2-乙酰吡嗪苯甲酰腙钴、锌和铜配合物的晶体结构及荧光性质
2018-01-04侯旭锋赵晓雷吴伟娜
侯旭锋 赵晓雷 张 露 吴伟娜 王 元
(1许昌学院化学化工学院,化学生物传感与检测重点实验室,许昌 461000)
(2河南理工大学化学化工学院,河南省煤炭绿色转化重点实验室,焦作 454000)
(3河南理工大学材料科学与工程学院,焦作 454000)
2-乙酰吡嗪苯甲酰腙钴、锌和铜配合物的晶体结构及荧光性质
侯旭锋1赵晓雷*,2张 露3吴伟娜*,2王 元2
(1许昌学院化学化工学院,化学生物传感与检测重点实验室,许昌 461000)
(2河南理工大学化学化工学院,河南省煤炭绿色转化重点实验室,焦作 454000)
(3河南理工大学材料科学与工程学院,焦作 454000)
合成了配合物[Co(L)2](1),[Zn(L)2](2)和[Cu2(L)2Cl2](3)(HL为2-乙酰吡嗪苯甲酰腙),并通过单晶衍射、元素分析及红外光谱表征了它们的结构。单晶衍射结果表明,配合物1和2同构,配体和金属的比例为2∶1。每个配合物中,中心金属离子与来自2个阴离子配体L-的N2O电子供体配位,形成扭曲的八面体配位构型。在双核配合物3中,Cuギ离子与1个阴离子三齿酰腙配体、2个μ2桥联的氯离子配位,拥有扭曲的四方锥配位构型。此外还研究了配合物的荧光性质。
酰腙;配合物;吡嗪;晶体结构;荧光
Acylhydrazones have attracted much more attention mainly due to their variable bonding modes towards transition metal ions and wide range of biological properties,such as antioxidant,anti-inflammatory,antibacterial and antitumor activities[1].Up to now,a number of acylhydrazone transition metals bearing pyridine heterocycle scaffold,possess potent anticancer activity[2-5].In fact,2-acetylpyrazine thiosemicarbazones have been reported to exhibit remarkable biological activity in vitro against K562 leukemia cell lines through the work of Li and co-workers[6-8].However,the investigation on the 2-acetylpyrazine acylhydrazone complexes is relatively scarce[9].Here,the structures of three transition metal complexes based on 2-acetylpyrazine benzoylhydrazone are described.In addition,the fluorescent properties of the ligand and the complexes were studied in detail.
1 Experimental section
1.1 Materials and measurement
Solvents and starting materials for synthesis were purchased commercially and used as received.Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1)were determined by the KBr pressed disc method on a BrukerV70 FT-IR spectrophotometer.1H NMR spectra of HL was acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMS as internal standard.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer,in the measurements of emission and excitation spectra the pass width is 10 nm.
1.2 Preparations of the ligand and complexes 1~3
As shown in Scheme 1,the ligand HL was produced by condension of 2-acetylpyrazine (1.22 g,0.01 mol)and benzohydrazide (1.36 g,0.01 mol)in anhydrous ethanol solution (30 mL)with continuous stirring at room temperature for 3 h.The white solid was filtered and washed three times by cold ethanol.Yield:1.92 g (80%).m.p.183~184 ℃.Elemental analysis Calcd.for C13H12N4O(%):C:64.99;H:5.03;N:23.32.Found(%):C:65.18;H:4.94;N:23.23.FTIR(cm-1):ν(C=O)1 687,ν(C=N)1 579,ν(C=N)pyrazine1 532.1H NMR(400 MHz,DMSO-d6):δ 11.07(1H,s,NH),9.27 (1H)and 8.65~8.67 (2H)for pyrazine-H,7.90(2H)and 7.52~7.63(3H)for phenyl-H,2.46(3H,s,CH3).
The complexes 1~3 were generated by reaction of the ligand HL (5 mmol)with equimolar of Co(NO3)2,Zn(OAc)2and CuCl2in ethanol solution(10 mL),respectively.Crystals suitable for X-ray diffraction analysis were obtained by evaporating the corresponding reaction solutions at room temperature.
1:brown blocks.Anal.Calcd.for C26H22N8O2Co(%):C:58.10;H:4.13;N:20.85.Found(%):C:58.15;H:4.00;N:20.63.FT-IR(cm-1):ν(N=C-O)1 638,ν(C=N)1 572,ν(C=N)pyrazine1 527.
2:brown blocks.Anal.Calcd.for(C26H22N8O2Zn)(%):C:57.42;H:4.08;N:20.60.Found(%):C:57.32;H:3.92;N:20.39.FT-IR(cm-1):ν(N=C-O)1 639,ν(C=N)1 573,ν(C=N)pyrazine1 526.
3:green blocks.Anal.Calcd.For(C13H11N4O ClCu)(%):C:46.16;H:3.28;N:16.56.Found(%):C:46.22;H:3.16;N:16.37.FT-IR(cm-1):ν(N=C-O)1 634,ν(C=N)1 565,ν(C=N)pyrazine1 525.

Scheme 1 Synthesis route of HL
1.3 X-ray crystallography
The X-ray diffraction measurement for complexes 1~3(size:0.15 mm×0.14 mm×0.12 mm,0.25 mm×0.22 mm×0.20 mm,and 0.14 mm×0.12 mm×0.08 mm,respectively)wereperformedona Bruker SMART APEXⅡ CCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[10].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[11].All nonhydrogen atoms were refined anisotropically.All the H atoms were positioned geometrically and refined using a riding model.Details of the crystal parameters,data collection and refinements for complexes 1~3 are summarized in Table 1.
CCDC:1552007,1;1552008,2;1552009,3.

Table1 Selected crystallographic data for complexes 1~3
2 Results and discussion
2.1 Crystal structure description
Selected bond distances and angles for complexes 1~3 are listed in Table 2.In each complex,C=O bond of the ligand HL is enolized,which could be confirmed by the bond lengths of C-O being 0.127 8(6),0.126 6(8)and 0.128 8(4)nm in complexes 1~3,respectively.The results are in excellent agreement with previously known acylhydrazone complexes in the literature[5].
Complexes 1 and 2 are isostructuraland crystallize in the orthorhombic,space group Aba2.Thus,the complex 1 is discussed in detail for an example.As shown in Fig.1a,the central Coギion in complex 1,which is situated on the two fold rational axis,is surrounded by two independent anionic ligands with N2O donor set,thus possessing a distorted octahedral coordination geometry.The distances of Co-N/O bonds were in the range of 0.204 4(4)~0.212 8(5)nm,which are shorter than those of the corresponding Zn-N/O bonds in complex 2,since the radius of the Znギion is greater than that of Coギion.
By contrast,complex 3 contains one discrete dimeric Cuギmolecule in the unit cell.Two Cu atoms of the dimer were separated by 0.338 1 nm and doubly bridged by two chloride anions to form an ideal planar four-membered Cu2Cl2core.Each of the Cuギions is penta-coordinated by one tridentate anionic ligand and two chloride anions(one of which acts as a μ2-bridge),thus giving a distorted square pyramid coordination geometry (τ=0.047)[9].As expected,there exist no classical hydrogen bonds in all three complexes.

Table2 Selected bond lengths(nm)and angles(°)in complexes 1~3
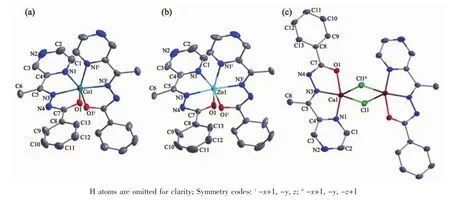
Fig.1 Diamond drawing of 1~3(a~c,respectively)with 30%thermal ellipsoids
2.2 IR spectra
The ν(C=O)of the free ligand is 1 687 cm-1,which is disappeared in complexes 1~3,meanwhile,new(N=C-O)stretching vibration absorption is observed at 1 634~1 639 cm-1,revealing that the C=O in O=C-N moiety has enolizated and the oxygen atom coordinates to each metal ion[12].The ν(C=N)bands of the imine group and pyrazine ring in the ligand HL shift to lower frequency values in the complexes,indicating that the N atoms of both units take part in the coordination[4].It is in accordance with the crystal structure study.
2.3 UV spectra
The UV spectra of HL,complexes 1 and 2 in CH3OH solution (concentration:2×10-5mol·L-1)were measured at room temperature (Fig.3).The spectra of HL features two bands located at 231(ε=3 889 L·mol-1·cm-1)and 297 nm(ε=10 473 L·mol-1·cm-1),which could be assigned to characteristic π-π*transition of benzene and pyrazine units[12]. Similar bands are observed at 257(ε=5 770 L·mol-1·cm-1)and 288 nm(ε=5 562 L·mol-1·cm-1);254 (ε=4 464 L·mol-1·cm-1)and 303 nm(ε=5 090 L·mol-1·cm-1)in the complexes 1 and 2,respectively.However,such two bands combined at 268(ε=8 775 L·mol-1·cm-1)in the spectra of 3.In addition,complexes 1~3 exhibit new absorbance bands at 374(ε=7 390 L·mol-1·cm-1),386(ε=7 650 L·mol-1·cm-1)and 403 nm(ε=7 496 L·mol-1·cm-1),respectively,probably due to the ligand-tometal charge transfer(LMCT)[13].This indicates that an extended conjugation is formed after complexation in complexes 1~3.

Fig.2 UV spectra of the ligand HL and complexes 1~3 in CH3OH solution at room temperature

Fig.3 Fluorescence emission spectra of ligand HL and complexes 1~3 in CH3OH solution at room temperature
2.4 Fluorescence spectra
The fluorescence spectra of the ligand HL,complexes 1~3 have been studied in CH3OH solution(concentration:2×10-5mol·L-1)at room temperature.The results show that complex 2 shows significant emission peak at 535 nm when excited at 380 nm,while HL,complexes 1 and 3 are free of fluorescence under same conditions.The ligand HL exhibits no fluorescence primarily due to C=N isomerization.Binding with Zn2+inhibits the isomerization of C=N,thereby increasing the fluorescence intensity through the CHEF mechanism[14].
[1]Mahmudov K T,Kopylovich M N,Pombeiro A J L.Coord.Chem.Rev.,2013,257:1244-1281
[2]Rodic'M V,Leovac V M,Jovanovic'L S,et al.Eur.J.Med.Chem.,2016,115:75-81
[3]Sathyadevi P,Krishnamoorthy P,Alagesan M,et al.Polyhedron,2012,31:294-306
[4]Hosseini-Monfared H,Bikas R,Szymczak R,et al.Polyhedron,2013,63:74-82
[5]Singh P,Singh D P,Singh V P.Polyhedron,2014,81:56-65
[6]Li M X,Zhang L Z,Yang M,et al.Bioorg.Med.Chem.Lett.,2012,22:2418-2423
[7]Li M X,Zhang D,Zhang L Z,et al.J.Organomet.Chem.,2011,696:852-858
[8]Li M X,Zhang L Z,Zhang D,et al.Eur.J.Med.Chem.,2011,46:4383-4390
[9]Xu J,Zhou T,Xu Z Q,et al.J.Mol.Struct.,2017,1128:448-454
[10]Sheldrick G M.SADABS,University of Göttingen,Germany,1996.
[11]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[12]WU Hao(吴浩),CHEN Ze-Hua(陈泽华),YU Ya-Ping(于亚平),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(4):699-704
[13]MAO Pan-Dong(毛盼东),ZHAO Xiao-Lei(赵晓雷),SHAO Zhi-Peng(邵志鹏),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(5):890-896
[14]Wu W N,Mao P D,Jia L,et al.Spectrochim.Acta A,2016,166:44-48
Coギ/Znギ/Cuギ Complexes Containing Hydrazone Ligand Bearing Pyrazine Unit:Syntheses,Crystal Structures and Fluorescence Properties
HOU Xu-Feng1ZHAO Xiao-Lei*,2ZHANG Lu3WU Wei-Na*,2WANG Yuan
(1Key Laboratory of Chemo/Bio-sensing and Detection,School of Chemistry and Chemical Engineering,Xuchang University,Xuchang,Henan 461000,China)(2College of Chemistry and Chemical Engineering,Henan Key Laboratory of Coal Green Conversion,Henan Polytechnic University,Jiaozuo,Henan 454000,China)(3School of Materials Science and Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three complexes,namely,[Co(L)2](1),[Zn(L)2](2)and[Cu2(L)2Cl2](3)based on HL(HL=2-acetylpyrazine benzoylhydrazone)were synthesized and characterized by X-ray diffraction analyses.The results show that complexes 1 and 2 are isostructural,in which the molar ratio between the metal and the ligand is 1∶2.The metal ion in each complex is surrounded by two enolizated ligands L-with N2O donor set,thus giving a distorted octahedral geometry.However,in the bi-nuclear complex 3,each Cuギion is coordinated with one monoanionic tridentate hydrazone ligand and two μ2-chloride anions,as [CuN2OCl2],indicating the coordination geometry is a distorted tetragonal pyramid.In addition,the luminescent properties of the complexes are discussed in detail.CCDC:1552007,1;1552008,2;1552009,3.
acylhydrazone;complex;pyrazine;crystal structure;fluorescence
O614.81+2;O614.24+1;O614.121
A
1001-4861(2018)01-0201-05
10.11862/CJIC.2018.005
2017-05-31。收修改稿日期:2017-10-09。
国家自然科学基金(No.21001040)、河南省科技厅基础与前沿项目(No.162300410011,162300410209)和河南省青年骨干教师项目(No.2014GGJS-045)资助。
*通信联系人。 E-mail:zhaoxiaolei@hpu.edu.cn,wuwn08@hpu.edu.cn;会员登记号:S06N6704M1112(吴伟娜)。
