Main chemical constituents and pharmacological properties of Harrisonia perforata (Blanco) Merr.
2018-04-02ShifngLiZhiyngYnXioxioHungShojingSong
Shifng Li, Zhiyng Yn, Xioxio Hung*, Shojing Song*
a Department of Natural Product Chemistry, Shenyang Pharmaceutical University, Shenyang 110016, China;
b Chinese People's Liberation Army 210 Hospital, Dalian 116021, People’s Republic of China
1 Introduction
Harrisonia perforata (Blanco) Merr., the only species of Harrisonia genus, belongs to the Simarubaceae family. It is mainly distributed in southeast of Asia and southern China [1]. Its roots and leaves are applied for the treatment of wound healing and malaria in Chinese folk medicine [2].In Vietnamese folk medicine, it is used to treat itching, and also used for diarrhea and dysentery in neighboring countries [3]. Extensive studies indicated that the main chemical constituents of this plant are structurally diverse chromones, rearranged limonoids, polyketides and triterpenes [4].According to the previous studies of H. perforata,it exhibited various pharmacological activities, such as anti-cancer, anti-inflammatory, antibacterial and insecticidal activities [5-8]. This paper summarized the chemical constituents and pharmacological activities of H. perforata, which provides a foundation for the further researches.
2 Studies of chemical constitutions
There have been some reports about the chemical compositions of H. perforata. And it is found that the main compounds of H. perforata are limonoids, chromones according to the previous researches.
2.1 Limonoids
There were about twenty-eight limonoids isolated from H. perforata (Fig. 1). Three compounds were isolated from the roots of this plant: harperfolide (1), harrisonin (2), obacunone(3) [6]. From the leaves and branches of H.perforata, kihadanin A (4), kihadanin B (5),6α-acetoxyobacunol acetate (6) [9], haperforine A (7), 12-desacetylhaperforine A (8), haperforine E (9) [3], perforanoid A (10) [10], perforatinolone(11) [11], perforatin (12) [12], haperforin B1(13), haperforin D (14) [13], haperforin C2(15), haperforin F (16), haperforin G (17) [14],harperspinoid A (18), harperspinoid B (19) [2] were isolated. Perforin A (20), 12β-acetoxyharrisonin(21), harrpernoid B (22), harrpernoid C (23),5,6-dehydrodesepoxyharperforin C2 (24), rutaevine(25) [15], haperforatin (26) [6] were extracted from fruits of H. perforata. Two limonoids were isolated from the twigs and stems of H. perforata:perforalactone A (27), perforalactone B (28) [8].
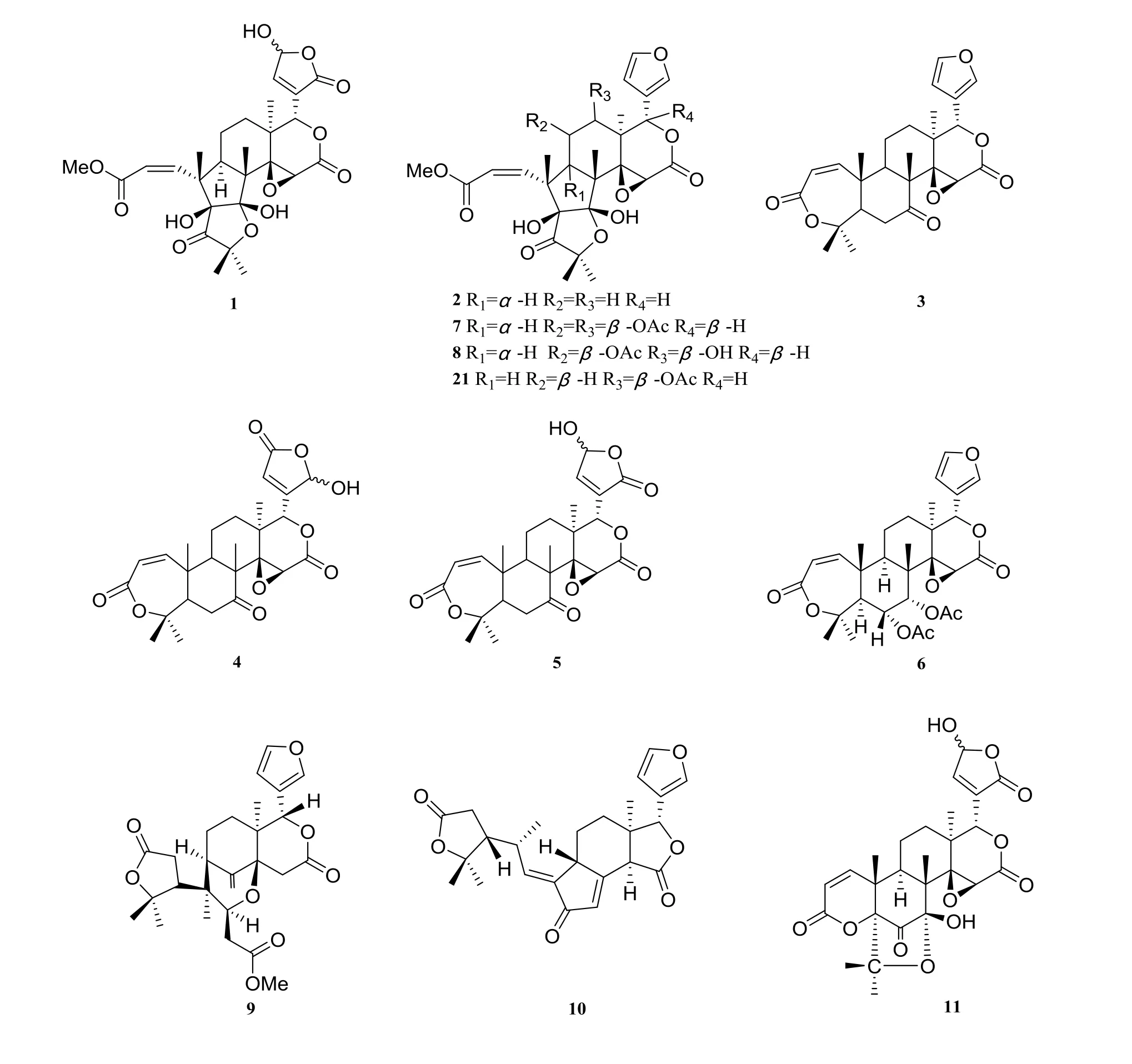
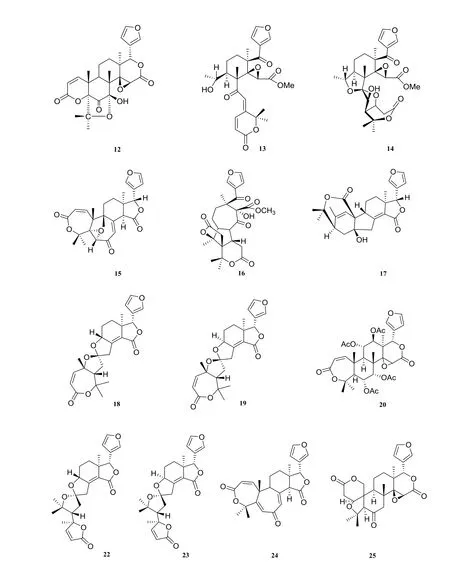
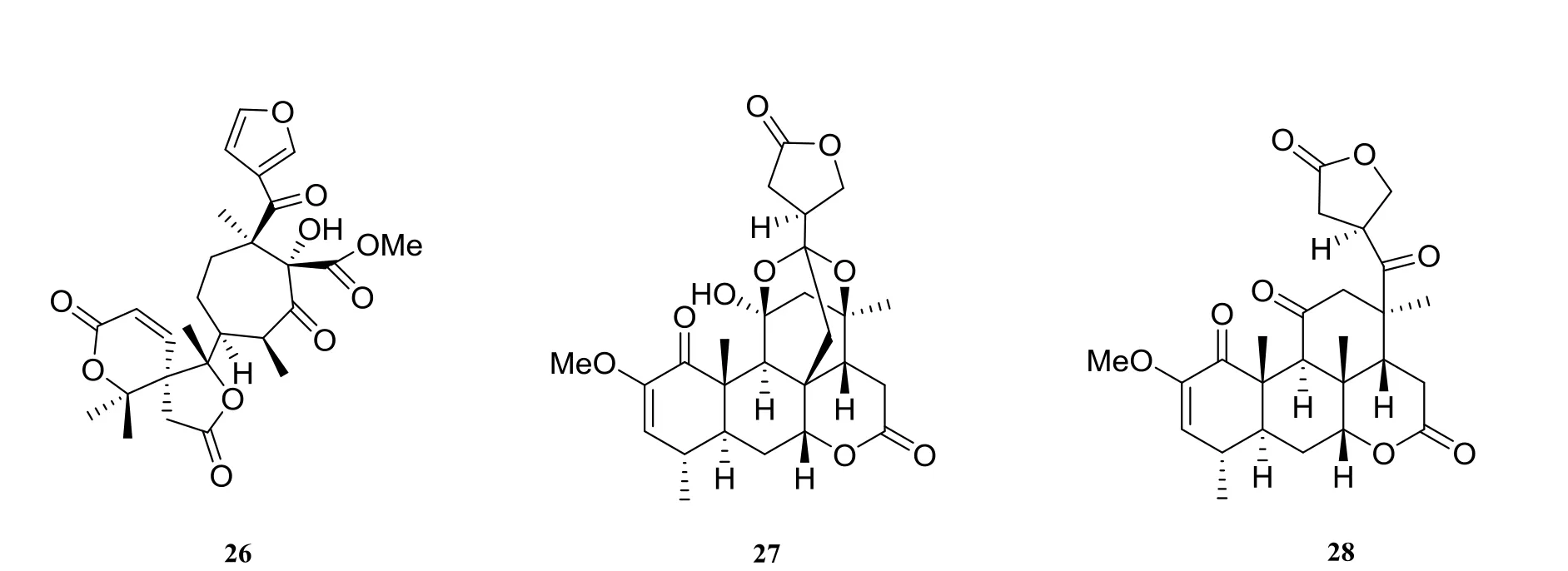
Fig. 1 Limonoids in Harrisonia perforata
2.2 Prenylated polyketides
Five compounds possessing an unusual spirocyclic skeleton (Fig. 2), harrisotone A(29), harrisotone B (30), harrisotone C (31),harrisotone D (32), harrisotone E (33) and a new hydroperoxypolyketide harrisonol A (34) [5] were isolated from the stems and leaves of H. perforata.
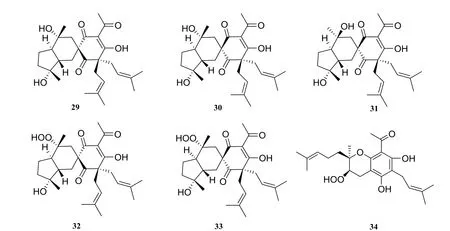
Fig. 2 Prenylated polyketides in Harrisonia perforata
2.3 Chromones
Previous studies have showed that the isolated chromones from H. perforata can be mainly divided into two classes: chromones and methyl chromones derivatives (Fig. 3). Chromones included heteropeucenin-5-methoxy-7-methyl ether (35),perforatin B (36) [16], perforatin D (37), perforatin E (38), perforatin F (39), heteropeucenin-5-methoxy-7-methyl ether (40), heteropeucenin-7-methyl ether(41) [17], heteropeucenin-7-methyl ether (42) [16],(±)-perforatin C (43a, 43b), (±)-2'-O-acetylperforatin C (44a, 44b), (±)-erythro-1'-hydroxyperforatin C (45a, 45b) [18], peucenin-7-methyl ether(46), perforamone A (47), perforamone B (48),perforamone C (49), eugenin (50), saikochromone A (51) [7], 5-hydroxy-7-methoxy-2-methyl-8-[(1E)-3-oxo-1-butenyl]chromone (52) [18], harriperfin E (53) [9]. And greveiglycol (54), greveiglycol 4'-methyl ether (55), (±)-horriperfin A (56a, 56b),(±)-horriperfin B (57a, 57b) [18], perforatic acid methyl ester (58), perforatic acid (59) [18], perforatin A (60) [16], 2-hydroxymethylallopataeroxylin-5-methyl ether (61), perforatic acid methyl ester(62), perforamone D (63) [17], umtatin (64) [15],greveichromenol (65) [7], perforatins G (66) [17]belong to methyl chromones derivatives.

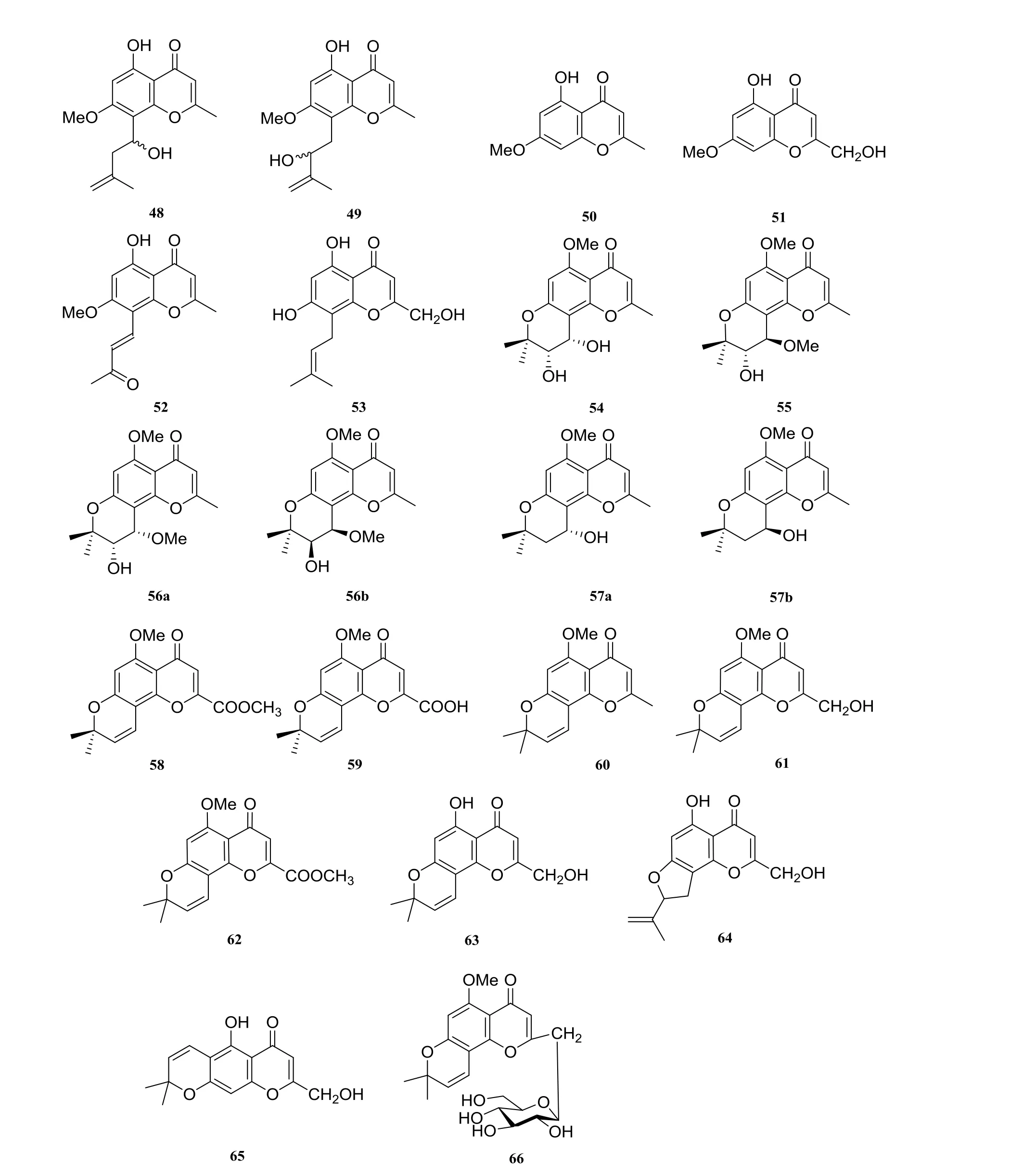
Fig. 3 Chromones in Harrisonia perforata
2.4 Other compounds
Other compounds mainly included gardaubryones C (67) [9], pachymic acid (68) [15],stigmasterol (69), β-sitosterol (70) [1], β-sitosterol methyl ether (71) [9], pinoresinol (72) [15],caryolane-1, 9β-diol (73), β-daucosterol (74) [1],palmitic acid (75), linoleic acid (76) [23] (Fig. 4).
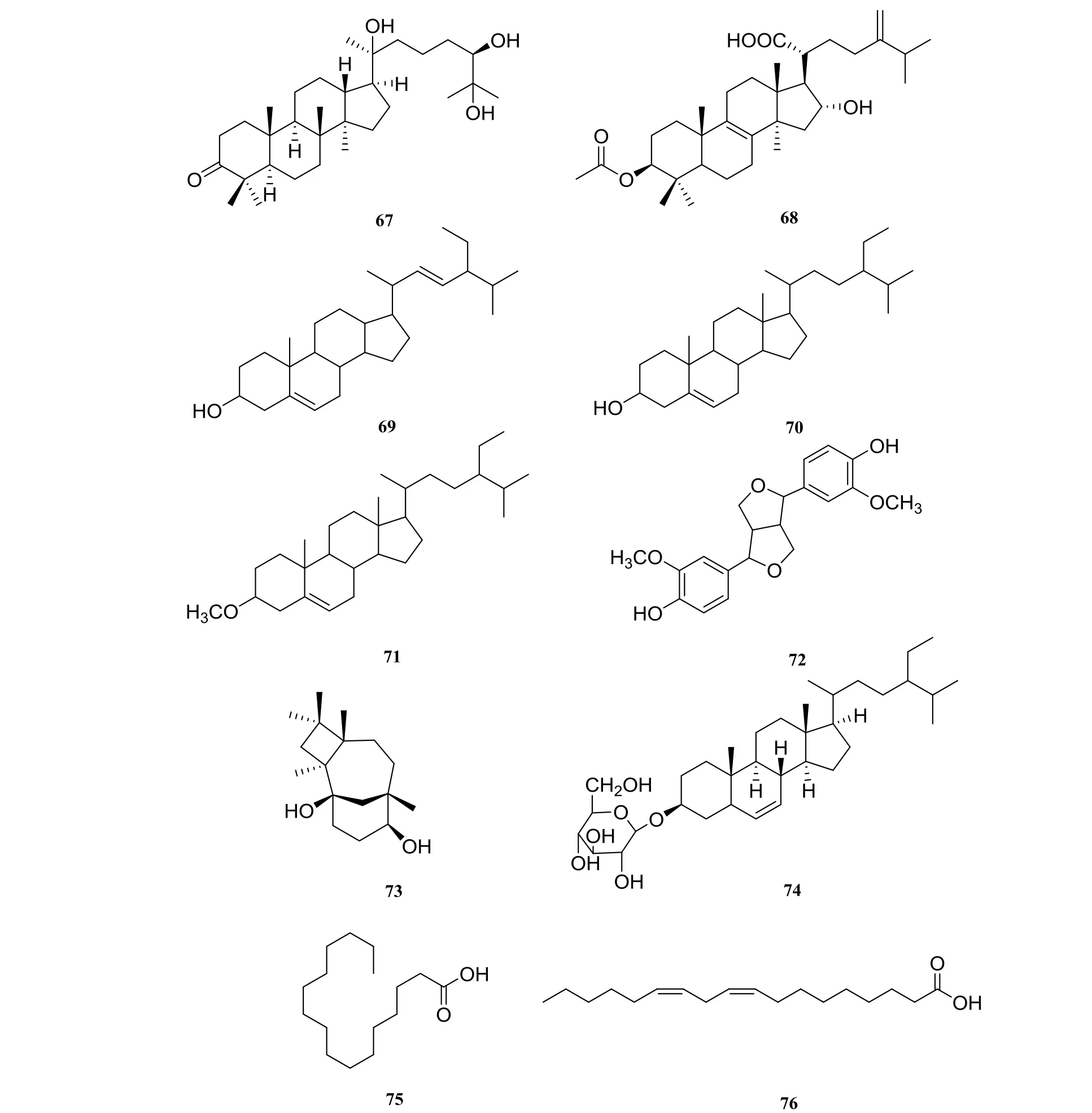
Fig. 4 Others in Harrisonia perforata
3 Studies of pharmacological activities
3.1 Anti-cancer activity
Yin [5] et al. isolated five novel prenylated polyketides, harrisotones A-E and a hydroperoxypolyketide harrisonol A from the stems and leaves of H. perforata. Harrisotones A-C (29-31) and the hydroperoxypolyketide harrisonol A (34) were evaluated for the cytotoxic activities against P-388 and A-549 tumor cell lines, using MTT and SRB methods. The results showed that harrisotone A, C and harrisonol A had significant cytotoxic activities against P-338 cell line, and the IC50values were 1.56,2.35 and 0.27 μM, respectively. Harrisotone A and harrisonol A also showed moderate activities against A-549 tumor cell line with IC50of 24.5 and 26.6 μM,respectively, as pseudolaric acid B was used to be the positive control with IC50values of 0.74 and 1.99 against P-338 and A-549, respectively.
Eight limonoids, isolated from the fruits of H. perforata by Yan [15] et al., were tested the cytotoxicities against the human leukaemia HL60 and human lung adenocarcinoma A-549 cell lines in vitro. Among these compounds, harrpernoid B (22)showed weak activity against HL60 and A-549 cell lines with the inhibition rates of 63.6% and 64.9%,respectively, at the concentration of 10 μM.
It was reported that perforatic acid (59) and heteropeucenin-7-methyl ether (41) extracted from H. perforata could inhibit effectively3H-TdR from permeating hepatoma ascites cells of mouse [19,20].
3.2 Anti-malarial activity
Nguyen-Pouplin [21] et al. investigated traditional medicinal plants used to treat malaria and identified forty-nine plants. 228 extracts from these plants were tested for their activities against Plasmodium falciparum in vitro. The results illuminated that the extracts of H. perforata had inhibitory effect on Plasmodium falciparum with IC50value of 5.1 μg/ml. Perforatin A (60)was isolated from the branches of H. perforata by Tuntiwachwuttikul [7] et al. and discovered that it had weak anti-plasmodial activity against Plasmodium falciparum, and the EC50value was 10.5 μg/ml.
3.3 Anti-inflammatory activity
Choodej [6] et al. isolated two new limonoids and a new chromone together with eight known compounds from fruits and roots of H. perforata.These compounds were evaluated for their antiinflammatory activity by testing the inhibition of NO production in LPS-activated murine macrophage J774. A1 cells. The results revealed that harperfolide(1) expressed the strong activity, with IC50value of 6.51 ± 2.10 μM, compared to control group with IC50of 28.42 ± 3.51 μM. Furthermore, the toxicity of harperfolide was investigated on unstimulated cell lines by measuring the cell viability. The outcome indicated that harperfolide did not showed notable cytotoxicity on macrophage J774. A1 cells. Thus,harperfolide inhibited NO production without causing cell death.
3.4 Antibacterial activity
Four new chromones together with six known compounds were isolated from the branches of H. perforata by Tuntiwachwuttikul [7] et al. The compounds were assessed for antimycobacterial activities against Mycobacterium tuberculosis H37Ra, using isoniazide (MIC of 0.040-0.090 μg/ml)and kanamycin sulfate (MIC of 2.0-5.5 μg/ml)as reference compounds. The results showed that perforamone B, D (48, 63) demonstrated weak antimycobacterial activities, with MIC of 25 μg/ml and 25 μg/ml, respectively.
Limsong [22] et al. studied the inhibiting action on adherence of Streptococcus mutans (S. mutans)ATCC 25175 and TPF-1 with the ethanol extracts of six herbs in vitro. In glass surface adherence assay,H. perforata showed the strongest inhibition for S. mutans ATCC 25175 with adherence of 4.7% to control, and was the strong inhibitor for S. mutans TPF-1 with adherence of 7.6% to control at 0.5%concentration. Subsequently, in bacterial adherence to hydroxyapatite assay, H. perforata expressed less than 50% adherence on S. mutans ATCC 25175.These findings indicated that H. perforata could inhibit the adherence of S. mutans in vitro, and may be effective at controlling dental caries.
3.5 Insecticidal activity
Fang [8] et al. isolated three quassinoids from H. perforata, and perforalactone A, B (27,28) were tested for insecticidal activity against Aphis medicaginis Koch. The result showed that perforalactone B had outstanding activity against Aphis medicaginis Koch, and the LC50values was 7.23 μM, but perforalactone A almost had no activity.In addition, the mechanism of their insecticidal effect was studied with nicotinic acetylcholine receptor (nAChR), a major and ubiquitous target for insecticide action. It showed that perforalactone B was more active than perforalactone A, with the IC50of 1.26 nM and 15.8 nM, respectively.Specially, the IC50of perforalactone B was similar to that of imidacloprid, the most commercially important insecticide. Besides, biochemical electrophysiological tests indicated that they were all antagonists at the nAChR of the insect.
4 Conclusion
Currently, the studies about H. perforata have been developed at home and abroad, but there are not many researches on its chemical components and pharmacological activities. We summarized the chemical constituents and pharmacological properties of H. perforata according to the previous reports, and the effective compounds to treat cancer,malaria, inflammation, etc. were discovered, which is meaningful for enriching the medicinal resources.This paper will be beneficial to the subsequent researches about H. perforata.
[1] Yan XX, Liang ZF, Wang MY, et al. Study on the chemical constituents in the fruit of Harrisonia perforata. Journal of Chinese Medinicinal Materials,2013, 36: 223-225.
[2] Yan XH, Yi P, Cao P, et al. 16-nor Limonoids from Harrisonia perforata as promising selective 11β-HSD1 inhibitors. Sci Rep-UK, 2016, 6: 1-9.
[3] Khuong-Huu Q, Chiaroni A, Riche C, et al. New rearranged limonoids from Harrisonia perforata. J Nat Prod, 2000, 63: 1015-1018.
[4] Lang ZF. Study on the bioactive constitutents from Harrisonia perforata. University of Hai Nan, 2010.
[5] Yin S, Chen X, Su ZS, et al. Harrisotones A–E, five novel prenylated polyketides with a rare spirocyclic skeleton from Harrisonia perforata. Tetrahedron, 2009,65: 1147-1152.
[6] Choodej S, Sommit D, Pudhom K. Rearranged limonoids and chromones from Harrisonia perforata and their antiinflammatory activity. Bioorg Med Chem Lett, 2013, 23:3896-3900.
[7] Tuntiwachwuttikul P, Phansa P, Pootaeng-on Y, et al.Chromones from the Branches of Harrisonia perforata.Chem Pharm Bull, 2006, 54: 44-47.
[8] Fang X, Di YT, Zhang Y, et al. Unprecedented Quassinoids with Promising Biological Activity from Harrisonia perforata. Angew Chem Int Edit, 2015, 127:5684-5687.
[9] Xiao H, Zheng RR, Zhang J, et al. Chemical constituents from the twigs and leaves of Harrisonia perforata. Acta Pharmaceutica Sinica, 2015, 12: 1622-1624.
[10] Lv C, Tu Q, Gong JX, et al. Asymmetric total synthesis of (-)-perforanoid A. Tetrahedron, 2017, 73: 3612-3621.
[11] Sung TV, Phuong NM, Kamperdick C, et al.Perforatinolone: a limonoid from Harrisonia perforata.Phytochemistry, 1995, 38: 213-215.
[12] Tri MV, Sargent M, Phuong NM, et al. Perforatin:a novel tetranortriterpenoid from Harrisonia perforata.Aust J Chem, 1991, 44: 165-169.
[13] Chiaroni A, Riche C, Khuong-Huu Q, et al. New limonoids from Harrisonia perforata (Blanco) Merr.Acta Crystallogr C, 2000, 31: 711-713.
[14] Khuong-Huu Q, Chiaroni A, Riche C, et al. New rearranged limonoids from Harrisonia perforata. III. J Nat Prod, 2001, 64: 634-637.
[15] Yan XH, Di YT, Fang Xin, et al. Chemical constituents from fruits of Harrisonia perforata. Phytochemistry,2011, 72: 508-513.
[16] Wang MX, Zhang MS, Zhu YL. Study on the chemical constituents of a chinese folk medicine niu-jin-guo(Harrisonia perforata Blano Merr). Acta Pharmaceutica Sinica, 1983, 18: 113-118.
[17] Tanaka T, Koike K, Mitsunaha K, et al. Chromones from Harrisonia perforata. Phytochemistry, 1995, 40:1787-1790.
[18] Liu G, Zheng RR, Liu ZW, et al. Enantiomeric chromones from Harrisonia perforata. Phytochem Lett,2014, 10: 295-299.
[19] Wang MX, Zhong MS, Liu WZ, et al. Isolation and structural determination of perforatic acid from chinese medicine niu-jin-guo (Harrisonia perforata). Acta Pharmaceutica Sinica, 1984, 19: 760-763.
[20] Wei XC, Gu XC, Jin XL, et al. The molecular and crystal structure of niu-jin I. Journal of peking university, 1986,4: 15-19.
[21] Nguyen-Pouplin J, Tran H, Tran H, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol, 2007, 109: 417-427.
[22] Limsong J, Benjavongkulchai E., Kuvatanasuchati J.Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J Ethnopharmacol, 2004, 92:281-289.
[23] Liang ZF, Wang ZN, Wang MY, et al. GC-MS analysis of the liposoluble components fruits of Harrisonia perforata. Journal of Chinese Medicinal Materials,2009, 32: 1697-1700.
杂志排行
Asian Journal of Traditional Medicines的其它文章
- Absolute configuration of curdione and its three isomers by NMR, ECD and DFT calculations: an insight into the scope of unsaturated ketone helicity rule based on an ECD study
- Exploring the active ingredients, potential targets and pathways of quassinoids in Simaroubaceae plants by network pharmacology approachs
- Chemical constituents of the genus Pithecellobium:a systematic review
- Chemical constituents and pharmacological effects of the fruits of Camptotheca acuminata: a review of its phytochemistry
- Studies on the Chemical Components and Biological Activities of Stellera chamaejasme L.
