 40 kV,40 mA using a Cu Kα radiation(λ=0.154 06 nm)in 2θrange of 5°~40°.IR spectra were measured in KBr pellets on a Nicolet AVATAR360 FT-IR spectrometer in the range of 4 000~400 cm-1.Fluorescence spectra were recorded on a Perkin-Elmer LS55 spectrometer.
1.2 Synthesis of the polymer 1
Zn(CH3COO)2·2H2O(0.439 g,2 mmol)was dissolved in 10 mL DMF solvent at room temperature.INH(0.274 g,2 mmol)was added to this solution with stirring,during which time white precipitate formed.Then,NH4SCN was added to the reaction mixture until the precipitate dissolved and a colorless solution formed.The colorless solution obtained was filtered and the filtrate was then transfered to two test tubes and carefully layered by i-PrOH.Three weeks later,the products were collected and colorless block crystals of[Zn(CH3COO)2(INH)]nsuitable for X-ray measurements were obtained.Anal.Calcd.for C10H13N3O5Zn(%):C,37.46;H,4.09;N,13.11.Found(%):C,37.22;H,4.20;N,13.28.IR(KBr,cm-1):3 445(m),3 308(m),3 098(m),3 004(m),1 666(s),1 620(s),1 559(vs),1 415(s),1 349(m),1 215(m),1 090(m),1 021(m),693(s),684(m).
1.3 Synthesis of the polymer 2
Compound 2 was synthesized by the same procedure as described for 1 except the addition of 2-APy(0.188 g,2 mmol)instead of INH(0.274 g,2 mmol).Colorless block crystals were obtained and collected in the same way as for compound 1.Anal.Calcd for C18H24N4O8Zn2(%):C,38.94;H,4.36;N,10.09.Found(%):C,38.62;H,4.55;N,10.21.IR(KBr,cm-1):3 432(s),3 328(s),3 312(s),1 653(s),1 614(s),1 589(vs),1 557(s),1 501(m),1 444(m),1 396(s),1 332(m),1 058(m),l 002(m),950(m),857(m),768(m),687(m),607(m),478(m),437(m).
1.4 Powder X-ray diffraction
The phase purity of the as-synthesized compounds was confirmed by PXRD patterns,which are consistent with the simulated patterns using the Mercury program(version 1.4.2,Cambridge Crystallographic Data Centre,Cambridge,UK)from the singlecrystal X-ray diffraction data.Fig.1(1 and 2)show the PXRD patterns of as-synthesized compounds and the simulated patterns on the basis of single-crystal structures of 1 and 2,respectively.
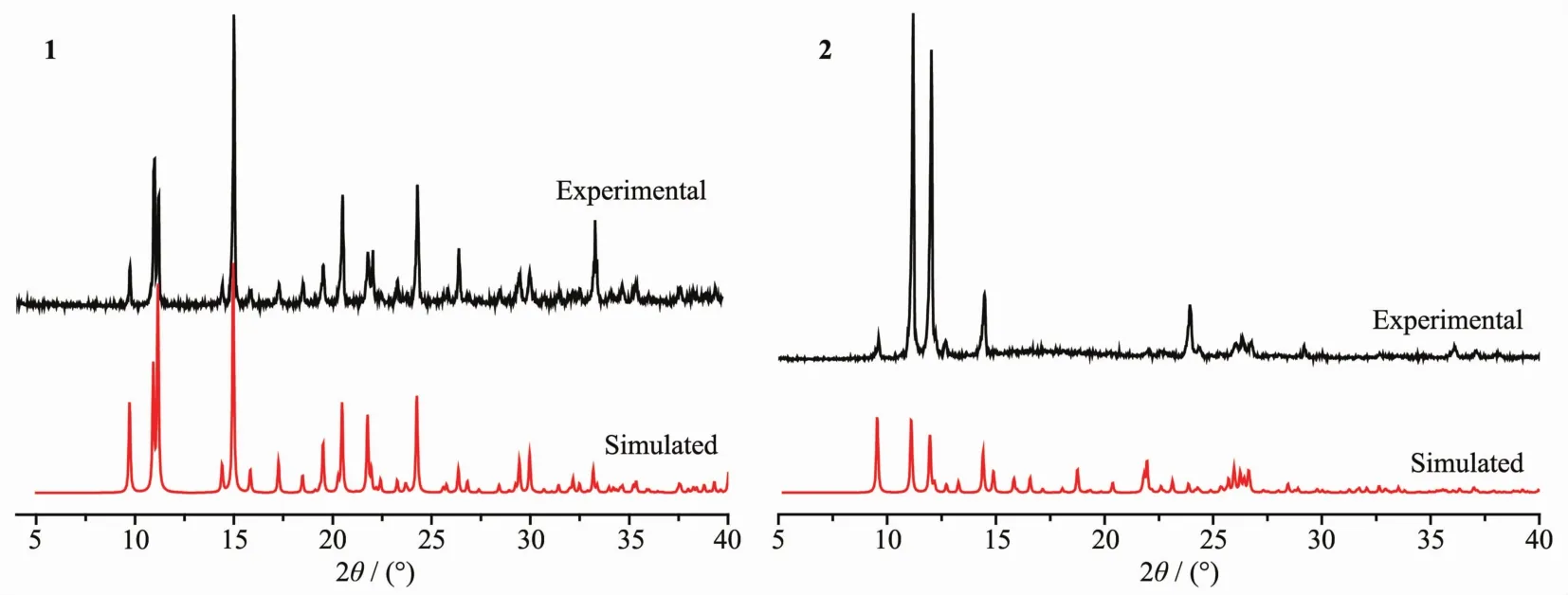
Fig.1 Experimental(upper trace)and simulated(lower trace)PXRD patterns of the polymers 1 and 2
1.5 Determination of crystal structures
The X-ray diffraction measurement for the two compounds were performed on a Bruker Smart APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation (λ=0.071 073 nm)by using φ-ω scan mode at 296(2)K.The crystal parameters and experimental details of the data collection are summarized in Table 1.Semi-empirical absorption correction was applied to the intensity data using the SADABSprogram[24].The structures were solved by direct methods of SHELXS-97 program and refined by full matrix least-square on F2using the SHELXTL-97 program[25-26].All non-hydrogen atoms were refined with anisotropic thermal parameters,and all hydrogen atoms were included in calculated positions and refined with isotropic thermal parameters riding on those of the parent atoms.
CCDC:870030,1;1524913,2.
2 Results and discussion
2.1 Description of crystal structure
Single-crystal structure analysis reveals that the polymer 1 crystallizes in the monoclinic system,space group P21/c with 4 asymmetric units in one unit cell,and exhibits a 2D layer network.The structure of the polymer 1 is depicted in Fig.2(1)and Fig.3.Some selected bond lengths and bond angles are listed in Table 2.As depicted in Fig.2,the asymmetric unit contains two acetate anions,an INH ligand and one Znギ ion.In the polymer 1,zincギ is coordinated byfour oxygen atoms from two bridging acetate groups,a non-bridge acetate group and a carbonyl group of INH ligand,a terminal hydrazide nitrogen atom and a pyridine nitrogen atom from two different INH ligands(Fig.3).The coordination geometry of zinc can be described as a distorted octahedral coordination geometry with Zn-N bond lengths ranging 0.215 1(5)~0.2159(4)nmand Zn-Obond lengthsranging0.2069(4)~0.218 0(4)nm,which are comparable to the values in related zincギpolymers[4,21].In the polymer 1,the Zn-N(pyridine nitrogen)bond length is 0.215 1(5)nm,which is similar to the Zn-N (from hydrazide)bond length(0.215 9(4)nm).Also,the Zn-O(from CH3COO-)bond lengths are 0.210 2(3),0.209 0(5),and 0.206 9(4)nm,respectively,which are similar to each other.However,the Zn-O bond(from carbonyl group of INH)length is 0.218 0(4)nm,which is slightly longer.As shown in Fig.3,in the polymer 1,one INH molecule is coordinated to a metal center by an oxygen atom of carbonyl group and a terminal nitrogen atom of the hydrazide,thereby forming a five-membered ring(Zn(1)-O(5)-C(10)-N(2)-N(3)).The pyridine nitrogen of this INH is coordinated to the other metal center.For the polymer 1,the bond angles of O-Zn-N and O-Zn-O (three atoms in the axial direction)are within 163.04(15)°(O(2)iv-Zn(1)-N(3))173.16(16)°(O(1)-Zn(1)-O(3)),which are decreased compared to the bond
angle of 180°for an ideal octahedron.Additionally,the bond angles of the cis O-Zn-N and O-Zn-O are within 75.47(15)°(O(5)-Zn(1)-N(3))101.47(17)°(N(1)ii-Zn(1)-N(3)),which are different from the bond angle of 90°for an ideal octahedron.These are in accordance with the distorted octahedron configuration.The deviation can be explained by steric effects because the INH acts as a bridge between Znギcenters and the approximation of the metal centers is accompanied by a geometric distortion in the INH molecule[21].In the polymer 1,there are two different types of CH3COO-groups,which are bridging(bidentate bridging)and non-bridging(monodentate)CH3COO-ligands.Two adjacent Znギatoms are bridged by a CH3COO-ligand,forming a 1D chain.The nonbonded Zn…Zn separation within the 1D chain is 0.504 67(11)nm.These 1D chains extend in the c direction.The INH molecules act as bridges to link the Znギ atoms(Zn…Zn separation is 0.914 44(19)nm)of two neighboring 1D chains,which further construct a layer network(Fig.4(1)).
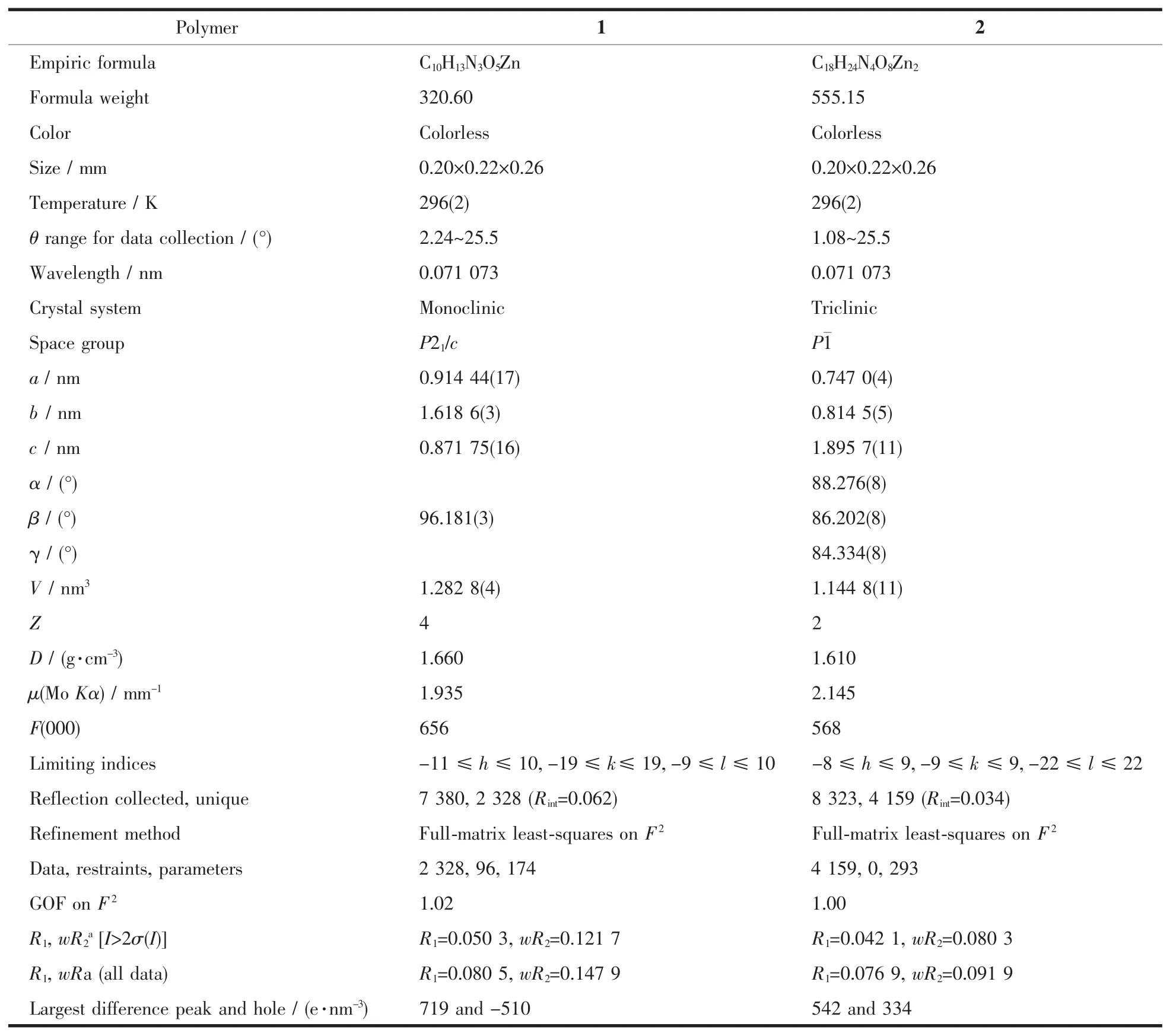
Table 1 Crystallographic collection and refinement parameters for the title polymers

Fig.2 Asymmetric units of the title polymers and the labeling scheme used with 30%ellipsoidal probability
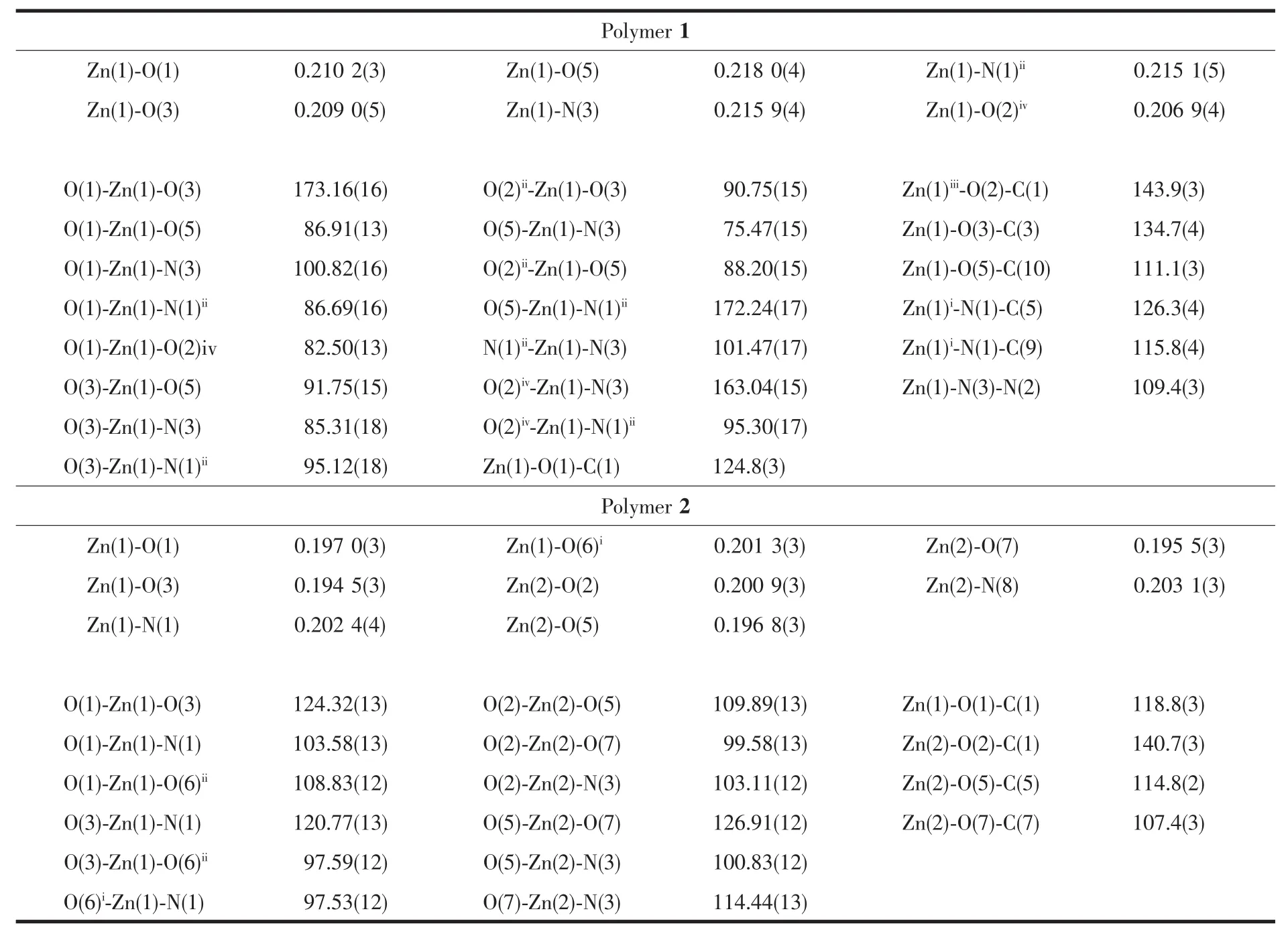
Table 2 Selected bond lengths(nm)and angles(°)for the polymers

Fig.3 Coordination environment of Znギatom in the polymer 1 with the ellipsoids drawn
The polymer 2 crystallizes in the triclinic system,space group P1 with 2 asymmetric units in one unit cell,and exhibits a 1D zig-zag chain.The structure of the polymer 2 is depicted in Fig.2.Some selected bond lengths and bond angles are listed in Table 2.As depicted in Fig.2,the asymmetric unit contains four acetate anions,two 2-APy ligands and two Znギions.In the polymer 2,the Znギ(2)is tetrahedrally surrounded by N(3)of 2-APy,O(7)of the monodentate acetate,O(2),O(5)of the two syn-anti bridging acetate.The Znギ(1)is also tetrahedrally surrounded by N(1)of 2-APy,O(3)of the monodentate acetate,O(1)of the syn-anti bridging acetate and another O of the synanti bridging acetate from the next repeating unit of the polymer.In the polymer 2,the coordination geometry of zinc can be described as a distorted tetrahedral coordination geometry with Zn-N bond lengths ranging 0.202 4(3)~0.203 1(3)nm and Zn-O bond lengths ranging 0.194 5(3)~0.201 3(3)nm,which are comparable to the values in related zincギpolymers[4].However,the Zn-N bond lengths and the Zn-O bond lengths are slightly longer than those of the polymer 1.The nonbonded Zn…Zn separations within the repeating unit and between the repeating units are 0.477 32(20)and 0.461 17(19)nm,and these distances are longer than those of the related reported(0.441 5(1)nm)[4].Another,in the polymer 2,the 2-APy is situated on the opposite side of the growing polymer and thus renders a syndiotactic stereo chemistry,as shown in Fig.4.This structure arise can due to the steric and basic properties of Lewis bases.
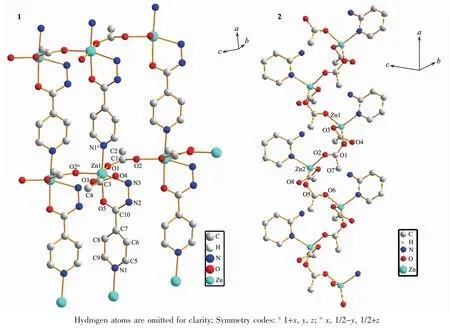
Fig.4 Single 2D coordination sheet for 1 and an 1D zig-zag chain for 2
Significant hydrogen-bond parameters observed in the polymer 1 and 2 are listed in Table 3.In the crystal lattice of the polymer 1,there exist some week intermolecular and intramolecular interactions,including non-classic C-H…O hydrogen bond.All of the N-terminals are involved in weak hydrogen bonds.The non-bridging CH3COO-ligands are involved in hydrogen bonding,with the hydrazide groups and pyridine ring of neighboring layers,thereby forming a 3Dsupramolecular arrangement.Thebridging CH3COO-ligands are only involved in hydrogen bonding with the N-terminals of the same layer(Fig.5(1)).These week interactions help to stabilize the crystal structure.Also,the polymer 2 is stabilized by some week intermolecular and intramolecular interactions in the crystal lattice(Fig.5(2)).In the crystal lattice of the polymer 2,including intramolecular H-bonding interactions(N(4)-H(4A)…O(2),N(4)-H(4A)…O(3),nonclassic C-H…O)and intermolecular interactions(N(2)-H(2A)…O(7)ii,N(2)-H(2B)…O(4)ii,N(4)-H(4B)…O(8)ii).These interactions exist in the zig-zag chain.The parameters of the H-bonds are listed in Table 3.

Fig.5 Selected hydrogen bonds for the polymers shown in dashed line
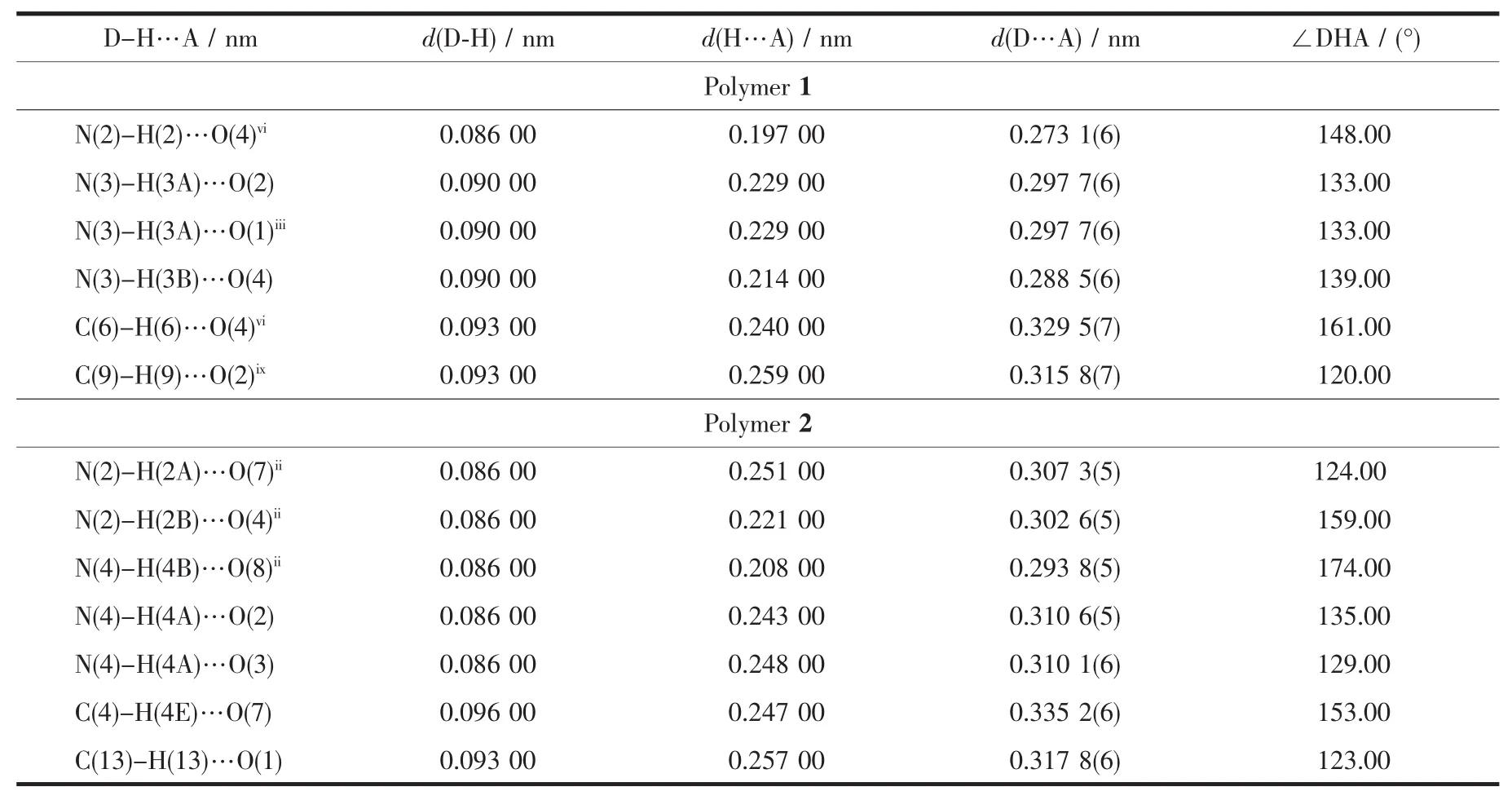
Table 3 Hydrogen bond parameters for polymers 1 and 2
2.2 Luminescent properties
Inorganic-organic hybrid coordination polymers,constructed with d10metal centers and conjugated organic linkers,have received remarkable attention in view of various potential applications,such as in chemical sensors,photochemistry,and so on[5,27-30].The fluorescence properties of the polymer 1 and 2,as well as free ligands INH and 2-APy,have been investigated in the solid state at room temperature,as depicted in Fig.6.Upon excitation at 211 nm,both the microcrystalline free ligand INH and the polymer 1 samples show broad emission bands with the maximum peaks at 369.5 nm for INH,382.6 nm for the polymer 1,respectively(Fig.6(1)).As shown in Fig.6,coordination of the INH ligand with Znギresults in a 13.1 nm red shift of the emission maximum.The emission spectra have broad peaks with maxima at 367.5 nm for polymer 2,and 396.5 nm for 2-APy(λex=250 nm),respectively.Coordination of the free ligand 2-APy with Znギresults in a 29.0 nm blue shift of the emission maximum.These emissions are neither metal-to-ligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)in nature,since the Zn2+ions are difficult to oxidize or to reduce due to their d10configuration.The emissions of polymers 1,2 may be a mixture of characters of intraligand and ligand-to-ligand charge transition(LLCT).And the observed red or blue shift of the emission maximum between the polymers and the ligands is considered to mainly originate from the influence of the coordination of the ligand to metal atom[27-30].The results indicate that these polymers may be potential candidates of fluorescent materials.

Fig.6 Solid-state emission spectra of the polymers and the free ligands
3 Conclusions
Two zincギcoordination polymers constructed by acetate and pyridyl-containing ligands have been successfully synthesized and structurally characterized.Polymer 1 is a 2D infinite layer framework,and the resulting 2D structure is interconnected by hydrogen-bond interactions to lead to a 3D supramolecular architecture.Polymer 2 is an infinite onedimensional (1D)zig-zag chain.The results of our present research,together with those in the previous study[21-23],demonstrate that the structural diversity in composition and dimensionality of the coordination compounds can be achieved by the adjustments of ligands,metal centers and/or coordinated anions.In addition,the photoluminescence investigation shows that two coordination polymers appear potentially applied as luminescent materials.
[1]Liu K,Shi W,Cheng P.Coord.Chem.Rev.,2015,289-290:74-122
[2]Zhou H C,Kitagawa S.Chem.Soc.Rev.,2014,43:5415-5418
[3]O′Keeffe M,Yaghi O M.Chem.Rev.,2012,112:675-702
[4]Kumar U,Thomas J,Thirupathi N.Inorg.Chem.,2010,49:62-72
[5]Zhang X,Hou L,Liu B,et al.Cryst.Growth Des.,2013,13:3177-3187
[6]Feng X,Ma L F,Liu L,et al.Cryst.Growth Des.,2013,13:4469-4479
[7]LI Hong-Jin(李红晋),GAO Zhu-Qing(高竹青),GU Jin-Zhong(顾金忠).Chinese J.Inorg.Chem.(无机化学学报),2014,30(11):2615-2620
[8]Huang Y M,Zheng X F,Duan J G,et al.Dalton Trans.,2014,43:6811-6818
[9]Kesanli B,Cui Y,Smith M R,et al.Angew.Chem.Int.Ed.,2005,44:72-75
[10]Zhang H M,Fu W F,Gan X,et al.Dalton Trans.,2008:6817-6824
[11]Xie Y B,Gan L,Saudo E C,et al.CrystEngComm,2015,17:4136-4142
[12]Hong D Y,Hwang Y K,Serre C,et al.Adv.Funct.Mater.,2009,19:1537-1552
[13]Karmakar A,Rubio G M D M,Silva M F C G,et al.Cryst.Growth Des.,2015,15:4185-4197
[14]Horcajada P,Chalati T,Serre C,et al.Nat.Mater.,2010,9:172-178
[15]Kumar U,Thomas J,Nagarajan R,et al.Inorg.Chim.Acta,2011,372:191-199
[16]Lan Y Q,Jiang H L,Li S L,et al.Inorg.Chem.,2012,51:7484-7491
[17]Zhao D,Timmons D J,Yuan D,et al.Acc.Chem.Res.,2011,44:123-133
[18]Hou L,Li D,Shi W J,et al.Inorg.Chem.,2005,44:7825-7832
[19]Chen S S,Sheng L Q,Zhao Y,et al.Cryst.Growth Des.,2016,16:229-241
[20]Lee T W,Lau J P K,Wong W T,et al.Polyhedron,2004,23:999-1002
[21]Freitas M CR,António JM S,Ziolli R L,et al.Polyhedron,2011,30:1922-1926
[22]Jiang Z J,Zhao P S,Li R Q,et al.J.Chem.Crystallogr.,2013,43:463-470
[23]JIANG Zheng-Jing(蒋正静),LU Lu-De(陆路德),WU Xiao-Dong(武晓东),et al.Chinese J.Inorg.Chem.(无机化学学报),2009,25(4):746-750
[24]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of Göttingen,Germany,1996.
[25]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution,University of Göttingen,Germany,1996.
[26]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1996.
[27]Kreno L E,Leong K,Farha O K,et al.Chem.Rev.,2012,112:1105-1125
[28]Su Z,Fan J,Chen M,et al.Cryst.Growth Des.,2011,11:1159-1169
[29]Wan X Y,Jiang F L,Chen L,et al.CrystEngComm,2015,17:3829-3837
[30]Wu G,Wang X F,Okamura T,et al.Inorg.Chem.,2006,45:8523-8532
