胶质瘤干细胞表型维持机制研究进展
2018-01-05任东妮涂艳阳
任东妮,王 震,2,刘 楠,涂艳阳
(1第四军医大学唐都医院实验外科,陕西西安710038;2西安交通大学生命科学院,陕西西安710049)
胶质瘤干细胞表型维持机制研究进展
任东妮1,王 震1,2,刘 楠1,涂艳阳1
(1第四军医大学唐都医院实验外科,陕西西安710038;2西安交通大学生命科学院,陕西西安710049)
0 引言
脑胶质瘤(glioma)是最常见的原发性脑肿瘤,根据组织学标准由世界卫生组织(World Health Organization,WHO)按恶性程度的增高依次分为四个等级[1].胶质母细胞瘤(glioblastoma, GBM, WHO gradeⅣ)是恶性程度最高,死亡率最高的肿瘤[2].近年来对恶性胶质瘤的发病机制和治疗研究取得了一定进展,制订了根治性切除术,术后予以放疗同步并序贯替莫唑胺(temozolomide,TMZ)化疗的标准治疗方案[3-5].尽管采用了严格的手术和药物治疗方法,患者的中位生存率仍只有 15~19个月[2,6].研究发现GBM中存在一个神经胶质瘤干细胞(glioma stem cells,GSCs)亚群,对化疗和放疗具有较强的抗性,表明GSCs可能是GBM治疗失败和高复发率的原因[7].GSCs被认为是胶质母细胞瘤治疗的重要靶标,杀伤GSCs对于治疗胶质母细胞瘤至关重要.临床上,靶向GSCs的策略主要是通过靶向维持GSCs干性所需的细胞表面标志物和特异性途径来直接杀伤GSCs.然而,另一种靶向胶质母细胞瘤的方法渐渐得到认可,即通过改变GSCs与其微环境相互作用的能力来特异性杀伤 GSCs[8-9].了解维持 GSCs 干性的分子通路以及与微环境相互作用的机制可以为胶质瘤的治疗提供新的思路.
1 维持胶质瘤干细胞干性相关分子
GSCs具有不同的标志物(如CD133,nestin)和分子谱,包括配体、受体、细胞内信号分子、microRNA以及转录因子和染色质修饰蛋白(表1).尽管这些分子不能绝对的、单独的表征干细胞表型,但它们对GSCs分离提供了重要筛选标志,对GSCs干细胞状态和特性的维持发挥着不可或缺的作用.迄今为止,已经鉴定了许多标记物和分子对GSCs具有不同程度的特异性,并且对GSCs表型具有不同程度的影响.
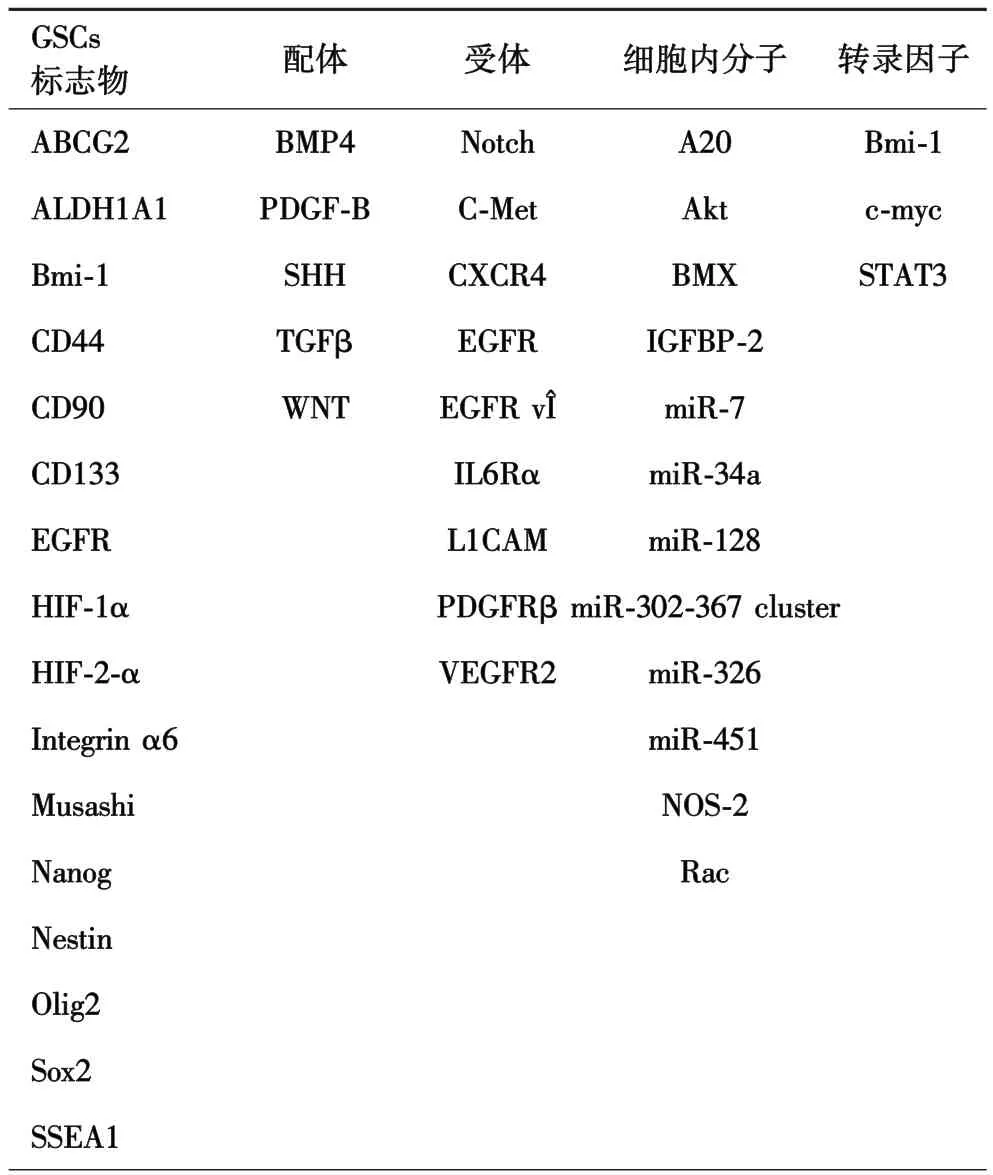
表1 胶质瘤干细胞标志物以及GSCs表型维持相关分子
1.1 GSCs标志分子 GSCs和正常的神经干细胞(neural stem cells,NSCs)具有相似的神经干细胞标志物,如 CD133/prominin-1, Sox2 和 Nestin[10-11].过去十几年的研究发现了许多其他的备选标志分子,包括 CD44[12], CD49f ( integrin a6)[13], Musashi[14],Nestin[15-16], Nanog[17-19], Oct4[20]和 Sox2[21-22].
CD133(prominin-1)是用于鉴定和分离恶性脑肿瘤中的癌干细胞的最早的干细胞表面标志物之一.然而,GBM分子研究结果中有许多CD133相关争议.例如,有研究[23]认为 GSCs中 CD133表达水平的变化与肿瘤发生潜力没有直接关系.研究[24-25]表明,从GBMs分离的CD133-肿瘤细胞在干细胞培养条件下也可以稳定地培养,类似于CD133+细胞,这些细胞还显示出“干细胞”特性,例如体外的自我更新,不同程度的异种移植物模型中形成可移植肿瘤.进一步的表型分析显示,与可以在培养物中形成浮游球体的CD133+细胞不同,CD133-细胞倾向于作为粘附球体生长.这一观察结果提示CD133+和CD133-细胞可能源于不同的 GSCs[26].最近有报道[27]称,少量的CD133-细胞可以产生CD133+细胞,这表明成球培养系统中,干细胞分级可能没有体内相关性.然而,对于CD133生物学研究仍在持续增多,因为它已经被重复证明是GSCs维持和神经球形成所必需的[28],是对常规疗法的抗性的良好指标.
1.2 microRNAs 已有研究[29-31]表明,microRNA(miRNA)参与胶质瘤的起始、发展、转移,在GSCs的形成和维持中发挥重要作用.据报道许多miRNA在GBM中异常表达,在胶质母细胞瘤干细胞中起重要作用.
miR-9/9∗,miR-17,miR-10b 和 miR-21 在人成胶质瘤细胞和组织中上调.miR-9/9∗和miR-17的抑制导致神经球形成的减少,并刺激细胞分化.干细胞相关转录因子基因 SOX2已被证明是 miR-9∗的靶点[32].此外,钙调蛋白结合转录激活因子 1(CAMTA1)是 miR-9/9∗和 miR-17 的靶标,可作为肿瘤抑制因子[33].据报道[34],miR-17 通过靶向 PTEN促进胶质母细胞瘤干性细胞的产生,PTEN调节胶质母细胞瘤干性细胞中的 PI3K/Akt和 STAT3信号转导[35].
miR-34a,miR-124,miR-125b 和 miR-137 在 GBM中与正常脑组织相比下调.miR-34a的过表达导致胶质母细胞瘤干细胞分化增加,并抑制胶质母细胞瘤干细胞恶性增殖[36-37].miR-34a 直接抑制胶质母细胞瘤干细胞中c-Met,CDK,Notch-1和Notch-2的表达;Notch 是干细胞维持的关键调节因子[38-40],抑制Notch途径可以降低GSCs的干细胞特性和放疗抵抗性[41].细胞周期蛋白依赖性激酶(cyclin dependent kinases,CDK)对于调节细胞周期是必不可少的.CDK4/6形成的复合物对G1/S相变至关重要,CDK6的丧失将导致 G1/S转换时细胞周期停滞[42].miR-124通过靶向SNAI2调节胶质母细胞瘤细胞的干性特征和侵袭性,过表达miR-124和敲低SNAI2能够抑制CD133+细胞亚群神经球的形成,并降低干细胞标记物的表达,如 BMI1,Nanog 和 Nestin[43].据报道[44],致癌基因NRAS和PIM3都是miR-124的下游靶点.miRNA-137在胶质母细胞瘤中下调,通过靶向CDK6和RTVP-1抑制胶质母细胞瘤干细胞的干性[45-46].据报道[47],RTVP-1 可以降低 CXCR4 的表达,抑制shh-GLI Nang信号通路,从而抑制GSCs的自我更新.miR-218的过表达导致稳定表达miR-218的胶质母细胞瘤神经球的体积显著减少,胶质母细胞瘤干细胞的自我更新能力降低.作为miR-218过表达的结果,胶质母细胞瘤神经球中的干细胞标志物如 CD133,SOX2,Nestin 和 Bmi1 的表达均降低[48].miR-218部分通过阻断Bmi1相关途径调节胶质瘤干细胞的干性[49].
2 与胶质瘤干细胞表型维持相关通路
GSCs中维持NSCs干性所需的许多信号通路均上调,增强了GSCs的干性以及异常细胞的存活,从而导致肿瘤发生[50-52].Notch, SHH (sonic hedgehog),血管内皮生长因子(vascular endothelial growth factor,VEGF),STAT3,Wnt和 BMP 信号通路对于调节GSC自我更新和分化非常重要.
2.1 Notch信号通路 在NSCs中,Notch信号调节细胞的增殖、分化、凋亡和细胞谱系决定[53-55].最近的研究[53]发现Notch信号传导在GSCs中高度活跃,能够制GSCs分化以及维持干细胞特性.Notch及其配体如Delta-like-1和Jagged-1的下调导致GSCs的致癌潜力降低,这表明Notch信号在GSCs的存活和增殖中具有重要作用[56-57].但 Notch信号通路参与GSCs维持调控的具体机制至今仍不清楚.有研究[12]提示Notch-1激活在GSCs的表型维持和增殖中起到重要作用.Notch-1信号通路激活 ERK,继而通过Shh-Gli-Nanog调控网络促进GSCs的自我更新.用小干扰RNA阻断GSCs的Notch-1表达,GSCs增殖能力明显下降,体外移植瘤明显减小,动物模型的存活时间明显延长[13].这些都说明 Notch-1信号通路的激活在GSCs的维持中起促进作用.在研究 GSCs与其周围血管微环境的关系中,用GSIs阻断Notch信号通路后,胶质瘤周围的血管内皮缺失,GSCs神经球生长明显受抑制,GSCs数量减少,功能受损[14],提示肿瘤周围血管内皮在 Notch信号通路调节 GSCs自我更新中起重要作用,而其作用的发挥是通过提供Notch配体,激活含有Notch受体的GSCs,促进肿瘤血管周围微环境形成,进而促进GSCs的自我更新和维持[15].至于内皮细胞表达Notch配体的机制,可能与GSCs分泌的VEGF促进内皮表达 Notch配体有关[16].
2.2 BMPs GSCs中的BMPs在引导星形胶质细胞分化从而抑制GSCs的致瘤性中发挥重要作用[58].具体来说,BMP-2通过引导星形胶质细胞分化减少GSCs增殖,并通过HIF-1α的不稳定使GSC对TMZ的敏感性增强[59-60].体内摄入 BMP-4能够抑制脑肿瘤生长,导致死亡率降低[58].BMP拮抗剂 Gremlin1通过调节内源性BMP水平来抑制GSCs的分化,以维持GSC的自我更新和致瘤潜力[61].
2.3 Wnt/β-Catenin β-Catenin 是 GSCs 增殖和分化的关键因素[62].GSCs中Wnt信号传导的异常活化导致肿瘤生长[63].FoxM1/β-Catenin 信号调节 c-Myc和其他Wnt靶基因的转录,导致胶质瘤的形成[64-65].此外,Wnt/β-Catenin信号调节PLAGL2的表达从而抑制 GSCs的分化,保持其干性[66].
2.4 EGFR信号通路 EGFR信号通路介导NSCs的增殖、迁移、分化和存活[67].EGFR 通过 β-连环蛋白的反式激活促进GSCs的增殖和肿瘤发生[68].此外,EGFR的过表达增加了GSCs的自我更新能力,从而诱导其致瘤潜能[69-70].
2.5 SHH信号通路 SHH信号在NSCs增殖、分化和存活中起关键作用[71].近来,有研究[72]表明,SHH途径在GSCs中具有高度活性,通过调节干细胞基因来维持自我更新并诱导肿瘤发生.SHH配体在胶质母细胞瘤的神经球中表达,将SHH抑制剂环巴胺作用于胶质母细胞瘤衍生的神经球,能够减少新的神经球形成,并且在SHH阻断后,颅内注射胶质母细胞瘤不能在免疫缺陷小鼠体内形成肿瘤.抑制SHH信号能够降低 GSCs自我更新和体内致瘤性[72-73].
2.6 STAT3通路 STAT3通路是通过上调TLR9表达来维持 GSCs 表型[74-75].Herrmann 等[76]报道了用CpG配体(CpG-ODN)刺激TLR9激活STAT3途径信号能够促进GSCs生长,而沉默 TLR9表达则抑制GSCs发展[76].
3 微环境与胶质瘤干细胞表型维持
在所有实体瘤中,高分级胶质瘤是血管化程度最高的一个.事实上,“微血管增生”是胶质母细胞瘤的一个特征.血管网络的改变导致血流的紊乱,导致肿瘤组织中氧的供应无法满足肿瘤的扩散,形成低氧或缺氧区,大部分的GSCs存在于这个区域[77].
胶质瘤干细胞需要特殊的微环境来维持“干性”,包括血管周围和低氧区.这些微环境对于提高GSCs的干性,促进GSCs的侵袭和转移具有重要作用.实验研究[78-80]证实,GSCs 富集在肿瘤血管周围的特定区域和坏死区域,后者与限制性氧水平相关.因此,GSCs与血管周围/增生性微环境以及缺氧/周围坏死性微环境相关,表现出共生关系.这些微环境在维持未分化的GSCs的干细胞状态及其体内平衡方面发挥了重要作用.GSCs不仅利用微环境存活,而且通过与肿瘤的近端和远端的各种组织成分的复杂相互作用积极形成这些微环境,从而参与复杂的双向互作(图 1).

图1 血管周围及周围坏死性微环境模拟图[81]
3.1 血管周围增生性微环境 在血管周围区域,GSCs富集,其中发现了大量区域性信号来促进干细胞表型[82].GSCs通常位于与毛细血管并行的内皮细胞(endothelial cells,ECs)附近,特别是在室管膜下层和海马区[83-84].这些区域性信号包括一些分子、细胞、细胞外基质等的相互作用已有一些相关研究.
据报道[83],GSCs释放高水平的促血管生成因子,如VEGF,促使新EC迁移到肿瘤并促进血管生成.此外,SHH被认为是通过激活HH信号通路促进GSCs特性获得的ECs分泌的中枢可溶性因子之一.GSCs显示出活跃的SHH-GLI1信号并调节GSCs自我更新和胶质瘤生长[73,85].成纤维细胞生长因子-2(fibroblast growth factor 2, FGF-2)有助于保持 GSCs的干性.当从GSCs细胞系中敲除FGF-2,导致GSCs分化;当细胞中存在这一生长因子时,则没有观察到这一点[86].FGF-2在C6胶质瘤细胞中能有效诱导巢蛋白(nestin)的表达,证明其对神经胶质瘤细胞的干性维持具有重要作用[87].FGF-2与EGF的自分泌生产也可能是维持GSCs自我更新潜力的原因[88].靶向FGF-2的治疗方法可能有效地杀伤GSCs,因为生长因子对于维持 GSCs的干细胞特征很重要[89].骨桥蛋白(osteopontin)来源于血管周围微环境,通过激活CD44促进GSCs表型,CD44是肿瘤干细胞(cancer stem cells,CSCs)的标志之一.CD44蛋白 C端胞内结构域通过提高缺氧诱导因子-2α(hypoxia inducible factor 2α, HIF-2α)的功能,对诱导 GSCs的特性至关重要[90].
血管微环境和GSCs之间的相互作用也涉及趋化因子及其受体.CXCR4是GSCs的生物标志物[91],其配体是CXCL12,由ECs和肿瘤微环境中的免疫细胞分泌[92],这突出了 CXCL12/CXCR4 轴在血管微环境中对于GSCs维持的重要性.
除上述分子外,GSCs可以直接刺激内皮细胞(endothelial cells, ECs)上 Notch 配体表达,ECs来源的一氧化氮(nitric oxide,NO)可以激活 GSCs中Notch信号通路和NO/cGMP/PKG信号,从而促进干细胞表型[93-94].
综上所述,GSCs与血管周围微环境之间存在双向交互性,血管周围微环境增强了GSCs的干细胞样特性,促进了这些细胞的侵袭和转移,使GSCs在治疗中幸存.
3.2 缺氧及周围坏死性微环境 缺氧促进GSCs自我更新、增殖以及致瘤性,并诱导非胶质瘤干细胞获取干细胞特性[95].缺氧刺激缺氧诱导因子(hypoxiainducible factor,HIF)家族的表达,导致促血管生成生长因子的产生[83].因此,研究[96]认为,缺氧微环境在GSCs的维持和扩增中具有关键作用.Li等[80]首先报道了HIF通路参与GSCs调节.使用异种移植胶质瘤起始细胞,通过体外神经球形成测定和CD133表达,观察到在缺氧环境下干细胞活性的显著增强.当HIF-1α或HIF-2α被shRNA沉默时,在正常氧和缺氧环境下的干细胞活性降低.考虑到HIF-2α mRNA水平与神经胶质瘤活动、进展和预后相关,强调HIF-2α对胶质瘤干细胞活性至关重要.由于HIF-1α蛋白水平可能受到转录后调控,进而导致HIF-1α mRNA水平与干细胞活性之间缺乏相关性[97].
在人胶质母细胞瘤活检中发现GSCs在周围坏死区富集.GSCs具有较低的氧气张力和激活的HIF-1α 和 HIF-2α[98].在体外培养中,缺氧情况下GSCs中的HIF-1α和HIF-2α上调.HIF-2α直接参与促进GSCs表型,而HIF-1α似乎在GSCs维持中不是至关重要的.此外,HIF-1α在GSCs和非GSCs细胞中均表达,而 HIF-2α 在 GSCs中特异性表达[80,98].值得注意的是,HIF-2α能够特异性调节干细胞维持的信号通路的激活[99].
血管退化加剧了微环境缺氧,被认为是胶质瘤侵袭性提高的重要原因[100].此外,缺氧导致GSCs的富集,使其具有更高侵袭性表型.因此,在探索新的抗血管生成策略时,应考虑在不加重缺氧前提下如何减少过量血管.HIF-1α诱导的Notch通路的激活对缺氧介导的GSCs维持至关重要.HIF-1α的消耗或Notch信号的失活部分抑制缺氧介导的GSCs维持[101].
3.3 免疫微环境 研究[96]表明GSCs与免疫细胞有直接相互作用.肿瘤相关巨噬细胞(tumor-associated macrophages, TAM)主要位于微血管周围[102]和缺氧区[103]的 CD133+GSC 附近,表明 GSCs和 TAM 之间有直接相互作用.在 GSCs的缺氧微环境中发现RAGE,COX2 和 NF-κB 等促炎基因的表达增强[104].与已分化的肿瘤细胞相比,GSCs显示较强的趋化作用活性以及募集TAMs能力,该过程由趋化因子和生长因子介导,包括VEGF,神经丝氨酸,SDF1和可溶性集落刺激因子1(sCSF-1).免疫细胞产生的分子/细胞因子如 TGFβ,VEGF,SDF1,bFGF和 NO 已被证明可以维持和促进GSCs[95],推测特异性炎性细胞的原促癌功能是通过直接刺激GSCs进行调控的.虽然上述分子证明了GSCs在免疫细胞调控中的重要作用,但免疫细胞对GSC维持的影响仍知之甚少.
4 结语
GSCs亚群最初只是构成肿瘤的少部分,但这些细胞自我更新,并对放疗和化疗具有抵抗性,使得它们能够持续存在,引起治疗后复发.GSCs表达干性标志物CD133、nestin以及受一系列分子如miRNA调控,激活notch、Wnt、EGFR等信号通路,并与周围增生血管、缺氧周围坏死微环境相互作用,是其维持干细胞特性,抑制分化,增强自我更新以及放化疗抵抗性和致瘤性的机制,为靶向GSCs治疗胶质瘤,减少复发提供新的靶点和思路.新型治疗方法应该破坏GSCs的保护性微环境、血管周围、缺氧和免疫逃逸,以改善甚至改变目前对胶质瘤的诊断和治疗.因此,GSCs微环境的有效控制可作为癌症传统治疗方法的补充.
[1]Gladson CL, Prayson RA, Liu WM.The pathobiology of glioma tumors[J].Annu Rev Pathol,2010,5:33-50.
[2]Linz U.Commentary on Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phaseⅢ study:5-year analysis of the EORTC-NCIC trial(Lancet Oncol.2009; 10: 459-466)[J].Cancer,2010,116(8):1844-1846.
[3]Walker MD, Green SB, Byar DP, et al.Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery[J].N Engl J Med,1980,303(23):1323-1329.
[4]DeAngelis LM.Brain tumors[J].N Engl J Med,2001,344(2):114-123.
[5]Oike T, Suzuki Y, Sugawara K, et al.Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma:Japanese mono-institutional results[J].PLoS One,2013,8(11):e78943.
[6]Grossman SA,Batara JF.Current management of glioblastoma multiforme[J].Semin Oncol,2004,31(5):635-644.
[7]Bao S, Wu Q, Mclendon RE, et al.Glioma stem cells promote radioresistance by preferential activation of the DNA damage response[J].Nature,2006,444(7120):756-760.
[8]Liebelt BD, Shingu T, Zhou X, et al.Glioma stem cells: signaling,microenvironment, and therapy[J].Stem Cells Int,2016,2016(18):7849890.
[9]Codrici E, Enciu AM, Popescu ID, et al.Glioma stem cells and their microenvironments: providers of challenging therapeutic targets[J].Stem Cells International,2016,2016:1-20.
[10]Singh SK, Clarke ID, Hide T, et al.Cancer stem cells in nervous system tumors[J].Oncogene,2004,23(43):7267-7273.
[11]Gangemi RM, Griffero F,Marubbi D,et al.SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity[J].Stem Cells,2009,27(1):40-48.
[12]Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, et al.TGF-β receptor inhibitors target the CD44 high/Id1 high, glioma-initiating cell population in human glioblastoma[J].Cancer Cell,2010,18(6):655-668.
[13]Lathia JD,Gallagher J,Heddleston JM,et al.Integrin alpha 6 regulates glioblastoma stem cells[J].Cell Stem Cell,2010,6(5):421-432.
[14]Thon N, Damianoff K, Hegermann J, et al.Presence of pluripotent CD133+, cells correlates with malignancy of gliomas[J].Mol Cell Neurosci,2010,43(1):51-59.
[15]Bexell D, Gunnarsson S, Siesjö P, et al.CD133+ and nestin+ tumor-initiating cells dominate in N29 and N32 experimental gliomas[J].Inter J Cancer,2009,125(1):15-22.
[16]Zhang M, Song T, Yang L, et al.Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients[J].J Exp Clin Cancer Res,2008,27:85.
[17]Guo Y, Liu S, Wang P, et al.Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas[J].Histopathology,2011,59(4):763-775.
[18]Mathieu J, Zhang Z, Zhou W, et al.HIF induces human embryonic stem cell markers in cancer cells[J].Cancer Res,2011,71(13):4640-4652.
[19]Niu CS, Li DX, Liu YH, et al.Expression of NANOG in human gliomas and its relationship with undifferentiated glioma cells[J].Oncol Rep,2011,26(3):593-601.
[20]Ikushima H, Todo T, Ino Y, et al.Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein[J].J Biol Chem,2011,286(48):41434-41441.
[21]Ge Y, Zhou F, Chen H, et al.Sox2 is translationally activated by eukaryotic initiation factor 4E in human glioma-initiating cells[J].Biochem Biophys Res Commun,2010,397(4):711-717.
[22]Hägerstrand D, He X, Bradic Lindh M, et al.Identification of a SOX2-dependent subset of tumor-and sphere-forming glioblastoma cells with a distinct tyrosine kinase inhibitor sensitivity profile[J].Neuro Oncol,2011,13(11):1178-1191.
[23]Gambelli F, Sasdelli F, Manini I, et al.Identification of cancer stem cells from human glioblastomas:growth and differentiation capabilities and CD133/prominin-1 expression [ J].Cell Biol Int,2012,36(1):29-38.
[24]Beier D, Hau P, Proescholdt M, et al.CD133(+) and CD133(-)glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles[J].Cancer Res,2007,67(9):4010-4015.
[25]Joo KM,Shi YK,Jun X,et al.Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas[J].Lab Invest,2008,88(8):808-815.
[26]Yang T, Rycaj K.Targeted therapy against cancer stem cells[J].Oncol Lett,2015,10(1):27-33.
[27]Chen R,Nishimura MC, Bumbaca SM,et al.A hierarchy of self-renewing tumor-initiating cell types in glioblastoma[J].Cancer Cell,2010,17(4):362-375.
[28]Brescia P, Ortensi B, Fornasari L, et al.CD133 is essential for glioblastoma stem cell maintenance[J].Stem Cells,2013,31(5):857-869.
[29]Huang Z, Cheng L, Guryanova OA, et al.Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting[J].Protein Cell,2010,1(7):638-655.
[30]Zhang Y,Dutta A,Abounader R.The role of microRNAs in glioma initiation and progression[J].Front Biosci(Landmank Ed),2012,17:700-712.
[31]Wang W, Dai LX, Zhang S, et al.Regulation of epidermal growth factor receptor signaling by plasmid-based microRNA-7 inhibits human malignant gliomas growth and metastasis in vivo[J].Neoplasma,2013,60(3):274-283.
[32]Jeon HM,Sohn YW,Oh SY,et al.ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9∗-mediated suppression of SOX2[J].Cancer Res,2011,71(9):3410-3421.
[33]Schraivogel D, Weinmann L, Beier D, et al.CAMTA1 is a novel tumour suppressor regulated by miR-9/9∗in glioblastoma stem cells[J].Embo J,2011,30(20):4309-4322.
[34]Li H, Yang BB.Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2[J].Oncotarget,2013,3(12):1653-1668.
[35]Moon SH, Kim DK, Cha Y, et al.PI3K/Akt and Stat3 signaling regulated by PTEN control of the cancer, stem cell population,proliferation and senescence in a glioblastoma cell line[J].Inter J Oncol,2013,42(3):921-928.
[36]Li Y, Guessous F,Zhang Y,et al.MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes[J].Cancer Res,2009,69(19):7569-7576.
[37]Guessous F, Zhang Y, Kofman A, et al.microRNA-34a is tumor suppressive in brain tumors and glioma stem cells[J].Cell Cycle,2010,9(6):1031-1036.
[38]Fan X, Khaki L, Zhu TS, et al.NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts[J].Stem Cells,2010,28(1):5-16.
[39]Zhen Y, Zhao S, Li Q, et al.Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas[J].Cancer Lett,2010,292(1):64-72.
[40]Fan X,Matsui W,Khaki L,et al.Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors[J].Cancer Res,2006,66(15):7445-7452.
[41]Wang J, Wakeman TP, Lathia JD, et al.Notch promotes radioresistance of glioma stem cells[J].Stem cells,2010,28(1):17-28.
[42]Chen SM, Chen HC, Chen SJ, et al.MicroRNA-495 inhibits proliferation of glioblastoma multiforme cells by downregulating cyclin-dependent kinase 6[J].World J Surg Oncol,2013,11:87.
[43]Xia H, Cheung WK, Ng SS, et al.Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells[J].J Biol Chem,2012,287(13):9962-9971.
[44]Sun L, Yan W, Wang Y, et al.MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10[J].Brain Res,2011,1389:9-18.
[45]Bier A,Giladi N,Kronfeld N,et al.MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1[J].Oncotarget,2013,4(5):665-676.
[46]Silber J, Lim DA, Petritsch C, et al.miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells[J].BMC Med,2008,6:14.
[47]Fareh M,Turchi L,Virolle V,et al.The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network [ J].Cell Death Differ, 2012,19(2):232-244.
[48]Tu Y, Gao X, Li G, et al.MicroRNA-218 inhibits glioma invasion,migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1[J].Cancer Res,2013,73(19):6046-6055.
[49]Gao X,Jin W.The emerging role of tumor-suppressive microRNA-218 in targeting glioblastoma stemness[J].Cancer Lett,2014,353(1):25-31.
[50]Hemmati HD, Nakano I, Lazareff JA, et al.Cancerous stem cells can arise from pediatric brain tumors[J].Pro Natl Acad Sci U S A,2003,100(25):15178-15183.
[51]Rich JN, Eyler CE.Cancer stem cells in brain tumor biology[J].Cold Spring Harb Symp Quant Biol,2008,73:411-420.
[52]Vescovi AL, Galli R, Reynolds BA.Brain tumour stem cells[J].Nat Rev Cancer,2006,6(6):425-436.
[53]Artavanis-Tsakonas S, Rand MD, Lake RJ.Notch signaling: cell fate control and signal integration in development[J].Science,1999,284(5415):770-776.
[54]Beatus P, Lendahl U.Notch and neurogenesis[J].J Neurosci Res,1998,54(2):125-136.
[55]Lasky JL, Wu H.Notch signaling, brain development, and human disease[J].Pediatr Res,2005,57(5 Pt 2):104R-109R.
[56]Kanamori M,Kawaguchi T,Nigro J M,et al.Contribution of Notch signaling activation to human glioblastoma multiforme[J].J Neurosurg,2007,106(3):417-427.
[57]Purow BW,Haque RM,Noel MW,et al.Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation[J].Cancer Res,2005,65(6):2353-2363.
[58]Piccirillo SG, Reynolds BA, Zanetti N, et al.Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells[J].Nature,2006,444(7120):761-765.
[59]Pistollato F, Chen HL, Rood BR, et al.Hypoxia and HIF-1α repress the differentiative effects of BMPs in high-grade glioma[J].Stem cells,2009,27(1):7-17.
[60]Persano L, Pistollato F, Rampazzo E, et al.BMP2 sensitizes glioblastoma stem-like cells to Temozolomide by affecting HIF-1α stability and MGMT expression[J].Cell Death Dis,2012,3:e412.
[61]Yan K, Wu Q, Yan DH, et al.Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy[J].Genes Dev,2014,28(10):1085-1100.
[62]Atkins RJ, Dimou J, Paradiso L, et al.Regulation of glycogen synthase kinase-3 beta (GSK-3β) by the Akt pathway in gliomas[J].J Clin Neurosci,2012,19(11):1558-1563.
[63]Hatten ME, Roussel MF.Development and cancer of the cerebellum[J].Trends Neurosci,2011,34(3):134-142.
[64]Bowman A, Nusse R.Location, location, location: FoxM1 mediates β-catenin nuclear translocation and promotes glioma tumorigenesis[J].Cancer Cell,2011,20(4):415-416.
[65]Zhang N, Wei P, Gong A, et al.FoxM1 promotes β-catenin nuclear localization and controls wnt target-gene expression and glioma tumorigenesis[J].Cancer Cell,2011,20(4):427-442.
[66]Zheng H, Ying H, Wiedemeyer R, et al.PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas[J].Cancer Cell,2010,17(5):497-509.
[67]Ayuso-Sacido A, Moliterno JA, Kratovac S, et al.Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells[J].J Neurooncology,2010,97(3):323-337.
[68]Yang W, Xia Y, Ji H, et al.Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation[J].Nature,2011,480(7375):118-122.
[69]Ayuso-Sacido A, Graham C, Greenfield JP, et al.The duality of epidermal growth factor receptor(EGFR) signaling and neural stem cell phenotype: cell enhancer or cell transformer[J].Curr Stem Cell Res Ther,2006,1(3):387-394.
[70]Sun Y, Goderie SK, Temple S.Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells[J].Neuron,2005,45(6):873-886.
[71]Agarwala S,Sanders TA,Ragsdale CW.Sonic Hedgehog Control of Size and Shape in Midbrain Pattern Formation[J].Science,2001,291(5511):2147-2150.
[72]Bar EE, Chaudhry A, Lin A, et al.Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma[J].Stem Cells,2007,25(10):2524-2533.
[73]Clement V, Sanchez P, de Tribolet N, et al.HEDGEHOG-GLI1 signaling regulates human glioma growth,cancer stem cell self-renewal, and tumorigenicity[J].Curr Biol,2007,17(2):165-172.
[74]Guryanova OA, Wu Q, Cheng L, et al.Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3[J].Cancer Cell,2011,19(4):498-511.
[75]Kortylewski M,Kujawski M,Herrmann A,et al.Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy[J].Cancer Res,2009,69(6):2497-2505.
[76]Herrmann A, Cherryholmes G, Schroeder A, et al.TLR9 is critical for glioma stem cell maintenance and targeting[J].Cancer Res,2014,74(18):5218-5228.
[77]Fidoamore A, Cristiano L, Antonosante A, et al.Glioblastoma stem cells microenvironment:the paracrine roles of the niche in drug and radioresistance[J].Stem Cells Int,2016,2016:6809105.
[78]Lathia JD, Gallagher J, Myers JT, et al.Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells[J].PloS One,2011,6(9):e24807.
[79]Calabrese C,Poppleton H,Kocak M,et al.A perivascular niche for brain tumor stem cells[J].Cancer Cell,2007,11(1):69-82.
[80]Li Z,Bao S,Wu Q,et al.Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells[J].Cancer Cell,2009,15(6):501-513.
[81]Mack SC, Hubert CG, Miller TE, et al.An epigenetic gateway to brain tumor cell identity[J].Nat Neurosci,2016,19(1):10-19.
[82]Christensen K, Schrøder HD, Kristensen BW.CD133+ niches and single cells in glioblastoma have different phenotypes[J].J Neurooncology,2011,104(1):129-143.
[83]Jain RK, Tomaso ED, Dan GD, et al.Angiogenesis in brain tumours[J].Nat Rev Neurosci,2007,8(8):610-622.
[84]Carmeliet P, Jain RK.Angiogenesis in cancer and other diseases[J].Nature,2000,407(6801):249-257.
[85]Ulasov IV, Nandi S,Dey M,et al.Inhibition of sonic hedgehog and notch pathways enhances sensitivity of CD133(+) glioma stem cells to temozolomide therapy[J].Mol Med,2011,17(1-2):103-112.
[86]Pollard SM,Yoshikawa K, Clarke ID,et al.Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens[J].Cell Stem Cell,2009,4(6):568-580.
[87]Chang KW, Huang YL, Wong ZR, et al.Fibroblast growth factor-2 up-regulates the expression of nestin through the Ras-Raf-ERK-Sp1 signaling axis in C6 glioma cells[J].Biochem Biophys Res Commun,2013,434(4):854-860.
[88]Li G, Chen Z, Hu YD, et al.Autocrine factors sustain glioblastoma stem cell self-renewal[J].Oncology Rep,2009,21(2):419-424.
[89]Haley EM,Kim Y.The role of basic fibroblast growth factor in glioblastoma multiforme and glioblastoma stem cells and in their in vitro culture[J].Cancer Lett,2013,346(1):1-5.
[90]Pietras A, Katz AM, Ekström EJ, et al.Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth[J].Cell Stem Cell,2014,14(3):357-369.
[91]Zheng X, Xie Q, Li S, et al.CXCR4-positive subset of glioma is enriched for cancer stem cells[J].Oncol Res,2011,19(12):555-561.
[92]Würth R, Bajetto A, Harrison JK, et al.CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment[J].Front Cell Neurosci,2014,8:144.
[93]Charles N, Ozawa T, Squatrito M, et al.Perivascular nitric oxide activates notch signaling and promotes stem-like character in pdgfinduced glioma cells[J].Cell Stem Cell,2010,6(2):141-152.
[94]Eyler CE, Wu Q, Yan K, et al.Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2[J].Cell,2011,146(1):53-66.
[95]Heddleston JM, Li Z, Mclendon RE, et al.The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype[J].Cell Cycle,2009,8(20):3274-3284.
[96]Filatova A, Acker T, Garvalov BK.The cancer stem cell niche(s):the crosstalk between glioma stem cells and their microenvironment[J].Biochim Biophys Acta,2013,1830(2):2496-2508.
[97]Peng G,Liu Y.Hypoxia-inducible factors in cancer stem cells and inflammation[J].Trends Pharmacol Sci,2015,36(6):374-383.
[98]Yang L, Lin C, Wang L, et al.Hypoxia and hypoxia inducible factors in glioblastoma multiforme progression and therapeutic implications[J].Exp Cell Res,2012,318(19):2417-2426.
[99]Holmquistmengelbier L, Fredlund E, Löfstedt T, et al.Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype[J].Cancer Cell,2006,10(5):413-423.
[100]Mackey TK, Cuomo R, Guerra C, et al.After counterfeit Avastin®--what have we learned and what can be done[J].Nat Rev Clin Oncol,2015,12(5):302-308.
[101]Qiang L, Wu T, Zhang HW, et al.HIF-1α is critical for hypoxiamediated maintenance of glioblastoma stem cells by activating Notch signaling pathway[J].Cell Death Differ,2012,19(2):284-294.
[102]Yi L, Xiao H, Xu M, et al.Glioma-initiating cells: A predominant role in microglia/macrophages tropism to glioma[J].J Neuroimmunol,2011,232(1-2):75-82.
[103]Wang SC, Hong JH, Hsueh C, et al.Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model[J].Lab Invest,2012,92(1):151-162.
[104]Tafani M,Di Vito M,Frati A,et al.Pro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma[J].J Neuroinflammation,2011,8:32.
Advances in phenotypic maintenance of glioma stem cells
REN Dong-Ni1, WANG Zhen1,2, LIU Nan1, TU Yan-Yang11Department of Experimental Surgery, Tangdu Hospital, Fourth Military Medical University, Xi'an 710038, China;2Department of Biology, Xi'an Jiaotong University, Xi'an 710049, China
Glioma stem cells (GSCs) have the characteristics of self-renewal, the formation of neurospheres, the expression of stem cell markers, multi-directional differentiation, higher invasion,radiotherapy and chemotherapy resistance,and these characteristics are considered to be the main cause of glioma recurrence.As a relevant target for glioblastoma therapy,the elimination of GSCs is crucial in treating glioblastoma.The strategy to target GSCs therapeutically is mainly focused on the direct ablation of GSCs by targeting cell surface markers and specific pathways that are required for maintaining GSCs stemness.However, it has been increasingly acknowledged that another way to specifically target GSCs is to alter the ability of GSCs to interact with their microenvironments.GSCs exist in specific niches (perivascular/proliferative niche and hypoxic/perinecrotic niche) that play a role in enhancing the stem-like features of GSCs,promoting invasion and metastasis of GSCs, and even making GSCs survive.Recognition of these mechanisms has opened doors for targeting GSCs.
glioma stem cells; Notch signaling; stemness markers; hypoxic niche; perivascular niche
胶质瘤干细胞(GSCs)具有自我更新、形成神经球、表达干细胞标志物、多向分化、较高侵袭力、放化疗抵抗等特性,这些特性被认为是胶质瘤复发的主要因素.GSCs作为胶质瘤治疗的重要靶标,主要是通过抑制维持GSC干性所需的细胞表面标志物的表达以及阻断相关特异性分子通路,从而减少GSCs增殖,促进其分化,降低其致瘤性来杀伤GSCs.近年来,GSCs与其所处的血管周围/增生性微环境以及缺氧/周围坏死性微环境的相互作用逐渐被关注.研究发现血管周围微环境及坏死周围缺氧区域中,存在一些分子和细胞,通过分子信号转导机制,增强GSCs的干细胞样特性,促进了这些细胞的侵袭和转移,使GSCs在治疗中幸存.因此深入了解这一机制,破坏这些微环境,寻找新的靶点,可以为胶质瘤的治疗开辟一条新的路径.
胶质瘤干细胞;Notch信号;干性标记物;缺氧微环境;血管微环境
R739.41
A
2095-6894(2017)12-57-07
2017-05-05;接受日期:2017-05-18
国家自然科学基金(81572983,81272419);陕西省社会发展科技攻关项目(2015SF027);唐都医院创新发展基金资助项目(2016JCYJ013)
任东妮.硕士.研究方向:胶质瘤治疗.E-mail:rendongni123@ 163.com
涂艳阳.博士,副主任医师,副教授.E-mail:tu.fmmu@ gmail.com
