手性稀土配合物在不对称催化应用中的研究进展
2018-01-11丑亚杰程探宇林静容
丑亚杰, 程探宇, 林静容
(上海师范大学 生命与环境科学学院,上海 200234)
手性稀土配合物在不对称催化应用中的研究进展
丑亚杰, 程探宇, 林静容*
(上海师范大学 生命与环境科学学院,上海 200234)
近年来随着光学纯化合物需求的不断增大,不对称催化逐渐成为科学研究的前沿领域之一.稀土元素则是由周期表中IIIB族Sc、Y和镧系元素(La~Lu)的17种元素组成.相比其他过渡金属与各类配体轨道间的作用,稀土金属有其独特之处,因此,近年来手性稀土配合物在不对称催化领域的应用,也逐渐引起人们的重视.根据各类手性配体中所含配位原子的不同,对手性稀土配合物的类型及相关的不对称催化反应作简要综述.
稀土元素; 镧系金属; 手性; 不对称催化
0 引 言
近年来金属不对称催化已成为一个巨大的研究领域,其范围随着有机催化的发展也在迅速扩大.对于过渡金属作路易斯酸进行不对称催化的反应已有大量研究,但是对于使用诸如Sc、Y和La等稀土元素作为路易斯酸进行催化,发展较为缓慢.Bednarski等[1]在1983年报道了第一例手性镧系元素络合物作为路易斯酸催化手性hetero-Diels-Alder反应,获得33%的对映选择性(ee值).此后,稀土元素配合物催化剂在Shibasaki等[2]的研究下,取得了飞跃性的发展.手性镧系元素络合物能够作为不对称合成中的新催化剂与其独特的化学和物理性质有关.镧系元素离子通常被认为是对氧原子具有高亲和力的硬路易斯酸.4f轨道是镧系元素的核心轨道,电子从La3+(4f0)到Lu3+(4f14)依次填充,但该轨道对配合物的键合作用没有贡献.因此,镧系金属-配体键合主要是静电,从而得到一些不规则配位几何形状的复合物.Lu3+的离子半径较大,其配位数比通常的d-过渡金属更高,出现8,9或10的配位数也很常见.这些性质对于稀土金属结合各种手性配体是非常有利的,并且可以构建一些结构复杂的复合物,从而有效地控制反应的立体化学.本文作者便根据配体中配位原子的不同,对手性稀土配合物的类型及催化的相关反应作简要综述.
1 含O给体的手性稀土金属配合物
1.1 β-二酮类配合物
β-二酮类镧系配合物,早在20世纪初就开始被关注,并且被Whitesides等[3-6]用于核磁共振位移试剂的研究.最早应用于立体选择性反应的斓系配合物,也是β-二酮类镧系配合物[1].随后优化反应条件(-10 ℃,无溶剂),仅用摩尔分数为1%的催化剂,将相应的环加成产物ee值提高至58%[7].也有使用铕的络合物[Eu(tfc)3]来催化不对称Michael加成反应的例子[8],反应中催化剂用量较高,但所获得的ee值并不是很理想.因此,之后该类配体主要被用于新型镧系发光配合物中[9-10].
通过L-甲氧基取代的二酮配体L1与Gd制备的手性配合物,可以催化酮的不对称硼氢化还原反应(图1),得到了较好的ee值[11].
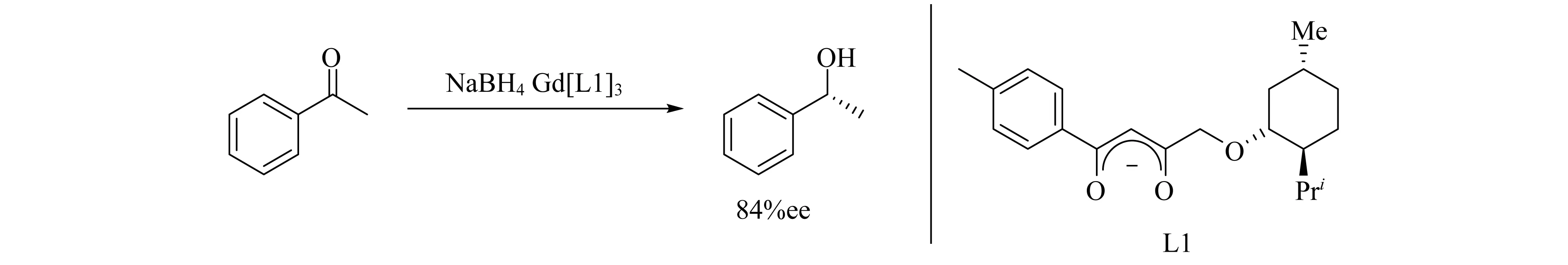
图1 Gd[L1]3催化酮的硼氢化还原反应
1,3-双(2-甲基二茂铁基)丙烷-1,3-二酮(HBMPD)与Y(OiPr)3配制得到二酮络合物,可以作为醛氰化反应的活性催化剂.研究初期其活性并不高,Abiko团队[12]通过筛选条件,发现BMPD-Y络合物对该反应具有很好的活性(图2).催化剂减少至摩尔分数为0.2%时,也可以得到产率95%,ee值87%的产物[13].
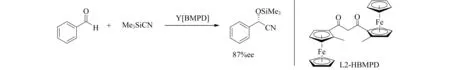
图2 Y[BMPD]催化醛的不对称氰化反应
1.2 醇盐配合物
合成手性稀土醇盐催化剂的一种方法是LnCl3( Ln为镧系金属)和碱金属醇盐之间的盐交换反应.该方法得到了不饱和酮烯烃不对称环氧化的有效催化剂:[Li2Cl] [Yb(L)2][14].
稀土醇盐与TMSCN(氰基三甲基硅烷)进行交换反应可以形成稀土氰化络合物,催化环氧乙烷的氰化,产率可达到99%[15].手性稀土醇盐便开始被用作不对称氰化反应的催化剂前体.Shibasaki团队[16]随后发现了一种氧化膦官能化二醇H2L3,其与Gd(OiPr)3反应形成Gd2[L3]3复合物,结构如图3所示.衍生自Gd2[L3]3的催化剂也已经用于酮[16-18]、亚胺[19-22]的对映选择性氰化反应和氮丙啶[23-24]的开环反应,获得了较高的活性和对映选择性.
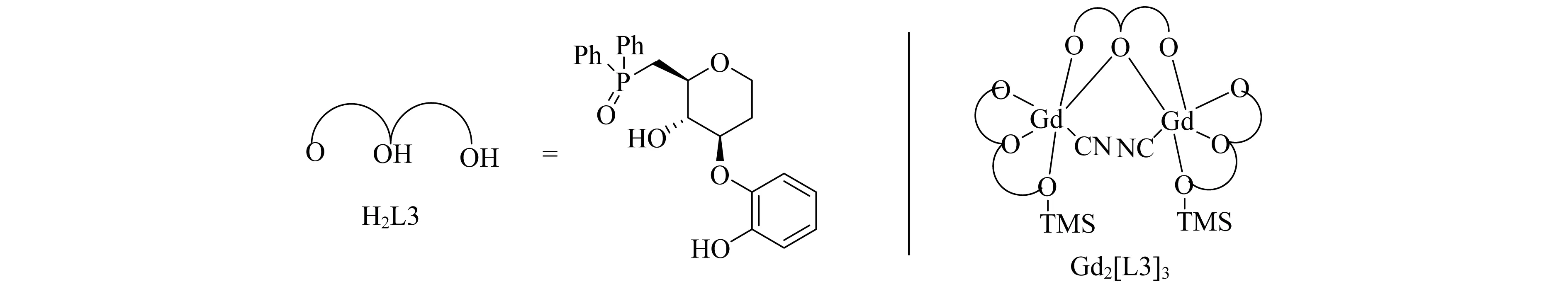
图3 Gd2[L3]3复合物
Evans团队[25]发现手性稀土醇盐可以实现酮的不对称还原反应(图4),通过催化剂的筛选发现SmI3[L4]可以得到较高的ee值.另外,Sm的醇盐可以催化Mukaiyama-aldol反应,但ee值较低,即使在2当量的催化剂时也只获得30%的ee值,产率仅为16%.在使用0.2当量的催化剂时获得了92%的产率,但此时,ee值却降低到了2%[26].
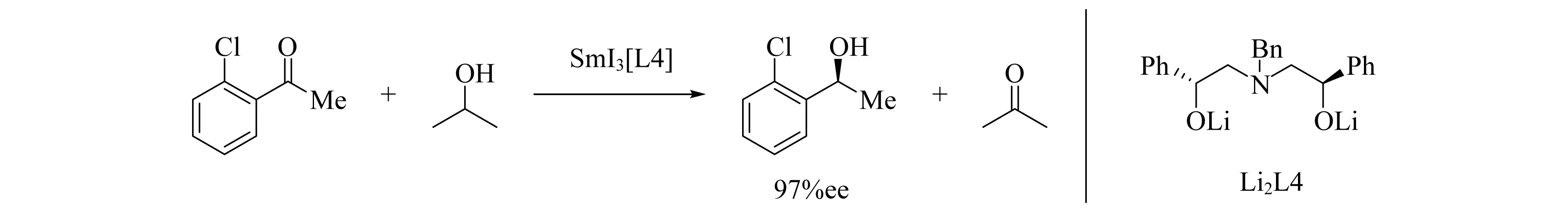
图4 SmI3[L4]催化酮的还原反应
1.3 联萘酚配合物
1.3.1 单金属联萘酚配合物
稀土醇盐Ln(OiPr)3和Ln(OtBu)3在商业上很容易获得的,并且是理想的合成手性醇盐的前体.早期,通过Ln(OiPr)3与1当量联萘酚制备的催化剂,催化烯酮的对映选择性环氧化获得了不错的ee值(94%)[27-29].随后,Shibasaki等[30-33]开发了高效不对称环氧化反应催化剂,如图5所示.其中,中性配体(Ph3As=O或Ph3P=O)是必不可少的,其作用可能是为了防止催化剂低聚.该反应的结果(特别是产率)强烈依赖于镧系金属的选择,通过金属的筛选,元素Y复合物得到了最好的结果,而Sc复合物则基本无活性[34].通过La(OiPr)3与联萘酚反应制备的催化剂也被用于催化酮的硼烷还原,得到61.8%的ee值[35].

图5 Y(OiPr)3[L5]催化烯烃不对称环氧化
Kobayashi[36-37]发现通过Yb(OTf)3与联萘酚反应获得的催化剂成功实现了Diels-Alder反应的不对称催化,并获得较好的ee值.Sm的联二萘酚碘化物络合物也被用于催化Diels-Alder反应[38].当使用催化剂Sm[L6]时,在-25 ℃下达到最高28%的ee值.在使用3和3′位置具有甲氧基苯基取代基的催化剂Sm[L7]时,在-28 ℃下得到81%的ee值(图6).再将温度降至-60 ℃时,观察到ee值的反转,得到30%的ee值[39].和联萘酚类似的联萘胺衍生配体也可以用于不对称D-A反应[40-41].

图6 Sm[L6]与Sm[L7]催化Diels-Alder反应
在联萘酚的3,3′位上连接手性噁唑啉基团形成新型的四齿配体,即BINOL-Box.该配体与稀土元素得到的催化剂对于图7中所示的1,3-偶极加成反应可以得到87%的ee值[42-44].

图7 Ln(OTf)3[L8]催化1,3-偶极加成反应
1.3.2 多金属联萘酚配合物

图8 多金属联萘酚配合物M3 [RE(binol)3]
20世纪90年代初,Shibasaki团队[45-46]发现了含稀土元素的多金属催化剂 Ln-M3-三(1,1′-双-2-萘酚)(M为第ⅠA族金属,碱金属),结构如图8所示.Ln的高配位数允许构建由一个稀土元素、三个碱金属和三个联萘酚组成的多金属配合物.RE是路易斯酸,二元配体则是布朗斯特碱,所以M3 [RE(binol)3]可以作为双功能催化剂.并且该催化剂修饰的潜力是巨大的:Ln 可以是任何一种稀土元素,M可以是Li、Na或K.通过改变一系列RE和M 的组合,可以获得具有不同O-金属键长度,离子半径和路易斯酸度的各种[RE(binol)3]络合物.该类催化剂的第一个复合物是Li3[La(binol)3],其首先被应用于催化硝基甲烷的不对称硝基Aldol反应并得到很好的ee值[47].随后发现加入催化量的BuLi或其他碱会得到更高的转化率[48-53].
催化不对称Aldol缩合反应时(图9),其中催化剂可以使供体烯醇化后,再对受体进行对映选择性进攻[54-56].这种催化方法,为Aldol缩合反应碳-碳键形成过程提供了简便的方法,并且避免了使用其他预烯醇化的试剂.实际上,这一发现引发了随后对于金属催化Aldol反应的广泛研究[57].在所用的各种RE中,发现La在大多数反应中可以得到很好的反应结果.

图9 Li3[La(R-binol)3(H2O)]催化Aldol缩合反应
丙二酸酯与环状不饱和酮的迈克尔加成如图10所示,该反应不仅对Ln有很大的依赖性,而且对碱金属的性质也有很大的依赖性,Li得到非常差的ee值,而Na却可以获得最好的ee值[58-60].虽然催化中间体的确切结构尚未确定,但有研究表明,脱质子化的丙二酸酯与手性催化剂配位,才能实现高对映体选择性[61].该催化剂同样可以实现查尔酮的不对称Michael加成[62],当Ln=Y或Dy时,产率和对映选择性最好;Tm,Yb和Lu的反应效率较差,可能因为Ln金属中心太小,无法有效地与查尔酮配位.
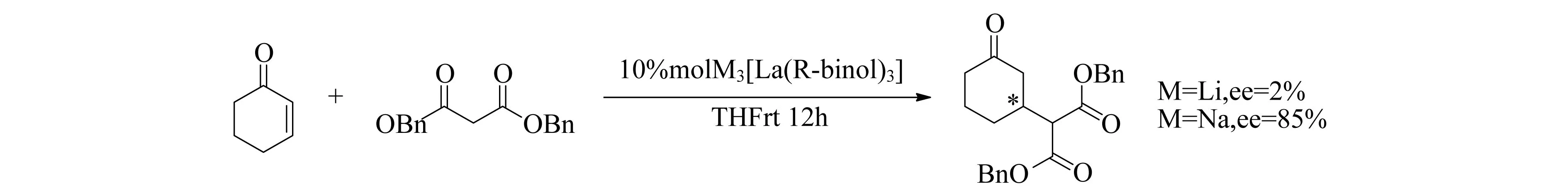
图10 M3[La(R-binol)3]催化Michael加成反应
催化剂Na3[La(R-binol)3]成功实现烯酮的不对称环氧化反应,得到83%的ee值,但该催化剂底物适应性较差[63-65].在非手性氧化膦添加剂的存在下,催化剂摩尔分数仅为1%~5%便可获得很好地ee值(91%~97%),同时达到 88%~99%的产率.乙基酮和丙基酮则得到略低的ee值(67%~88%),这可能是空间位阻的原因[66].
[La(R-binol)3]和Li形成的催化剂在醛的氢化膦酰化催化中获得较好的催化活性[67],而在亚胺的氢化膦酰化过程中,K获得了最佳结果[68](图11).醛的不对称氢化膦酰化反应也可以由Li2Binol与LaCl3的反应产生的络合物[69]或3,3′-双(甲氧基乙基)联萘酚与NaOBut和LaCl3反应得到的络合物催化.
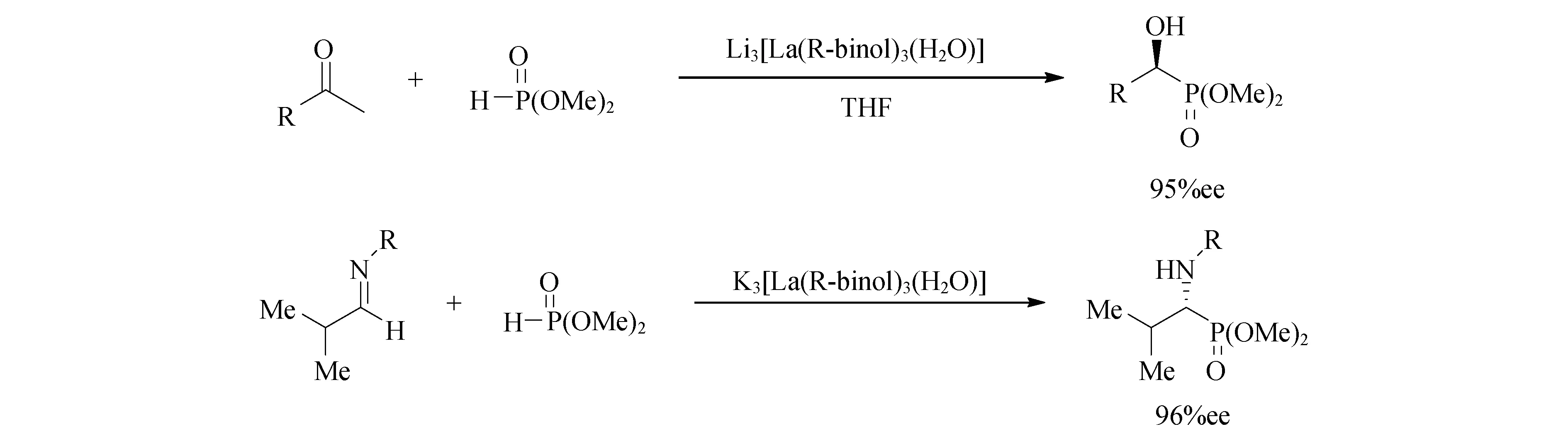
图11 M3[La(R-binol)3]催化醛/亚胺的氢化膦酰化反应
2 含N给体的手性稀土金属配合物
2.1 双噁唑啉配合物(BOX)
双噁唑啉配体可以与镧系元素配位形成手性催化剂,Tian等[70]利用这种催化剂催化氨基烯烃的分子内胺化反应(图12),得到了中等ee值(67%),但是对于1,3偶极环加成[71]和Michael加成[72]均得到消旋产物.
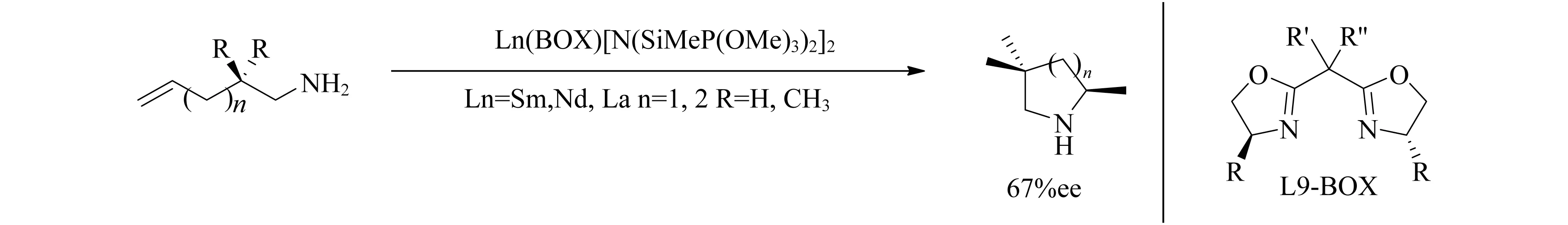
图12 Ln(BOX)[N(SiMe3)2]2催化氨基烯烃分子内胺化反应
2.2 吡啶双噁唑啉配合物(pybox)
1989年Nishiyama等[73]首次介绍了pybox配体,pybox是一种三齿N型配体,吡啶和亚胺的N原子都是强供电体,并且配体与金属结合后会形成刚性平面结构,该系列配体结合各种稀土元素,可以得到多种催化剂,现已广泛用于镧系元素的催化应用.
Fukuzawa最初发现使用镧系元素pybox复合物可以催化Diels-Alder反应[74],并且用[Sc(iPr-pybox)(OTf)3]获得了82%的ee值.随后使用(S)-TIPSOCH2-pybox Sc催化剂又得到了93%的ee值[75].(R,R)-Ph4-pybox Yb[76],和(R)-iPr-pybox Sc[77],也能得到84%的ee值.该反应中较大的镧系元素(Sm,La和Yb)具有较低的对映体选择性,并且产物都以内型产物为主.值得注意的是,使用配体iPr-pybox时,分子筛的存在可以提高对映选择性,而对于Ph-pybox配体,分子筛则降低对映体选择性.
Schaus等[78]报道了由LnCl3·6H2O/pybox催化环氧化物的开环氰化反应.筛选了几种水合的LnCl3,发现ee值随着Ln3+半径的减小而增加.还筛选了几个pybox配体(tBu-,iPr-,Ph-,Bn-);(S)-Ph-pybox获得最佳的ee(91%值).而LnCl3·6H2O-Ph2-pybox催化剂也可以应用于腙的氰化反应[79].在这个反应中,前面的元素Ln(La,Ce,Pr)活性非常差,Er获得很好ee值后,从Er到Lu,ee值又开始快速下降.
Desimoni等[80-81]研究了pybox配体和Ln对不对称Mukaiyama-Michael加成反应的对映选择性影响.对于大多数反应,使用Sc或Yb得到最佳对映选择性,但该反应中金属La和Ce是最有效的催化剂,如图13所示.这表明对于特定反应优化Ln和配体是很重要的.
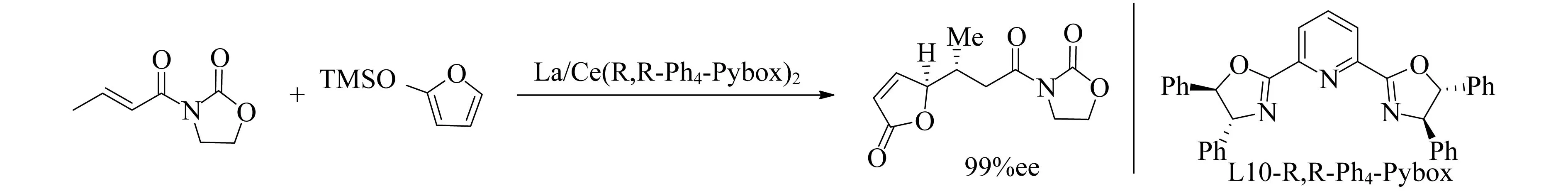
图13 La/Ce(R,R-Ph4-Pybox)2催化Mukaiyama-Michael加成反应
Pybox衍生配体(R)-inda-pybox与Yb配合物高效地催化了β-酮酸的脱羧加成反应(图14),获得了一系列有生物价值的3-羟基吲哚,产率高达98%,最高ee值达了99%[82].
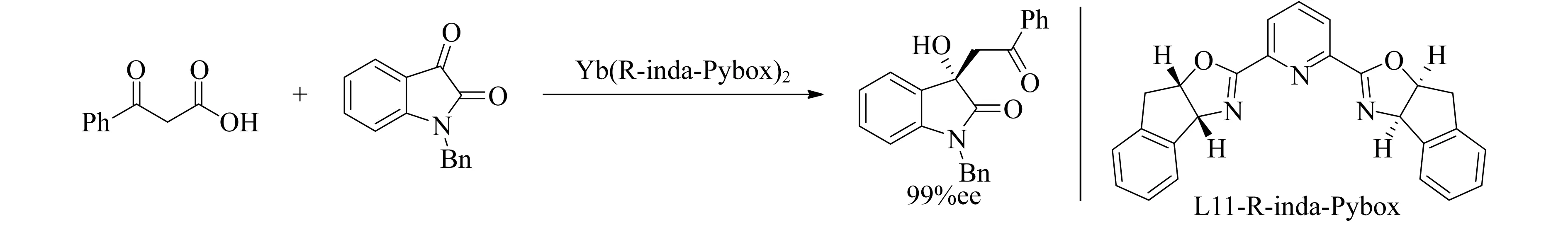
图14 Yb(R-inda-Pybox)2催化β-酮酸的脱羧加成反应
3 含N/O混合给体的手性稀土金属配合物
3.1 含O Schiff碱配合物

图15 含O Schiff碱体L12
稀土金属与Schiff碱配体的广泛配位具有几乎无限的修饰潜力.Fukuzawa等[83]筛选了几种Schiff碱二醇基手性配体与Sc(OTf)形成配合物(图15)催化Diels-Alder反应,配体L12获得85%的ee值.
双金属催化的另一种方法是设计具有两个不同结合位点的双核配体.较小的结合位点具有N2O2的供电子体,其将有利于较小的金属离子如d-过渡金属,而O4供电子体将有利于Ln离子.这些双金属催化剂已经证明了具有一些独特的反应性:[(L13)Cu/Sm]是第一个催化顺式选择性硝基-Mannich反应的催化剂(图16[84-87]).[(L13)Pd/Sm]催化硝基Aldol反应也得到了84%的ee值[88-89].

图16 Cu/Sm(OAr)[L13]催化硝基-Mannich反应
稀土元素与二烷基胺(R2N-)配体的配位化学很普遍,二烷基胺是比相应的醇盐更强的布朗斯特碱,并且它们在空间上要求更高,导致Ln(NR2)3通常具有更简单的结构.RajanBabu团队通过H2L13与Y[N(SiHMe2)2]3]的反应制备得到催化剂,在环氧化物的开环反应中显示出很好的活性,催化剂负载量摩尔分数仅为0.01%(图17)[90].RajanBabu团队[91]后来又将Y[L13][N(SiHMe2)2]应用于更具挑战性的氮丙啶开环的催化,并获得88%的ee值.
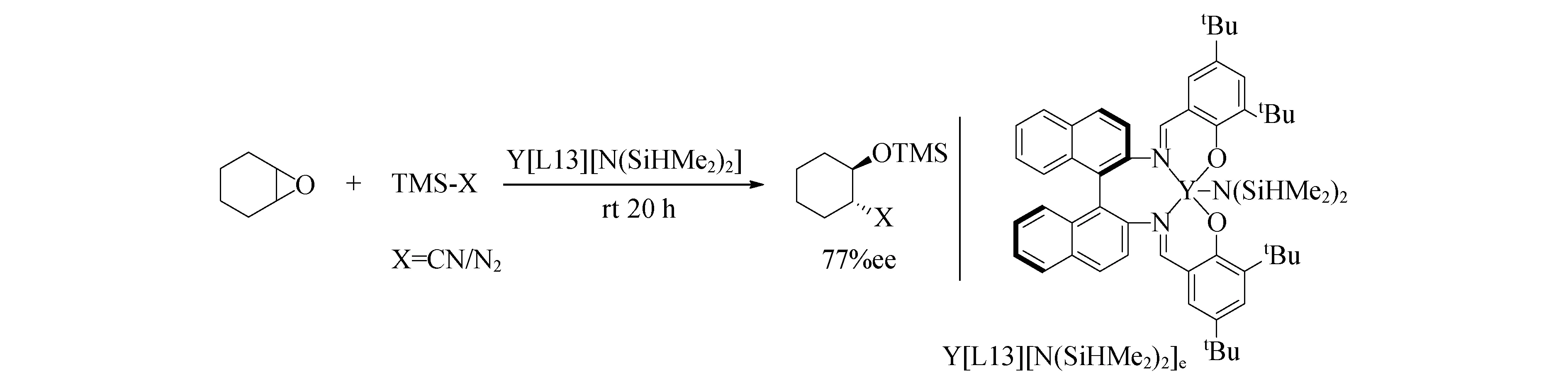
图17 Y[L13][N(SiHMe2)2]催化环氧化物的开环反应
3.2 N,N′-二氧配体
C2轴对称的N,N′-二氧化物很容易从氨基酸合成,并且获得各种的结构修饰类似物.N-氧化物官能团是稀土离子的良好供体,2003年,手性双喹啉N,N′-二氧化物配体(L14)与稀土三氟甲磺酸盐Sc(OTf)3结合使用催化不对称Michael加成反应获得良好的产率和ee值[92](图18).Feng团队[93-96]也已经成功地应用[Ln(OTf)3(N,N′-二氧化物)]催化了各种不对称反应.
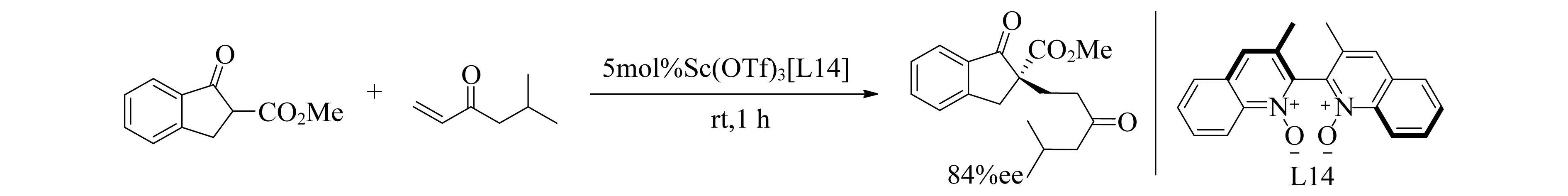
图18 Sc(OTf)3[L14]催化Michael加成反应
将Sc(OTf)3与配体L15结合,催化Aldol反应(图19)获得了高产率和高ee值[97].还发现该反应中加入3Å分子筛会增加反应速率,但对对映体选择性没有影响.
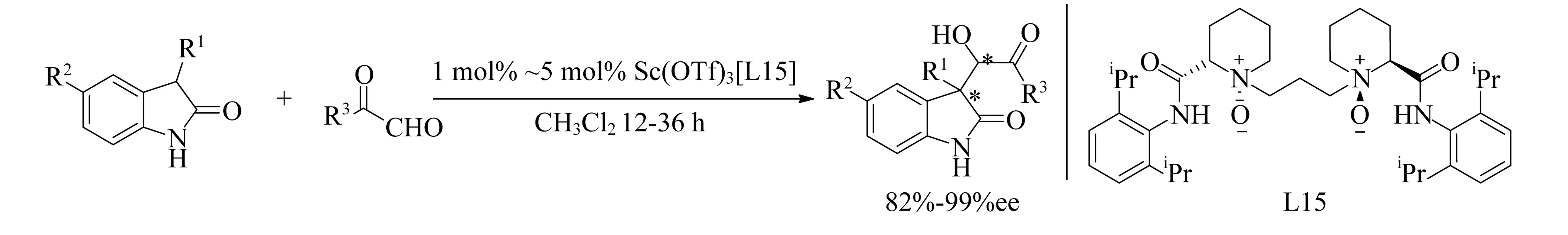
图19 Sc(OTf)3[L15]催化Aldol反应
在硫代乙醇酸酯与查尔酮的Michael加成中,当Ln=La(最大的稀土金属)时获得最高ee值,而较小的金属Sc,Yb和Y获得很低的ee值(分别为12%、4%和10%ee)[98].而在另一个Michael加成中(图20),Sc(OTf)3[L16]获得的对映选择性与Y(OTf)3[L16]相反[99].并且,La、Sm、Gd、Dy、Er、Yb和Y均具有相同的高ee值.同时发现乙醇在Sc(OTf)3催化中对于实现高ee有重要作用.
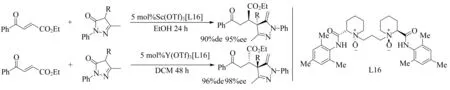
图20 Sc(OTf)3[L16]与Y(OTf)3[L16]催化Michael加成反应
3.3 大环N/O配合物
已经有报道使用大环化合物与Ln(OTf)3的复合物催化Aldol反应并获得较高的ee值(图21)[100].该反应的对映体选择性随着镧系元素半径的减小而减小:用[Ce(OTf)3]获得最佳结果.该大环配体L17的空腔尺寸应该与排在前面的镧系金属离子Ln3+半径有很好匹配,这也可能是ee值随半径减少的原因,随后的研究包括测定配体结合常数证实了这一假设[101].配体L18在环中具有N2O2供电子体,且N原子上连有手性基团.该配体对于Ln3+离子太小,亲和力较低,所以不能共面结合.为了使Ln3+结合充分,需要2当量的配体L17[102-104].

图21 Ce(OTf)3[L17]与Nd(OTf)3[L18]催化Aldol反应
4 含P给体的手性稀土金属配合物
衍生自联萘酚的手性膦酸酯配体与稀土离子形成稳定的络合物.这些配合物即使在室温下也显示出良好的活性和对映选择性.如图22所示,在催化hetero-Diels-Alder反应时添加2,6-二甲基吡啶后得到89%ee值[105].该类催化剂也可催化查尔酮Michael加成,在该反应中,Sc获得最好的ee值69%,并且明显优于Yb[106].

图22 Yb[L19]催化hetero-Diels-Alder反应
5 环戊二烯基配合物
由于Ln3+的大尺寸以及镧系双(环戊二烯基)复合物的非刚性结构,合成具有明确手性结合位点的此类复合物是一个挑战.1992年,Marks团队[107]首先使用烷基络合物L20催化烯烃氢化(图23).催化过程中的对映选择性步骤推测是烯烃不可逆地嵌入到了Ln-H键中.
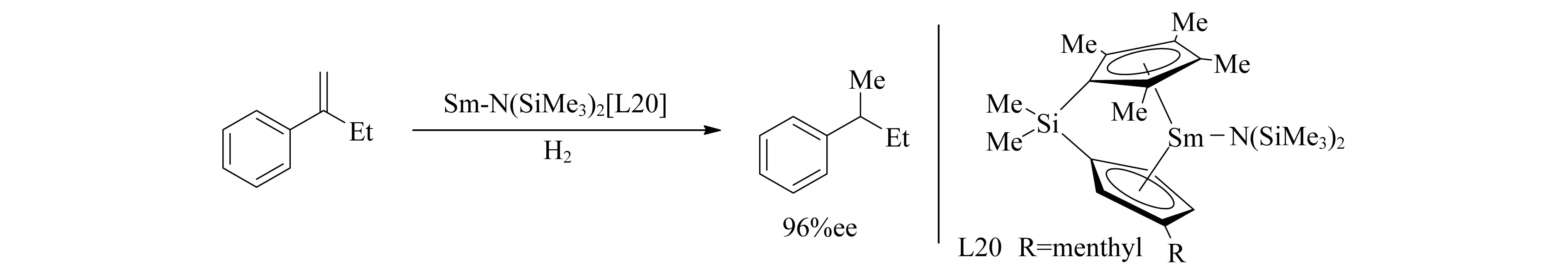
图23 Sm-N(SiMe3)2[L20]催化烯烃氢化反应
CH(SiMe3)2或N(SiMe3)2的络合物可用作对映选择性氢胺化/环化反应的催化中[108].CH(SiMe3)2或N(SiMe3)2可以被底物的氨基通过质子分解快速置换,该过程会加速整个反应.然而产物的绝对构型对催化剂的手性结构几乎不敏感,而仅仅取决于手性取代基R 的手性结构[109].该类催化剂也可以催化烯烃硅烷化反应,并且得到了68%的ee值[110].
6 展 望
本文作者简要综述了各类手性稀土配合物在不对称催化中的应用.已经认识到Ln镧系金属的半径较大,由于配位层的流动性,从而想要得到足够刚性的结构产生高对映选择性,是一个很大的挑战.可以看到联萘酚、pybox尤其是Shibasaki的多金属M3[RE(binol)3]催化剂已经取得了很大的成功.中国是稀土资源大国,可以预见在今后的发展中,稀土金属在不对称催化中的应用还会不断深入.
[1] Bednarski M,Danishefsky S.Mild lewis acid catalysis:eu(fod)3-mediated hetero-diels-alder reaction [J].Journal of the American Chemical Society,1983,105:3716-3717.
[2] Shibasaki M,Sasai H,Arai T.Asymmetric catalysis with heterobimetallic compounds [J].Angewandte Chemie International Edition,1997,36:1236-1256.
[3] Whitesides G M,Lewis D W.Tris [b(tert-butylhydroxy methylene)-d-camphorato] europium(iii).a reagent for determining enantiomeric purity [J].Journal of the American Chemical Society,1970,92:6979-6980.
[4] Whitesides G M,Lewis D W.The determination of enantiomeric purity using chiral lanthanide shift reagents [J].Journal of the American Chemical Society,1971,93:5914-5916.
[5] Goering H L,Eikenberry J N,Koermer G S.Tris[3-(trifluoromethyl hydroxymethylene) dcamphorato] europium(iii).a chiral shift reagent for direct determination of enantiomeric compositions [J].Journal of the American Chemical Society,1971,93:5913-5914.
[6] Mccreary M D,Lewis D W,Wernick D L,et al.The determination of enantiomeric purity using chiral lanthanide shift reagents [J].Journal of the American Chemical Society,1974,96:1038-1054.
[7] Bernarski M,Maring C,Danishefsky S,et al.Chiral induction in the cyclocondensation of aldehydes with siloxydienes [J].Tetrahedron Letters,1983,24:3451-3454.
[8] Bonadies F,Lattanzi A,Orelli L R,et al.Lanthanides in organic synthesis:eu3+-catalyzed michael addition of 1,3=dicarbonyl compounds [J].Tetrahedron Letters,1993,34:7649-7650.
[9] Sun O,Gao T,Sun J W,et al.A series of lanthanide(III) complexes constructed from Schiff base and β-diketonate ligands [J].CrystEngComm,2014,16:10460-10468.
[10] George T M,Krishna M S,Reddy M L P.Alysosome targetable luminescent bioprobe based on a europium β-diketonate complex for cellular imaging applications [J].Dalton Transactions,2016,45:18719-18729.
[11] Okawa H,Tokunaga H,Katsuki T,et al.Noncovalent interactions in metal complexes.16.Stereoselectivity of 1:3 complexes of Sc,Y,La,Al,Ga,and In ions with 4-(l-menthyloxy)-1-phenyl-1,3-butanedione and 4-(l-menthyloxy)-1-p-tolyl-1,3-butanedione [J].Inorganic Chemistry,1988,27:4373-4377.
[12] Abiko A,Wang G Q.A BMPD-Yttrium Isopropoxide Complex:Highly Efficient Chiral Lewis Acid Catalyst for Asymmetric Silylcyanation [J].The Journal of Organic Chemistry,1996,61:2264-2265.
[13] Abiko A,Wang G Q.BMPD,a Novel C2-Chiral 1,3-Diketone Ligand;Synthesis and Application to an Asymmetric Catalytic Reaction1 [J].Tetrahedron,1998,54:11405-11420.
[14] Qian Q Q,Tan Y F,Zhao B,et al.Asymmetric Epoxidation of Unsaturated Ketones Catalyzed by Heterobimetallic Rare Earth-Lithium Complexes Bearing PhenoxyFunctionalized Chiral Diphenylprolinolate Ligand [J].Organic Letters,2014,16:4516-4519.
[15] Matsubara S,Onishi H,Utimoto K.Reaction of cyanotrimethylsilane with oxiranes under Yb(CN)3catalysis [J].Tetrahedron Letters,1990,31:6209-6212.
[16] Yabu K,Masumoto S,Yamasaki S,et al.Switching Enantiofacial Selectivities Using One Chiral Source:Catalytic Enantioselective Synthesis of the Key Intermediate for (20S)-Camptothecin Family by (S)-Selective Cyanosilylation of Ketones [J].Journal of the American Chemical Society,2001,123:9908-9909.
[17] Masumoto S,Suzuki M,Kanai M,et al.A practical synthesis of (S)-oxybutynin [J].Tetrahedron Letters,2002,43:8647-8651.
[18] Yabu K,Masumoto S,Kanai M,et al.Studies toward practical synthesis of (20S)-camptothecin family through catalytic enantioselective cyanosilylation of ketones:improved catalyst efficiency by ligand-tuning [J].Tetrahedron Letters,2002,43:2923-2926.
[19] Kato N,Mita T,Kanai M,et al.Assembly State of Catalytic Modules as Chiral Switches in Asymmetric Strecker Amino Acid Synthesis [J].Journal of the American Chemical Society,2006,128:6768-6769.
[20] Masumoto S,Usuda H,Suzuki M,et al.Catalytic Enantioselective Strecker Reaction of Ketoimines [J].Journal of the American Chemical Society,2003,125:5634-5635.
[21] Kato N,Suzuki M,Kanai M,et al.General and practical catalytic enantioselective Strecker reaction of ketoimines:significant improvement through catalyst tuning by protic additives [J].Tetrahedron Letters,2004,45:3147-3151.
[22] Kato N,Suzuki M,Kanai M,et al.Catalytic enantioselective Strecker reaction of ketoimines using catalytic amount of TMSCN and stoichiometric amount of HCN [J].Tetrahedron Letters,2004,45:3153-3155.
[23] Mita T,Fujimori I,Wada R,et al.Catalytic Enantioselective Desymmetrization of meso-N-Acylaziridines with TMSCN [J].Journal of the American Chemical Society,2005,127:11252-11253.
[24] Fujimori I,Mita T,Maki K,et al.Key Role of the Lewis Base Position in Asymmetric Bifunctional Catalysis:Design and Evaluation of a New Ligand for Chiral Polymetallic Catalysts [J].Journal of the American Chemical Society,2006,128:16438-16439.
[25] Evans D A,Nelson S G,Gagne M R,et al.A Chiral Samarium-Based Catalyst for the Asymmetric Meenvein-Ponndorf-Verley Reduction [J].Journal of the American Chemical Society,1993,115:9800-9801.
[26] Makioka Y,Nakagawa I,Taniguch Y,et al.Lanthanoid(I1) or-(III) Alkoxide-Promoted Reactions of Silyl Ketene Acetals with Aldehydes [J].The Journal of Organic Chemistry,1993,58:4771-4774.
[27] Bougauchi M,Watanabe S,Arai T,et al.Catalytic Asymmetric Epoxidation of αβ-Unsaturated Ketones Promoted by Lanthanoid Complexes [J].Journal of the American Chemical Society,1997,119:2329-2330.
[28] Chen R F,Qian C T,Vries J G.Highly efficient enantioselective epoxidation of α,β-enones catalyzed by cheap chiral lanthanum and gadolinium alkoxides [J].Tetrahedron,2001,57:9837-9842.
[29] Chen R F,Qian C T,Vries J G.Asymmetric epoxidation ofα,β-unsaturated ketones catalyzed by chiral ytterbium complexes [J].Tetrahedron Letters,2001,42:6919-6921.
[30] Hara K,Park S Y,Yamagiwa N,et al.Catalytic Asymmetric Epoxidation of a,b-Unsaturated Phosphane Oxides with a Y(O-iPr)3/Biphenyldiol Complex [J].Chemistry-An Asian Journal,2008,3:1500-1504.
[31] Kakei H,Tsuji R,Ohshima T,et al.Catalytic Asymmetric Epoxidation of α,β-Unsaturated Esters Using an Yttrium-Biphenyldiol Complex [J].Journal of the American Chemical Society,2005,127:8962-8963.
[32] Ohshima T,Nemoto T,Tosaki S,et al.Catalytic asymmetric epoxidation of a,b-unsaturated carboxylic acid imidazolides and amides by lanthanide-BINOL complexes [J].Tetrahedron,2003,59:10485-10497.
[33] Tosaki S,Horiuchi Y,Nemoto T,et al.Strategy for Enantio-and Diastereoselective Syntheses of All Possible Stereoisomers of 1,3-Polyol Arrays Based on a Highly Catalyst Controlled Epoxidation of a,β-Unsaturated Morpholinyl Amides:Application to Natural Product Synthesis [J].Chemistry-A European Journal,2004,10:1527-1544.
[34] Kakei H,Tsuji R,Ohshima T,et al.Catalytic Asymmetric Epoxidation of a,β-Unsaturated Esters with Chiral Yttrium-Biaryldiol Complexes [J].Chemistry-An Asian Journal,2007,2:257-264.
[35] Zhang F Y,Yip C W,Chan A S C.Enantioselective Borane Reduction of Ketones Catalyzed by Chiral Lanthanum Alkoxides [J].Tetrahedron:Asymmetry 1996,7:2463-2466.
[36] Kobayashi S,Hachiya I,Ishitani H,et al.Asymmetric Diels-Alder Reaction Catalyzed by a Chiral Ytterbium Trifluoromethanesulfonate [J].Tetrahedron Letter,1993,34:4535-4538.
[37] Kobayashi S,Ishitani H.Lanthanide(III) Catalyzed Enantioselective Diels-Alder Reactions.Stereoselective Synthesis of Both Enantiomers by Using a Single Chiral Source and a Choice of Achiral Ligands [J].Journal of the American Chemical Society,1994,116:4083-4084.
[38] Collin J,Giuseppone N,Weghe P V.Lanthanide iodides,a new family of efficient Lewis acid catalysts [J].Coordination Chemistry Reviews,1998,180:117-144.
[39] Giuseppone N,Santos I,Collin J,et al.Enantioselective Diels-Alder reactions catalyzed by samarium iodo binaphthoxides [J].Tetrahedron Letters.2000,41:639-642.
[40] Harada S,Toudou N,Hiraoka S,et al.Highly enantioselective Diels-Alder reaction of Danishefsky-type diene and electron-deficient olefins catalyzed by an Yb(III)/chiral bis-urea complex [J].Tetrahedron Letter,2009,50:5652-5655.
[41] Sudo Y,Shirasaki D,Harada S,et al.Highly Enantioselective Diels-Alder Reactions of Danishefsky Type Dienes with Electron-Deficient Alkenes Catalyzed by Yb(III)-BINAMIDE Complexes [J].Journal of the American Chemical Society,2008,130:12588-12589.
[42] Kodama H,Ito J,Hori K,et al.Lanthanide-catalyzed asymmetric 1,3-dipolar cycloaddition of nitrones to alkenes using 3,3%-bis(2-oxazolyl)-1,1%-bi-2-naphthol (BINOL-Box) ligands [J].Journal of Organometallic Chemistry,2000,603:6-12.
[43] Kobayashi S,Kawamura M.Catalytic Enantioselective 1,3-Dipolar Cycloadditions between Nitrones and Alkenes Using a Novel Heterochiral Ytterbium(III) Catalyst [J].Journal of the American Chemical Society,1998,120:5840-5841.
[44] Kawamura M,Kobayashi S.A Switch of Enantiofacial Selectivity in Chiral Ytterbium Catalyzed 1,3-Dipolar Cycloaddition Reactions [J].Tetrahedron Letters,1999,40:3213-3216.
[45] Shibasaki M,Sasai H,Arai T.Asymmetric Catalysis with Heterobimetallic Compounds [J].Angewandte Chemie International Edition,1997,36:1236-1256.
[46] Shibasaki M,Sasai H,Arai T,et al.Heterobimetallic asymmetric catalysts.Developments and applications [J].Pure and Applied Chemistry,1998,70:1027-1034.
[47] Sasai H,Suzuki t,Arai S,et al.Basic Character of Rare Earth Metal Alkoxides.Utilization in Catalytic C-C Bond-FormingReactions and Catalytic Asymmetric Nitroaldol Reactions [J].Journal of the American Chemical Society,1992,114:4418-4420.
[48] Sasai H,Suzuki T,Itoh N,et al.Catalytic Asymmetric Nitroaldol Reactions.A New Practical Method for the Preparation of the Optically Active Lanthanum Complex [J].Tetrahedron Letters.1993,34:851-854.
[49] Sasai H,Itoh N,Suzuki T,et al.Catalytic Asymmetric Nitroaldol Reaction:An Efficient Synthesis of (s) Propranolol Using the Lanthanum Binaphthol Complex [J].Tetrahedron Letters.1993,34:855-858.
[50] Sasai H,Kim W S,Suzuki T,et al.Diastereoselective Catalytic Asymmetric Nitroaldol Reaction Utilizing Rare Earth-Li-(R)-BINOL Complex.A Highly Efficient Synthesis of Norstatine [J].Tetrahedron Letters.1994,35:6123-6126.
[51] Sasai H,Tokunaga T,Watanabe S,et al.Efficient Diastereoselective and Enantioselective Nitroaldol Reactions from Prochiral StartingMaterials:Utilization of La-Li-6,6-Disubstituted BINOL Complexes as Asymmetric Catalysts [J].The Journal of Organic Chemistry,1995,60:7388-7389.
[52] Arai T,Yamada Y M A,Yamamoto N,et al.Self-Assembly of Heterobimetallic Complexes and Reactive Nucleophiles:A General Strategy for the Activation of Asymmetric Reactions Promoted by Heterobimetallic Catalysts [J].Chemistry-A European Journal,1996,2:1368-1371.
[53] Sasai H,Hiroi M,Yamada Y M A,et al.The First Tandem Inter-Intramolecular Catalytic Asymmetric Nitroaldol Reaction Utilizing a LnLi3tris((R)-binaphthoxide) Complex ((R)-LnLB) (Ln:Lanthanoid) [J].Tetrahedron Letters,1997,38:6031-6034.
[54] Yamada Y M A,Yoshikawa N,Sasai H,et al.Direct Catalytic Asymmetric Aldol Reactions of Aldehydes with Unmodified Ketones [J].Angewandte Chemie International Edition,1997,36:1871-1873.
[55] Yoshikawa N,Yamada Y M A,Das J,et al.Direct Catalytic Asymmetric Aldol Reaction [J].Journal of the American Chemical Society,1999,121:4168-4178.
[56] Yoshikawa N,Kumagai N,Matsunaga S,et al.Direct Catalytic Asymmetric Aldol Reaction:Synthesis of Either syn-or anti-αβ-Dihydroxy Ketones [J].Journal of the American Chemical Society,2001,123:2466-2467.
[57] Trost B M,Brindle C S.The direct catalytic asymmetric aldol reaction [J].Chemical Society Reviews,2010,39:1600-1632.
[58] Sasai H,Arai T,Satow Y,et al.The First Heterobimetallic Multifunctional Asymmetric Catalyst [J].Journal of the American Chemical Society,1995,117:6194-6198.
[59] Sasai H,Arai T,Shibasaki M.Catalytic Asymmetric Michael Reactions Promoted by a Lithium-Free Lanthanum-BINOL Complex [J].Journal of the American Chemical Society,1994,116:1571-1572.
[60] Shimizu S,Ohori K,Arai T,et al.A Catalytic Asymmetric Synthesis of Tubifolidine [J].The Journal of Organic Chemistry,1998,63:7547-7551.
[61] Robinson J R,Gu J,Carroll P J,et al.Exchange Processes in Shibasaki′s Rare Earth Alkali Metal BINOLate Frameworks and Their Relevance in Multifunctional Asymmetric Catalysis [J].Journal of the American Chemical Society,2015,137:7135-7144.
[62] Yamagiwa N,Qin H,Matsunaga S,et al.Lewis Acid-Lewis Acid Heterobimetallic Cooperative Catalysis:Mechanistic Studies and Application in Enantioselective Aza-Michael Reaction [J].Journal of the American Chemical Society,2005,127:13419-13427.
[63] Bougauchi M,Watanabe S,Arai T,et al.Catalytic Asymmetric Epoxidation of α,β-Unsaturated Ketones Promoted by Lanthanoid Complexes [J].Journal of the American Chemical Society,1997,119:2329-2330.
[64] Sone T,Yamaguchi A,Matsunaga S,et al.Catalytic Asymmetric Synthesis of 2,2-Disubstituted Terminal Epoxides via Dimethyloxosulfonium Methylide Addition to Ketones [J].Journal of the American Chemical Society,2008,130:10078-10079.
[65] Sone T,Lu G,Matsunaga S,et al.Catalytic Asymmetric Synthesis of 2,2-Disubstituted Oxetanes from Ketones by Using a One-Pot Sequential Addition of Sulfur Ylide [J].Angewandte Chemie International Edition,2009,48:1677-1680.
[66] Sone T,Yamaguchi A,Matsunaga S,et al.Enantioselective Synthesis of 2,2-Disubstituted Terminal Epoxides via Catalytic Asymmetric Corey-Chaykovsky Epoxidation of Ketones [J].Molecules,2012,17:1617-1634.
[67] Sasai H,Bougauchi M,Arai T,et al.Enantioselective Synthesis of cx-Hydroxy Phosphonates Using the LaLi3tris(binaphthoxide) Catalyst (LLB),Prepared by an Improved Method [J].Tetrahedron Letters,1997,38:2717-2720.
[68] Sasai H,Arai S,Tahara Y,et al.Catalytic Asymmetric Synthesis of a-Amino Phosphonates Using Lanthanoid-Potassium-BINOL Complexes [J].The Journal of Organic Chemistry,1995,60:6656-6657.
[69] Rath N P,Spilling C D.The enantioselective addition of dialkylphosphites to aldehydes:Catalysis by a lanthanum binaphthoxide complex [J].Tetrahedron Letters,1994,35:227-230.
[70] Hong S,Tian S,Metz M V,et al.C2-Symmetric Bis(oxazolinato)lanthanide Catalysts for Enantioselective Intramolecular Hydroamination/Cyclization [J].Journal of the American Chemical Society,2003,125:14768-14783.
[71] Blanco A I S,Gothelf K V,Jorgensen K A.Lanthanide-Catalyzed Endo and Enantioselective 1,3-Dipolar Cycloaddition Reactions of Nitrones with Alkenes [J].Tetrahedron Letter,1997,38:7923-7926.
[72] Desimoni G,Faita G,Filippone S,et al.A new and highly efficient catalyst for the enantioselective Mukaiyama-Michael reaction between (E)-3-crotonoyl-1,3-oxazolidin-2-one and 2-trimethylsilyloxyfuran [J].Tetrahedron,2001,57:10203-10212.
[73] Nishiyama H,Sakaguchi H,Nakamura T,et al.Chiral and C,-Symmetrical Bis(oxazollnylpyrldlne) rhodlum( III ) Complexes:Effective Catalysts for Asymmetric Hydrosilylatlon of Ketones [J].Organometallics,1989,8:846-848.
[74] Fukuzawa S,Matsuzawa H,Metoki K.Scandium(III) Triflate/Isopropyl-Pybox Complex as an Efficient Catalyst for Asymmetric Diels-Alder Reaction [J].Synlett,2001,5:0709-0711.
[75] Desimoni G,Faita G,Guala M,et al.A New Pyridine-2,6-bis(oxazoline) for Efficient and Flexible LanthanideBased Catalysts of Enantioselective Reactions with 3-Alkenoyl-2-Oxazolidinones [J].Chemistry-A European Journal,2005,11:3816-3824.
[76] Desimoni G,Faita G,Guala M,et al.An efficient catalyst for highly enantioselective exo-Diels-Alder reaction between alkenoyl-1,3-oxazolidin-2-ones and cyclopentadiene [J].Tetrahedron,2002,58:2929-2935.
[77] Desimoni G,Faita G,Guala M,et al.Different Lanthanide Ions and the Pybox Substituents Induce the Reverse of the Sense of Induction in the Enantioselective Diels-Alder Reaction between Acryloyloxazolidinone and Cyclopentadiene [J].The Journal of Organic Chemistry,2003,68:7862-7866.
[78] Schaus S E,Jacobsen E N.Asymmetric Ring Opening of Meso Epoxides with TMSCN Catalyzed by (pybox) lanthanide Complexes [J].Organic Letters,2000,2:1001-1004.
[79] Keith J M,Jacobsen E N.Asymmetric Hydrocyanation of Hydrazones Catalyzed by Lanthanide-PYBOX Complexes [J].Organic Letters,2004,6:153-155.
[80] Desimoni G,Faita G,Guala M,et al.A New Pyridine-2,6-bis(oxazoline) for Efficient and Flexible Lanthanide Based Catalysts of Enantioselective Reactions with 3-Alkenoyl-2-Oxazolidinones [J].Chemistry-A European Journal,2005,11:3816-3824.
[81] Desimoni G,Faita G,Filippone S,et al.A new and highly efficient catalyst for the enantioselective Mukaiyama-Michael reaction between (E)-3-crotonoyl-1,3-oxazolidin-2-one and 2-trimethylsilyloxyfuran [J].Tetrahedron,2001,57:10203-10212.
[82] Duan Z Q,Han J L,Qian P,et al.Enantioselective synthesis of 3-hydroxy oxindoles by ytterbium-catalysed decarboxylative addition of β-ketoacids to isatins [J].Organic & Biomolecular Chemistry,2013,11:6456-6459.
[83] Fukuzawa S,Komuro Y,Nakano N,et al.New chiral scandium(III)/bisimine and diol complexes catalyzed asymmetric Diels-Alder reaction [J].Tetrahedron Letter,2003,44:3671-3674.
[84] Handa S,Gnanadesikan V,Matsunaga S,et al.syn-Selective Catalytic Asymmetric Nitro-Mannich Reactions Using a Heterobimetallic Cu-Sm-Schiff Base Complex [J].Journal of the American Chemical Society,2007,129:4900-4901.
[85] Shepherd N E,Tanabe H,Xu Y,et al.Direct Catalytic Asymmetric Vinylogous Mannich-Type and Michael Reactions of an α,β-Unsaturated γ-Butyrolactam under Dinuclear Nickel Catalysis [J].Journal of the American Chemical Society,2010,132:3666-3667.
[86] Chen Z H,Morimoto H,Matsunaga S,et al.A Bench-Stable Homodinuclear Ni2-Schiff Base Complex for Catalytic Asymmetric Synthesis of r-Tetrasubstituted anti-α,β-Diamino Acid Surrogates [J].Journal of the American Chemical Society,2008,130:2170-2171.
[87] Xu Y,Lu G,Matsunaga S,et al.Direct anti-Selective Catalytic Asymmetric Mannich-Type Reactions of a-Ketoanilides for the Synthesis of g-Amino Amides and Azetidine-2-amides [J].Angewandte Chemie International Edition,2009,48:3353-3356.
[88] Handa S,Nagawa K,Sohtome Y,et al.A Heterobimetallic Pd/La/Schiff Base Complex for anti-Selective Catalytic Asymmetric Nitroaldol Reactions and Applications to Short Syntheses of b-Adrenoceptor Agonists [J].Angewandte Chemie International Edition,2008,47:3230-3233.
[89] Shibasaki M,Kanai M,Matsunaga S,et al.Recent Progress in Asymmetric Bifunctional Catalysis Using Multimetallic Systems [J].Accounts of Chemical Research,2009,42:1117-1127.
[90] Saha B,Lin M H,Rajanbabu T V.Exceptionally Active Yttrium-Salen Complexes for the Catalyzed Ring Opening of Epoxides by TMSCN and TMSN3[J].The Journal of Organic Chemistry,2007,72:8648-8655.
[91] Wu B,Gallucci J C,Parquette J R,et al.Bimetallic catalysis in the highly enantioselective ring-opening reactions of aziridines [J].Chemical Science,2014,5:1102-1117.
[92] Nakajima M,Yamamoto S,Yamaguchi Y,et al.Enantioselective Michael additions of b-keto esters to α,β-unsaturated carbonyl compounds catalyzed by a chiral biquinoline N,N-dioxide-scandium trifluoromethanesulfonate complex [J].Tetrahedron,2003,59:7307-7313.
[93] Liu X,Lin L,Feng X.Chiral N,N-Dioxides:New Ligands and Organocatalysts for Catalytic Asymmetric Reactions [J].Accounts of Chemical Research,2011,44:574-587.
[94] Liu X,Lin L,Feng X.Chiral N,N′-dioxide ligands:synthesis,coordination chemistry and asymmetric catalysis [J].Organic Chemistry Frontiers,2014,1:298-302.
[95] Shen K,Liu X,Lin L,et al.Recent progress in enantioselective synthesis of C3-functionalized oxindoles:rare earth metals take action [J].Chemical Science,2012,3:327-334.
[96] Liu Y,Shang D,Zhou X,et al.Enantioselective Friedel-Crafts Alkylation of Indoles with Alkylidene Malonates Catalyzed by N,N-Dioxide-Scandium(III) Complexes:Asymmetric Synthesis of b-Carbolines [J].Chemistry-A European Journal,2009,15:2055-2058.
[97] Hu X,Lin L,Feng X,et al.Catalytic Asymmetric Synthesis of 3-(a-Hydroxy-b-carbonyl) Oxindoles by a ScIII-Catalyzed Direct Aldol-Type Reaction [J].Chemistry-A European Journal,2010,16:3736-3742.
[98] Hui Y,Jiang J,Wang W,et al.Highly Enantioselective Conjugate Addition of Thioglycolate to Chalcones Catalyzed by Lanthanum:Low Catalyst Loading and Remarkable Chiral Amplification [J].Angewandte Chemie International Edition,2010,49:4290-4293.
[99] Wang Z,Yang Z,Chen D,et al.Highly Enantioselective Michael Addition of Pyrazolin-5-ones Catalyzed by Chiral Metal/N,N′-Dioxide Complexes:Metal-Directed Switch in Enantioselectivity [J].Angewandte Chemie International Edition,2011,50:4928-4932.
[100] Kobayashi S,Hamada T,Nagayama S,et al.Lanthanide Trifluoromethanesulfonate-Catalyzed Asymmetric Aldol Reactions in Aqueous Media [J].Organic Letters,2001,3:165-167.
[101] Hamada T,Manabe K,Ishikawa S,et al.Catalytic Asymmetric Aldol Reactions in Aqueous Media Using Chiral Bis-pyridino-18-crown-6-Rare Earth Metal Triflate Complexes [J].Journal of the American Chemical Society,2003,125:2989-2996.
[102] Mei Y,Averill D J,Allen M J.Study of the Lanthanide-Catalyzed,Aqueous,Asymmetric Mukaiyama Aldol Reaction [J].The Journal of Organic Chemistry,2012,77:5624-5632.
[103] Mei Y,Dissanayake P,Allen M J.A New Class of Ligands for Aqueous,Lanthanide-Catalyzed,Enantioselective Mukaiyama Aldol Reactions [J].Journal of the American Chemical Society,2010,132:12871-12873.
[104] Hatanaka M,Morokuma K.How Can Fluctional Chiral Lanthanide (III) Complexes Achieve a High Stereoselectivity in Aqueous Mukaiyama-Aldol Reaction? [J].ACS Catalysis,2015,5:3731-3739.
[105] Hanamoto T,Furuno H,Sugimoto Y,et al.Asymmetric Hetero Diels-Alder Reaction Catalyzed by Chiral Ytterbium(III) Phosphate{Yb[(R)-(-)-BNP]3}:Remarkable Ligand Effect on the Enantioselectivity [J].Synlett,1997,1:79-80.
[106] Sugihara H,Daikai K,Jin X L,et al.Catalytic conversion of conjugated enones into optically active α-keto aziridines using chiral rare earth metal complexes [J].Tetrahedron Letter,2002,43:2735-2739.
[107] Conticello V P,Brard L,Giardello M A,et al.Chiral Organolanthanide Complexes for Enantioselective Olefm Hydrogenation [J].Journal of the American Chemical Society,1992,114:2761-2762.
[108] Gagne M R,Brard L,Conticello V P,et al.Stereoselection Effects In the Catalytic Hydroamlnatlon/Cyclization of Amlnoolefins at Chiral Organolanthanide Centers [J].Organometallics,1992,11:2003-2005.
[109] Giardello M A,Conticello V P,Brard L,et al.Chiral Organolanthanides Designed for Asymmetric Catalysis.A Kinetic and Mechanistic Study of Enantioselective Olefin Hydroamination/Cyclization and Hydrogenation [J].Journal of the American Chemical Society,1994,116:10241-10254.
[110] Fu P F,Brard L,Li Y W,et al.Regioselection and Enantioselection in Organolanthanide-Catalyzed Olefin Hydrosilylation.A Kinetic and Mechanistic Study [J].Journal of the American Chemical Society,1995,117:7157-7168.
Researchprogressofchiralrareearthcomplexesinasymmetriccatalysis
ChouYajie,ChengTanyu,LinJingrong*
(College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
With the increasing demand of the optical compounds,asymmetric catalysis becomes a hot research area.The rare earth elements are defined as the elements of lanthanide from La to Lu,together with Sc and Y.Compared to the orbital interaction between the other transition metals and ligands,the one between rare earth metals and ligands is unique.Therefore,the application of chiral rare earth complexes in asymmetric catalysis has also attracted much attention.This paper reviewed research progress of chiral rare earth complexes in asymmetric catalysis.
rare earth elements; lanthanides; chiral; asymmetric catalysis
10.3969/J.ISSN.1000-5137.2017.06.012
2017-09-18
丑亚杰(1991-),男,硕士研究生,主要从事不对称催化方面的研究.E-mail:choumars@163.com
*通信作者: 林静容(1968-),女,博士,副教授,主要从事天然产物合成等方面的研究.E-mail:jrlin@shnu.edu.cn
丑亚杰,程探宇,林静容.手性稀土配合物在不对称催化应用中的研究进展 [J].上海师范大学学报(自然科学版),2017,46(6):865-879.
formatChou Y J,Cheng T Y,Lin J R.Research progress of chiral rare earth complexes in asymmetric catalysis [J].Journal of Shanghai Normal University(Natural Sciences),2017,46(6):865-879.
O 621.3
A
1000-5137(2017)06-0865-15
郁 慧)
