基于半刚性二羧酸配体的两个三维镉ギ配位聚合物
2018-01-04洪梦如刘莹莹王智会侯红卫张裕平
刘 露 洪梦如 刘莹莹 王智会 李 英 侯红卫 张裕平
(1河南科技学院化学化工学院,新乡 453003)
(2郑州大学化学与分子工程学院,郑州 450052)
基于半刚性二羧酸配体的两个三维镉ギ配位聚合物
刘 露*,1洪梦如1刘莹莹1王智会1李 英1侯红卫*,2张裕平1
(1河南科技学院化学化工学院,新乡 453003)
(2郑州大学化学与分子工程学院,郑州 450052)
通过引入2个不同的氮杂环配体,得到了2个Cdギ配位聚合物[Cd(m-phda)(btbb)0.5]n(1)和[Cd(m-phda)(hbmb)0.5]n(2)(m-H2phda=间苯二乙酸),并对它们的结构进行了表征。结构分析表明配合物1是一个6-连接的pcu拓扑网络,拓扑符号为412·63。配合物2是一个5-连接的sqp拓扑网络,拓扑符号为(44·66)。另外,配合物1和2表现出较好的热稳定性和不同的荧光行为。
Cdギ;结构;拓扑;荧光
Over the past few decades,the metal-organic framework(MOF)materials,by reason of their unique structures,have motivated the chemicists to achieve the disparate multifunctional applications in the areas of gas storage and separation[1-2],ion exchange[3-4],heterogeneous catalysis[5-6], electrical conduction[7],molecular magnets[8]and luminescence materials[9].The solvothermal method has been broadly adopted to build MOFs or coordination polymers(CPs),since it not only can ease the matters of ligand solubility,butalso can boost the reactivity of the reactant during the crystallization process.Nevertheless,the crystallization process of CPs is influenced by many factors,such as the structural characteristics of ligand,the metal-ligand ratio,the temperature and the solvent systems[10-11],in this method.Among those factors mentioned above,the selection of ligands is of vital importance in the fabrication ofCPs.So far,numerous polybasic carboxylic acids ligands have been hired to construct CPs on account of their affluent coordination modes.Here,we choose semi-rigid m-phenylenediacetic acid(m-H2phda)as main ligand.m-H2phda possesses the following characters:firstly,it possess two carboxylic groups with diversified coordination mode;secondly,the existence of two-CH2-groups can make carboxylic groups twist,leading to product unlike conformations.Besides,the synthetic strategy of mixed polycarboxylate and N-donor ligand has been proved to be a fruitful way for the formation of multidimensional structures[12].
Inspired by the above ideas,the reactions of Cdギsalts and m-H2phda were executed in the presence of auxiliary ligands 1,4-bis(2-(4-thiazolyl)benzimidazole-1-ylmethyl)benzene(btbb)and 1,1′-(1,6-hexane)bis-(2-methylbenzimidazole) (hbmb)in thisessay.Two different three-dimensional (3D)Cdギcomplexes,[Cd(m-phda)(btbb)0.5]n(1)and[Cd(m-phda)(hbmb)0.5]n(2),have successfully been acquired.And then,the thermal stability and photoluminescence of the complexes 1 and 2 were also reported.
1 Experimental
1.1 Materials and methods
All reagents and solvents were commercially available without any further purification in addition to ligand btbb and hbmb,which were synthesized according to the literature[13].The data of FT-IR spectra were calendared on a Bruker-ALPHA spectrophotometer with KBr pellets in the scale of 400~4 000 cm-1.Elemental analyses(C,H,and N)were carried out on a FLASH EA 1112 elemental analyzer.Powder X-ray diffraction patterns were accomplished on a PANalytical X′Pert PRO diffractometer making use of Cu Kα1radiation(λ=0.154 06 nm)at 40 kV and 40 mA.The XRD patterns were collected in the range of 5°~50°.Thermogravimetric analyses were performed on a Netzsch STA 449C thermal analyzer with a 10℃·min-1heating rate in the air flow.The measurements of the luminescent spectra of the powdered solid samples were conducted on a Hitachi 850 fluorescence spectrophotometer at ambient temperature.
1.2 Synthesis of[Cd(m-phda)(btbb)0.5]n(1)
A mixture of Cd(NO3)2·4H2O(0.061 7 g,0.2 mmol),m-H2phda (0.009 6 g,0.05 mmol),btbb (0.050 4 g,0.1 mmol),EtOH(6 mL)and H2O(4 mL)was placed in a 25 mL Teflon-lined stainless steel container.The mixture was sealed and heated at 170℃for three days.Afterthe mixture wascooled to ambient temperature at a rate of 5℃·h-1,yellow block-shaped crystals of 1 were obtained with a yield of 43%(based on Cd).Anal.Calcd.for C24H18CdN3O4S(%):C,51.76;H,3.25;N,7.54.Found(%):C,51.74;H,3.31;N,7.58.IR(KBr,cm-1):3 434(m),2 923(m),2 854(w),1 629(m),1 573(w),1 475(w),1 425(s),1 384(vs),1 303(w),927(w),831(w),744(w),657(w),619(w),485(w).
1.3 Synthesis of[Cd(m-phda)(hbmb)0.5]n(2)
A mixture of Cd(Ac)2·2H2O(0.053 3 g,0.2 mmol),m-H2phda(0.009 6 g,0.05 mmol),hbmb(0.034 6 g,0.1 mmol),EtOH(5 mL)and H2O(5 mL)was placed in a 25 mL Teflon-lined stainless steel container at 160℃for three days.After the mixture was cooled to room temperature at a rate of 5℃·h-1,yellow blockshaped crystals suitable for X-ray diffraction were obtained with a yield of 55% (based on Cd).Anal.Calcd.for C21H21CdN2O4(%):C,52.78;H,4.42;N,5.86.Found(%):C,52.84;H,4.46;N,5.79.IR(KBr,cm-1):3 434(w),3 052(w),3 025(w),2 938(w),2 917(w),2 852(w),1 560(s),1 475(m),1 459(m),1 396(s),1 292(w),1 560(s),1 475(m),1 459(m),1 396(s),1 292(w),1 272(s),1 241(w),1 162(s),1 089(w),1 016(m),941(s),914(w),844(w),798(w),763(w),742(m),727(s),611(w),580(w),437(w).
1.4 Crystal structural determination
The collections of crystallographic data of 1 and 2 were fulfilled at room temperature adopting a Rigaku Saturn 724 CCD diffractometer,which was equipped with Mo Kα radiation (λ=0.071 073 nm).Crystal size of 1 is 0.20 mm ×0.19 mm ×0.15 mm.Crystal size of 2 is 0.21 mm ×0.19 mm ×0.17 mm.Absorption corrections were enforced via utilizing multi-scan program.The data were corrected on the basis of Lorentz and polarization effects.The structures were handled by firsthand methods and then refined by means of F2with a full-matrix least-squares technique that adopted the SHELXL-97 crystallographic software package[14].All of the non-hydrogen atoms were refined anisotropically.The hydrogen atoms of ligands were assigned at perfect positions capitalizing on a riding model and then refined isotropically.Crystallographic data and structure refinement details for 1 and 2 are generalized in Table 1.Selected bond lengths and bond angles of 1 and 2 are listed in Table 2.
CCDC:988559,1;1524429,2.
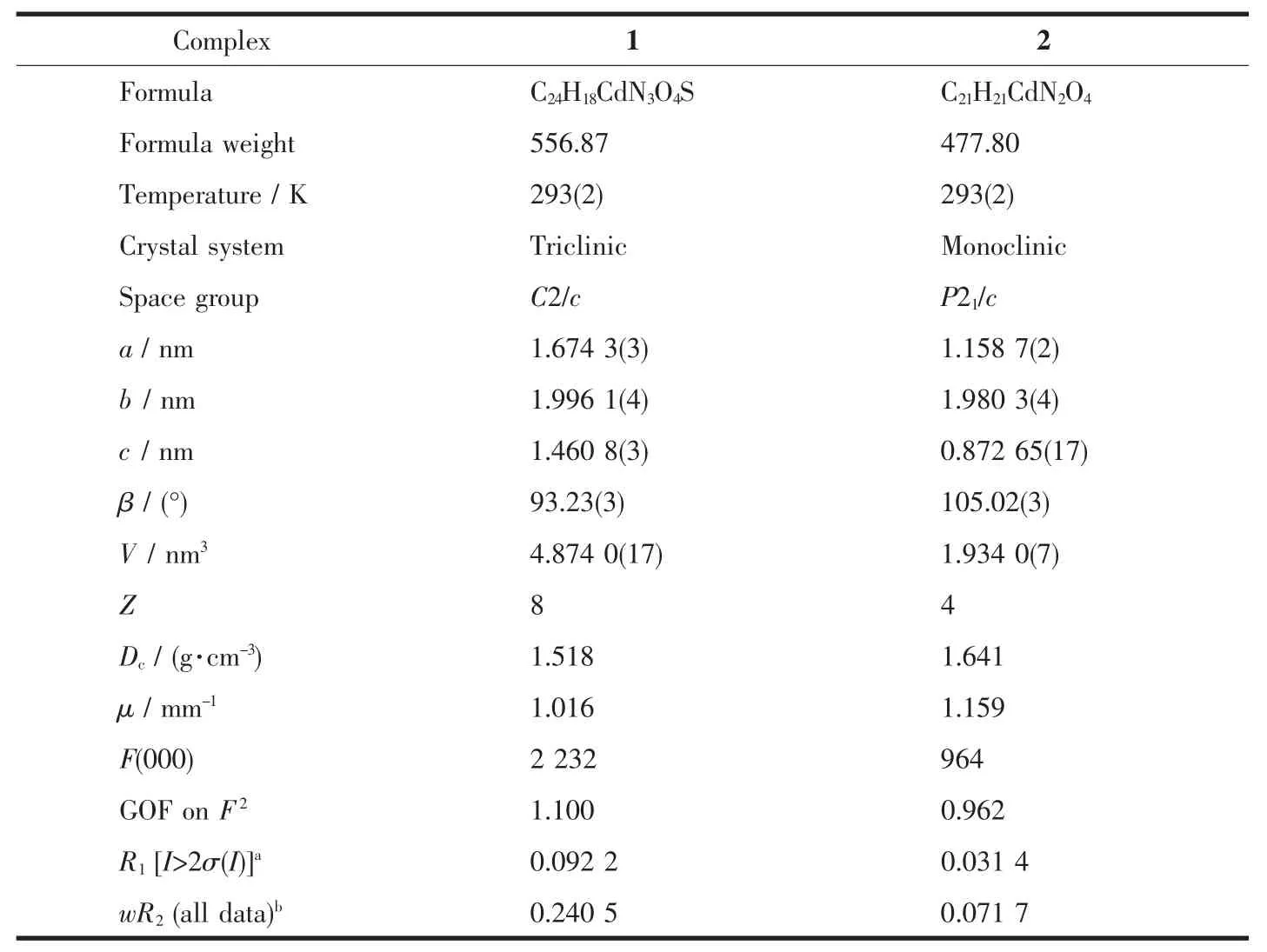
Table1 Crystal data and structure refinement details for complexes 1 and 2
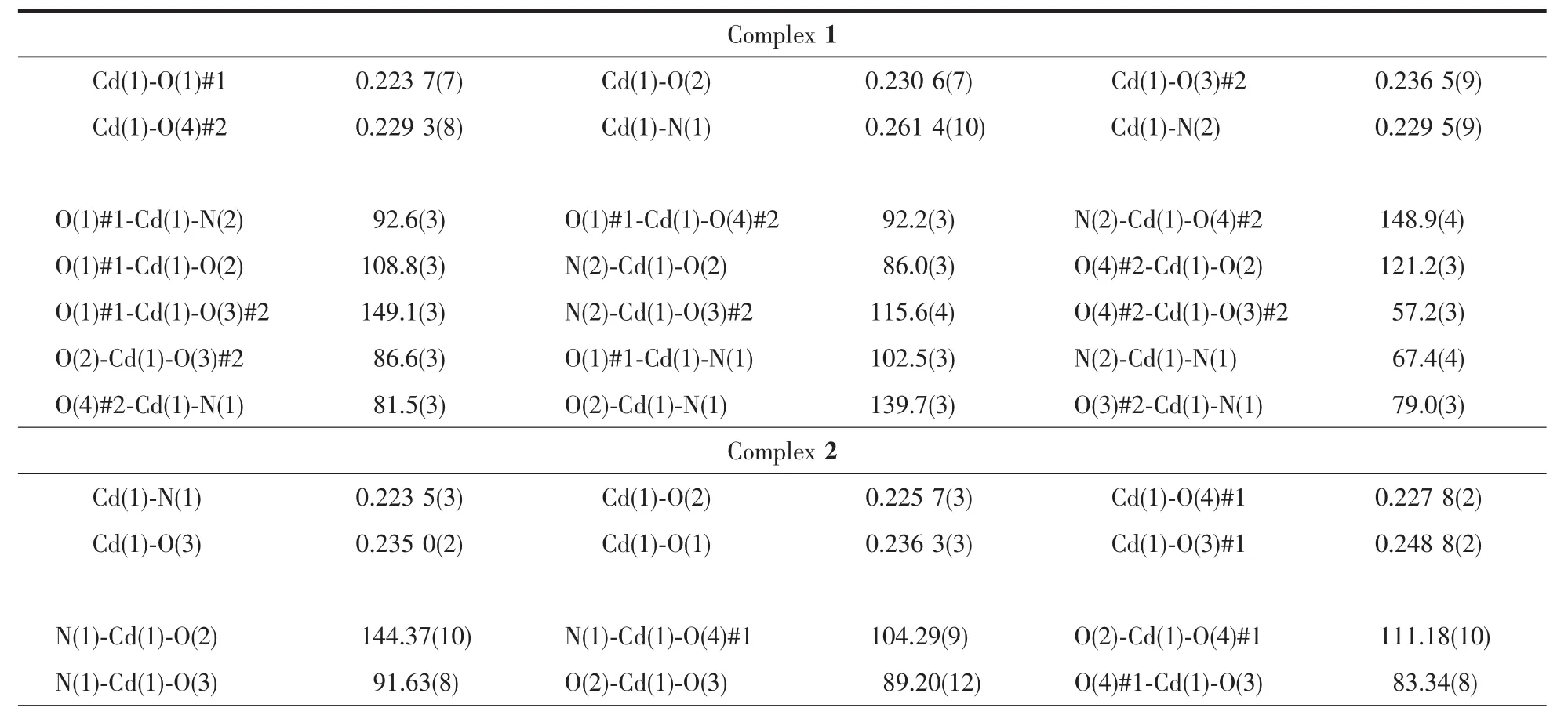
Table2 Selected bond lengths(nm)and bond angles(°)for 1 and 2
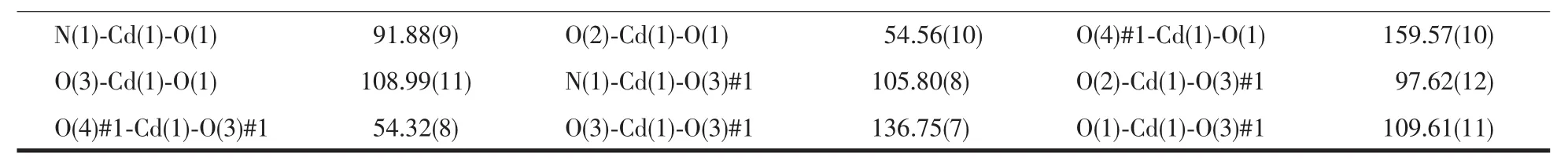
Continued Table 2
2 Results and discussion
2.1 Structuredescriptionof[Cd(m-phda)(btbb)0.5]n(1)
The results of crystallographic analysis reveal that 1 crystallizes in monoclinic system with C2/c space group.The asymmetric unit of complex 1 consists of one Cdギion,half btbb ligand and one m-phda2-ligand.As shown in Fig.1a,each Cdギion is in the distorted octahedral geometry with CdO4N2coordination environment finished by two nitrogen atoms(N1 and N2)from one btbb ligand and four oxygen atoms(O1A,O2,O3B,O4B)from three different m-phda2-ligands.The distances of Cd-O/N bonds range from 0.223 7(7)to 0.261 4(10)nm,which are similar to other Cd-based complexes[15].
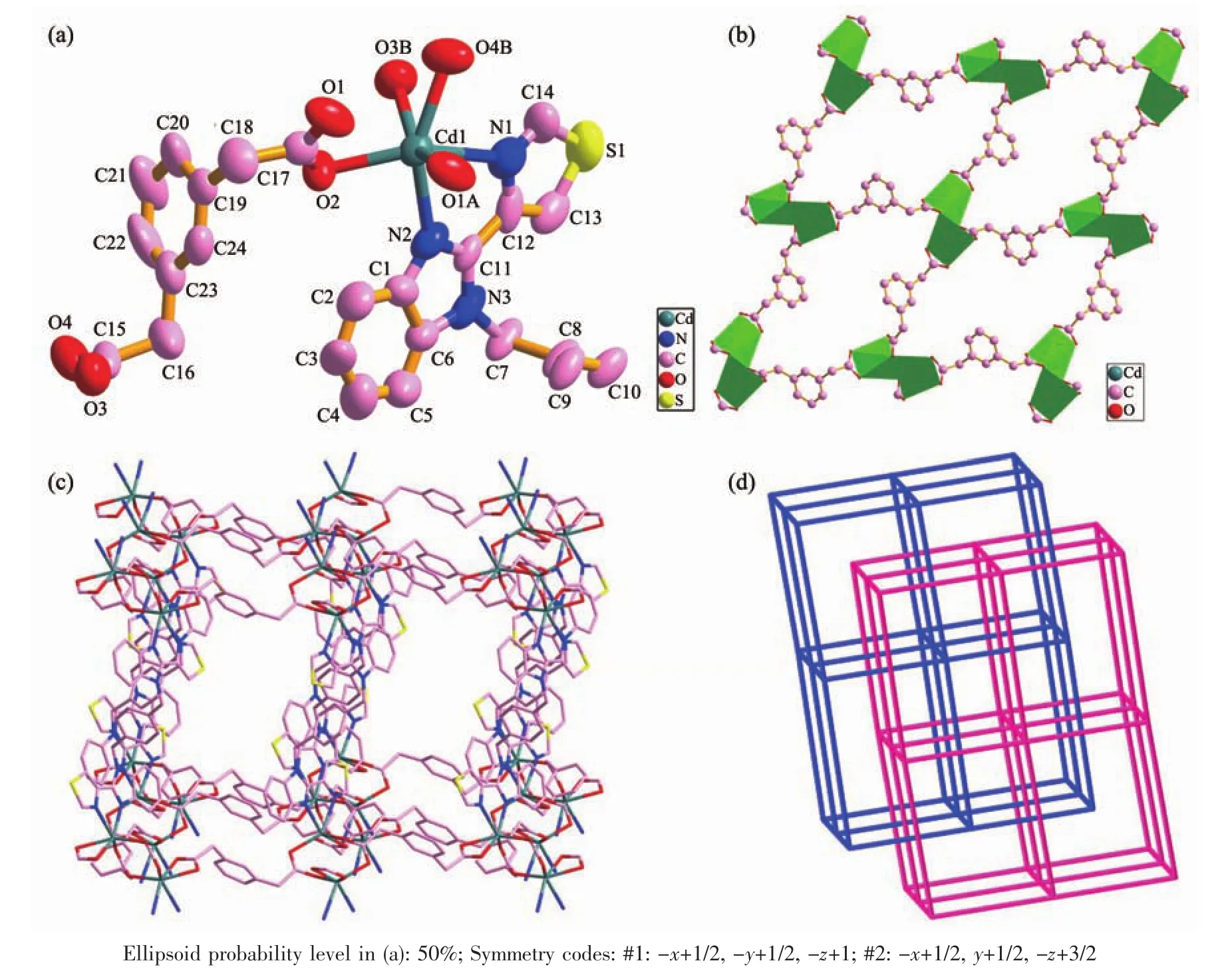
Fig.1 (a)Coordination environment of Cdギion with hydrogen atoms omitted for clarity;(b)Two-dimensional(2D)sheet generated by Cdギ ions and m-phda2-linkers in 1;(c)Schematic view of the 3D framework built by 2D Cdギ/m-phda2-layers and btbb pillars in 1;(d)Schematic representation of the 2-fold interpenetrated topology nets for 1
The btbb ligand adopts asymmetric trans-conformation with Ndonor…Ntorsion angles of 69.578°.Ligand m-phda2-also adopts asymmetric transconformation,and the anglesoftwo -CH2-are 114.255°and 111.979°.The two carboxylate groups of the m-phda2-ligand show different coordination modes:one carboxylate group adoptsbidentate chelating mode and the other adopts bidentate bridging mode.Each m-phda2-anion links three Cdギions resulting in a 2D sheet structure parallel to the bc plane(Fig.1b).The architecture of the 2D sheet is composed of the dinuclear unit[Cd2(COO)2]with Cd…Cd distance of 0.392 06 nm.The dinuclear units are further pillared upward and downward by two trans-conformational btbb ligands into a 3D pillar-layered framework(Fig.1c).
Better insight into the present 3D framework can be accessed by the topologicalmethod.Ifthe dinuclear unit[Cd2(COO)2]is viewed as a 6-connected node,and the btbb and m-phda2-serves as linkers,the 3D structure can be classified as a 6-connected pcu topology with point symbol of 412·63(Fig.1d).The void space in the single framework is so large that two identical 3D frameworks interpenetrate each other to form a 2-fold interpenetrating architecture(Fig.1d).
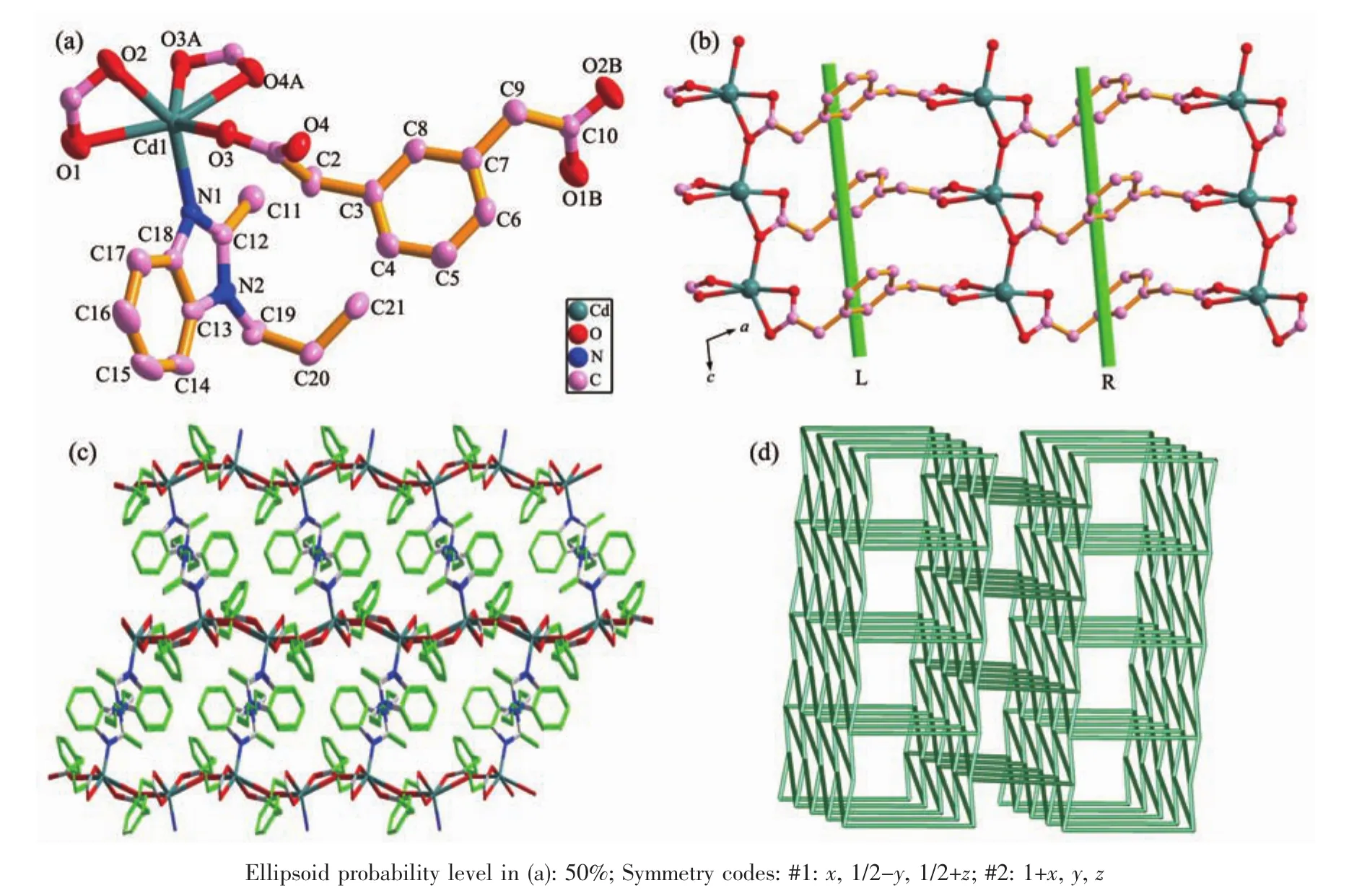
Fig.2 (a)Coordination environment of Cdギion with hydrogen atoms omitted for clarity;(b)View of the 2D sheet structure with alternately arranged left-and right-handed helical chains parallel to the ac plane;(c)Schematic view of the 3D framework built by 2D Cdギ/m-phda2-layers and hbmb pillars in 2;(d)Schematic view of the 3D topology network with a vertex symbol of 44·66for 2
2.2 Structure description of[Cd(m-phda)(hbmb)0.5]n(2)
The single crystal X-ray diffraction analysis for 2 shows that 2 crystallizes in monoclinic system with P21/c space group.The asymmetric unit of complex 2 consists of one Cdギion,one m-phda2-ligand,half hbmb ligand.As depicted in Fig.2a,each Cdギion is six-coordinated by five carboxylate oxygen atoms(O1,O2,O3,O3A and O4A)from three m-phda2-anions and one nitrogen atom(N1)from one hbmb ligand.The Cd-O bond distances vary from 0.225 7(3)to 0.248 8(2)nm,while the Cd-N1 bond length is 0.223 5(3)nm.The m-phda2-anions adopt asymmetric trans-conformation,and the angles of two-CH2-are 113.385°and 115.813°.Ligand hbmb adopts symmetric trans-conformation with an Ndonor…N-Csp3…Csp3torsion angle of 97.555°.
In 2,the ligand m-phda2-adopts(κ2-κ1-μ2)-(κ2)-μ3coordination information.The Cdギions are connected by m-phda2-anions to form a 2D sheet structure with alternately arranged left- and right-handed helical chains (Fig.2b).Both the helical pitches are 0.872 65(17)nm corresponding to the length of c-axis.Then the 2D nets are connected by hbmb ligands in a trans-conformation via the Cd-N connections to product a 3D pillar-layered framework(Fig.2c).The topological analysis is performed on 2(Fig.2d).We can consider each [CdCO3]unit as a 5-connecting node,which is linked to two [CdCO3]units by two m-phda2-ligands and one hbmb ligand.The m-phda2-and hbmb can be defined as linkers,the 3D framework of 2 can be described as a 5-connected sqp topology net with point symbol of 44·66(Fig.2d).
2.3 Effects of the coordination modes of the mphda2-anion and N-donor co-ligands on the structures of complexes 1 and 2
As described above,the semi-rigid m-phda2-ligand presents asymmetric trans-conformation in 1 and 2.The free conformations of-CH2-spacer vastly enrich the structures of 1 and 2.Although the m-phda2-ligand all acts as a three-connector to link three Cdギions in 1 and 2,their coordination modes are different.For 1,the angles of two-CH2-are 114.255°and 111.979°,singly.The ligand m-phda2-adopts(κ1-κ1)-(κ2)-μ3coordination information,in which two carboxyl groups adoptsyn-anti-μ2-η1:η1and μ1-η1∶η1coordina-tion modes,severally.This kind of m-phda2-anion links three Cdギions forming an ordinary 2D net.For 2,the angles of two-CH2-are 113.385°and 115.813°,respectively.The ligand m-phda2-adopts(κ2-κ1-μ2)-(κ2)-μ3coordination information,in which two carboxylic groups act as μ2-η2∶η1and μ1-η1∶η1modes,singly.This type of m-phda2-anion connects three Cdギ ions building a 2D net with alternately arranged left-and right-handed helical chains.
The introduction of N-donor ligands into Cdギ/mphda2-system will make the structure of complexes more complicated and more beautiful.In 1,btbb adopts asymmetric trans-conformation with Ndonor…N-torsion angles of 69.578°.btbb acts as pillars linking the adjacent Cdギ/m-phda2-nets into a 2-fold interpenetrating pcu 3D framework with point symbol of 412·63.In 2,hbmb adopts symmetric transconformation with an Ndonor…N-Csp3…Csp3torsion angle of 97.555°.Hbmb serves as pillars connecting the neighboring Cdギ/m-phda2-nets into a sqp 3D framework with point symbol of 44·66.
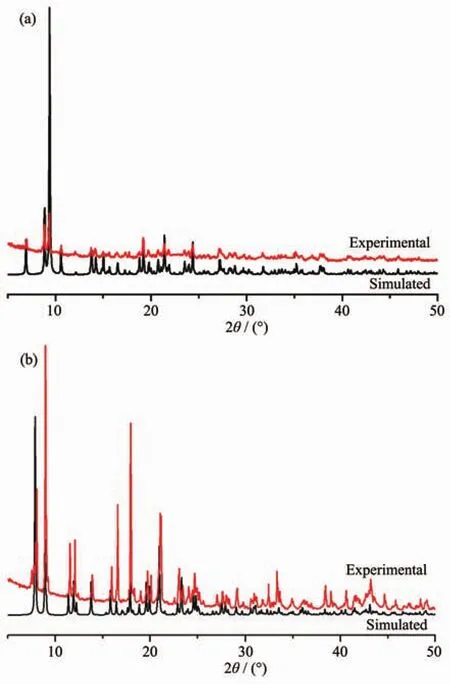
Fig.3 Experimental(top)and simulated(bottom)PXRD patterns of complexes 1(a)and 2(b)
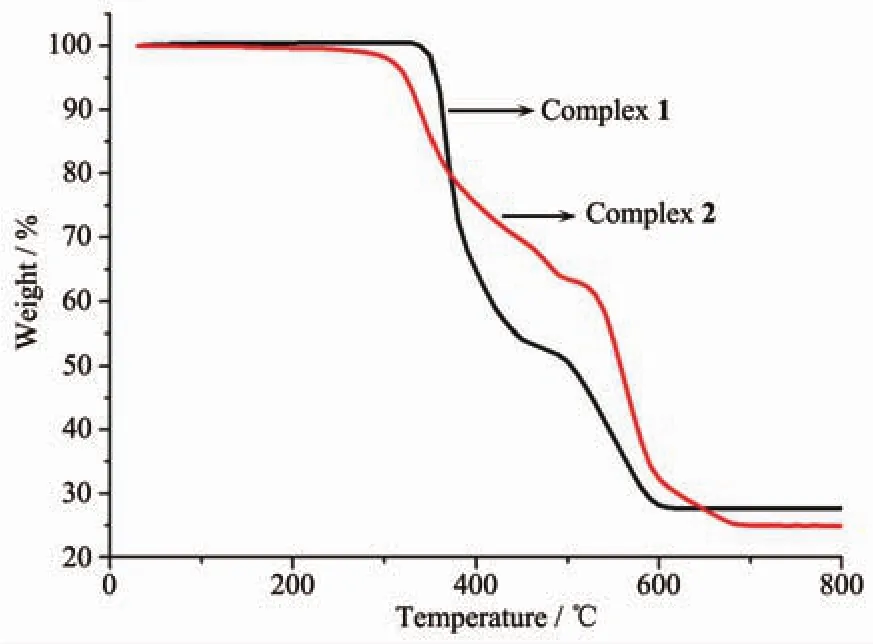
Fig.4 TGA curves of complexes 1 and 2
2.4 XRD patterns and thermal analyses
The PXRD patterns of complexes 1 and 2 are shown in Fig.3.The PXRD patterns of the two complexes determined by experiment are in line with the simulated ones of single crystals,which indicate that each of the two complexes is pure phase.To assess the stability of the coordination architectures,thermogravimetric analyses (TGA)of 1 and 2 were carried out(Fig.4).For 1,the weight loss from 336 to 607℃corresponds to the decomposition of half btbb ligand and one m-phda2-ligand.A residue of CdO(Obsd.27.69%,Calcd.26.87%)is discovered.As for complex 2,the weight loss in the range of 302~676 ℃represents the decomposition of the whole framework.A residue of CdO (Obsd.24.23%,Calcd.23.06%)is discovered.
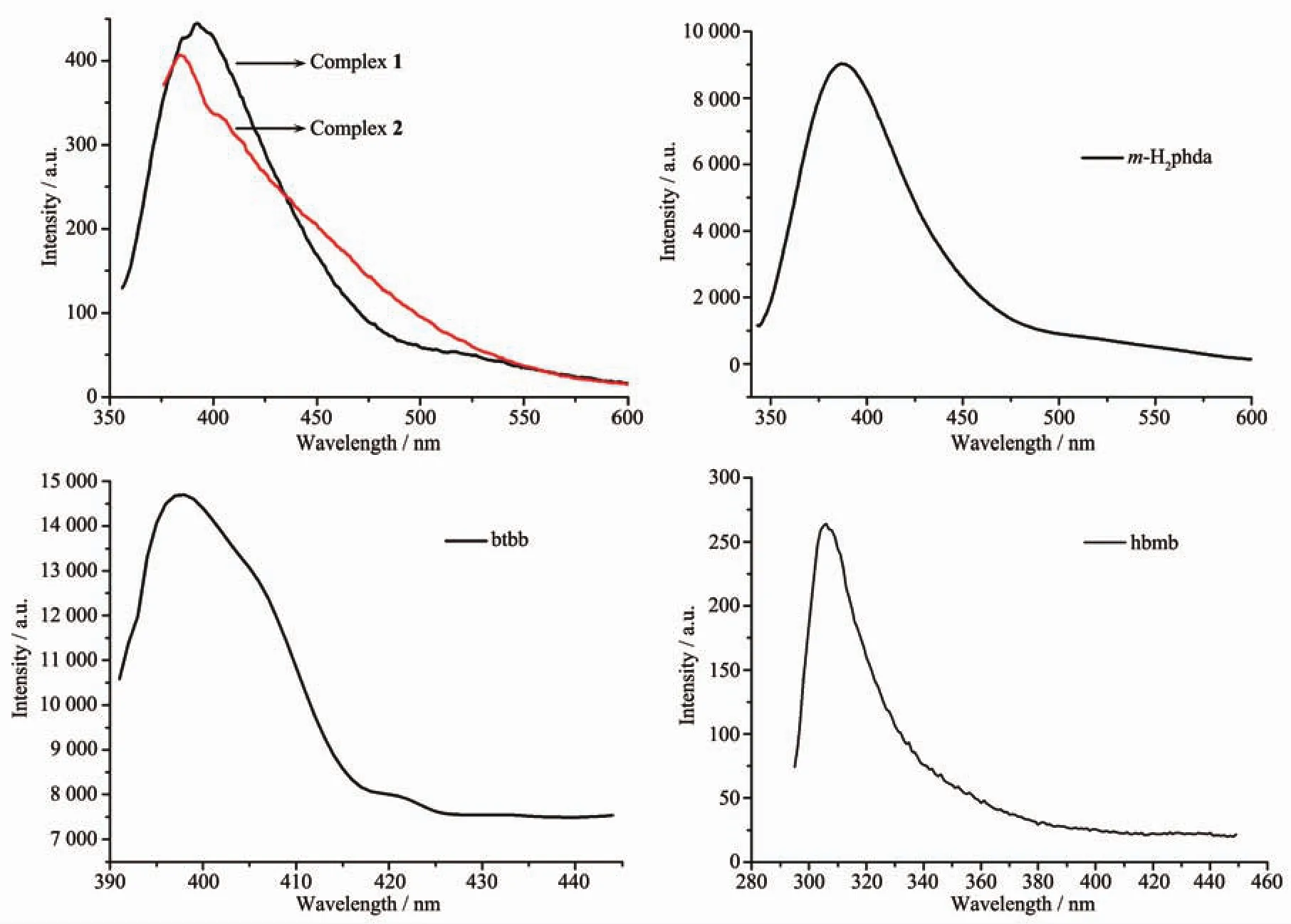
Fig.5 Solid-state photoluminescent spectra of complexes 1 and 2 and free ligand m-H2phda,btbb,hbmb
2.5 Photoluminescence properties
The solid-state luminescence spectra of complexes 1 and 2,the free ligand m-H2phda and the relevant auxiliary ligands were investigated at room temperature (Fig.5).The spectrum of m-H2phda and btbb shows one strong emission at 387 nm (λex=330 nm)and 399 nm (λex=385 nm),respectively,and the spectrum of hbmb shows one weaker emission at 306 nm (λex=275 nm).For complex 1,excited at 335 nm,it gives rise to an emission band at 391 nm.As compared to the btbb ligand,a little hypsochromic shift(8 nm)is observed.As compared to the m-H2phda ligand,a little red shift(4 nm)is observed.For complex 2,the emission spectrum displays a emission band centered at 384 nm with excitation maximum at 337 nm and the emission band presents little hypsochromic shift(3 nm)compared with that of the ligand m-H2phda.Obviously,the emission spectra of 1 can be mainly ascribed to the intraligand emission of mphda2-and btbb,while that of 2 can be mainly attributed to the intraligand emission of m-phda2-and hbmb.
3 Conclusions
In a nutshell,the synchronous use of the m-H2phda ligand and related N-donor co-ligands to react with Cdギions affords two CPs.These two complexes present enthralling structural motifs. This work manifests that the hire of mixed-ligands is a feasible strategy to adjust and control the formation of highdimensionality frameworks.The structural multiformity of 1 and 2 also imply that the conformation of the mphda2-ligand and additional N-donors play pivotal roles in the assembly of the final skeleton.
[1]Sumida K,Rogow D L,Mason J A,et al.Chem.Rev.,2012,112:724-781
[2]Qin L,Ju Z M,Wang Z J,et al.Cryst.Growth Des.,2014,14:2742-2746
[3]Yao R X,Xu X,Zhang X M.Chem.Mater.,2012,24:303-310
[4]Dalrymple S A,Shimizu G K H.Chem.Eur.J.,2002,8:3010-3015
[5]Huang S L,Jia A Q,Jin G X.Chem.Commun.,2013,49:2403-2405
[6]Huang S L,Weng L H,Jin G X.Dalton Trans.,2012,41:11657-11662
[7]Gándara F,Uribe-Romo F J,Britt D K,et al.Chem.Eur.J.,2012,18:10595-10601
[8]Whlert S,Wriedt M,Fic T,et al.Inorg.Chem.,2013,52:1061-1068
[9]Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012,112:1126-1162
[10]Tong M L,Ye B H,Cai J W,et al.Inorg.Chem.,1998,37:2645-2650
[11]Zhang W L,Liu Y Y,Ma J F,et al.Cryst.Growth Des.,2008,8:1250-1256
[12]Liu L,Lyu X F,Zhang L,et al.CrystEngComm,2014,16:8736-8746
[13]Bronisz R.Inorg.Chem.,2005,44:4463-4465
[14]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,A64:112-122
[15]Shi X J,Wang X,Li L K,et al.Cryst.Growth Des.,2010,10:2490-2500
Two 3D CdギCoordination Polymers Based on Semi-rigid Dicarboxylic Acid Ligand
LIU Lu*,1HONG Meng-Ru1LIU Ying-Ying1WANG Zhi-Hui1LI Ying1HOU Hong-Wei*,2ZHANG Yu-Ping1
(1School of Chemistry and Chemical Engineering,Henan Institute of Science and Technology,Xinxiang,Henan 453003,China)(2College of Chemistry and Molecular Engineering,Zhengzhou University,Zhengzhou 450052,China)
Two novel Cdギcoordination polymers(CPs),formulated as[Cd(m-phda)(btbb)0.5]n(1)and[Cd(m-phda)(hbmb)0.5]n(2)(m-H2phda=m-phenylenediacetic acid,btbb=1,4-bis(2-(4-thiazolyl)benzimidazole-1-ylmethyl)benzene,hbmb=1,1′-(1,6-hexane)bis-(2-methylbenzimidazole))have been prepared by hydrothermal reactions of the semirigid ligand m-H2phda with Cdギions in the presence of two disparate N-heterocyclic co-ligands and further characterized by infrared spectra(IR),powder X-ray diffraction(PXRD),elemental analyses and thermogravimetric(TG)analyses.1 possesses a 6-connected 2-fold interpenetrating 3D pcu topology net with point symbol of 412·63.2 exhibits a 5-connected 3D sqp network with a Schläfli symbol of 44·66.Additionally,complexes 1 and 2 present better thermal stabilities,as well as 1 and 2 also show different photoluminescence behaviors in the solid state.CCDC:988559,1;1524429,2.
Cdギ;structure;topology;fluorescence
O614.24+2
A
1001-4861(2018)01-0187-08
10.11862/CJIC.2018.006
2017-06-15。收修改稿日期:2017-09-27。
河南科技学院高层次人才科研启动项目、河南科技学院大学生创新训练计划项目、河南省博士后科研项目、河南省高等学校重点科研项目(No.16A150007)和河南科技学院标志性创新工程项目(No.2015BZ02)资助。
*通信联系人。 E-mail:liululiulu2012@126.com,houhongw@zzu.edu.cn
