在离子液体[BMIm][BF4]中电沉积光亮金镀层
2018-01-04宋云鹤杨培霞冯忠宝张锦秋安茂忠
宋云鹤 杨培霞 连 叶 冯忠宝 张锦秋 安茂忠
(哈尔滨工业大学化工与化学学院,城市水资源与水环境国家重点实验室,哈尔滨 150001)
在离子液体[BMIm][BF4]中电沉积光亮金镀层
宋云鹤 杨培霞 连 叶 冯忠宝 张锦秋 安茂忠*
(哈尔滨工业大学化工与化学学院,城市水资源与水环境国家重点实验室,哈尔滨 150001)
在离子液体1-丁基-3-甲基咪唑四氟硼酸盐 ([BMIm][BF4])中以HAuCl4·3H2O为主盐、通过添加5,5-二甲基乙内酰脲(DMH)和胞嘧啶可得到色泽光亮、厚度达1.5 μm的金镀层,沉积过程中阴极电流效率可达到100%。SEM和XRD测试结果表明,DMH和胞嘧啶具有细化晶粒、平整镀层的作用。电化学测试结果表明,DMH可分别与Au3+、Au+形成配合物[Au(DMH)4]-、[Au(DMH)2]-,抑制了还原过程的表面转化步骤,从而增加了阴极极化,起到光亮镀层、细化晶粒的作用;胞嘧啶可在金核表面吸附,从而可以进一步光亮镀层、细化晶粒,与DMH有协同作用。循环伏安测试研究了镀液的电化学行为,研究表明在此体系中Au3+的还原为两步还原过程,分别为Au3+→Au+和Au+→Au。此外DMH和胞嘧啶的添加不会带来副反应。
金镀层;离子液体;电沉积;5-5二甲基乙内酰脲;胞嘧啶
0 Introduction
Gold deposits have numerous excellent physical and chemical properties,such as the golden yellow appearance,excellent electrical conductivity,topping thermal conductivity and outstanding chemical stability,and they are widely used as decorative deposits[1],protective deposits[2]and functional deposits[3].Gold deposits have extensive applications in decorations,electronic devices and aerospace.Gold electroplating baths including cyanide are the most widely used nowadays.Nowadays,cyanidebaths,which have excellently stable electrolytes,refined grains and good adhesion of gold deposits[4-5],are the most widely used gold electroplating Unfortunately,cyanide brings immense hazards to the environment and biology[6].To solve the problem,golden-bright gold deposits were electrodeposited in ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate([BMIm][BF4]),a free-cyanide bath.
Ionic liquids (ILs)are a kind of conductive organiccompoundssaltin liquid stateatroom temperature.Moreover,ILs have chemical stability,non-toxic,negligible vapor pressure,especially wide electrochemical window,high solubilityof metal salts and high conductivity[7-9].Thus,ILs attract much attention in electrodeposition.Many active metals are unavailable in aqueous solution,but those can be obtained in ionic liquids by electrodeposition,such as Al[10],Mg[11],Na[12]and Li[13].Ionic liquid[BMIm][BF4]has the excellent properties above mentioned,and it can be applied as a solvent in electroplating field[14-15].[BMIm][BF4]has a high solubility for chloroauric acid.The cations and anions of [BMIm][BF4]have no reducibility,so that Au3+will not be reduced spontaneously.Therefore,[BMIm][BF4]was used as electrolytes for electrodeposition of gold in this work.
5,5-Dimethylhydantoin(DMH)has very important applications in electrodeposition,due to its low-cost,non-toxic and environmentally friendlyproperties.DMH can be used as a stable complexing agent in aqueous solution and it can combine with many metal ions to compose stable complexes,including Zn2+[16],Ag+[17],Au3+[18-19].Therefore,DMH was added into[BMIm][BF4]as the complexing agent,and it had a good effect,as expected.However,the gold deposits obtained in the bath involving DMH as the complexing agent were insufficiently bright,whose surfaces were unsmooth.It was essential that appropriate additives were selected to improve the brightness of deposits and refine crystal grains.Cytosine can be used as an additive in metal electrodeposition,and it is adsorbed on the surface of electrodes[20-21].
Bright gold deposits were obtained in IL by electrodeposition in this work,and there were some other studies carried out in ILs to electrodeposite gold.De Sá et al.[22]electrodeposited gold deposits in 1-butyl-1-methyl-pyrrolidinium dicyanamide ionic liquid at open circuit and constant potential conditions.The grains of the deposits obtained in the two conditions were spherical and dendritic,respectively.The gold films obtained were golden-brown.Katayama et al.[23]used 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide as solvent,and gold tribromide was chosen as the main salt on this basis.The Au nano-particles with a diameter of 3 nm were obtained,which almost dispersed in the bath.The compact and bright gold deposits were not obtained in the work of other researchers.
In this work,[BMIm][BF4]was chosen as the solvent and chloroauric acid (HAuCl4·3H2O)as the main salt.DMH and cytosine were added into the baths as the complexing agent and the additive,respectively.The gold deposits obtained in the abovementioned bathswere characterized by scanning electron microscope (SEM) and X-ray diffraction(XRD).The influences of DMH and cytosine in the electrodeposition were investigated by linear sweep voltammetry (LSV).Furthermore,cyclic voltammetry(CV)were performed to study the electrochemical behavior of the baths.
1 Experimental
1.1 Materials and methods
[BMIm][BF4]was commercially available,and dried under vacuum for 24 h at 353 K before used.HAuCl4·3H2O was added to[BMIm][BF4]with a concentration of 0.05 mol·L-1,which was prepared by Au and hydrochloric acid and nitric acid.A magnetic stirrer stirred the solution under dry N2at 328 K for 6 h until HAuCl4·3H2O added was completely dissolved.The baths with DMH (Aladdin,98%)and cytosine(Aladdin,98%)were prepared in the same way.
The surface morphology of gold deposits was characterized by scanning electron microscopy(SEM,Zeiss Supra55 SAPPHIRE)at 10 kV.The crystal structure was investigated by X-ray diffractometry(XRD,Bruker D8 Advance)with Cu Kα radiation at 40 kV and 40 mA (λ=0.154 06 nm).The 2θ ranged from 20°to 90°at a scan rate of 0.02°·s-1.The type of counting X-ray was counts·s-1,and the detector used was LynxEye.
The electrochemicalmeasurements including linear sweep voltammetry and cyclic voltammetry were carried out in N2atmosphere at 328 K.A threeelectrode electrolytic cell and an electrochemical workstation CHI750d were used.A glassy carbon electrode (GCE,Φ=3 mm)was used as the working electrode(WE)of cyclic voltammetry.The WE was a glassy carbon rotating disk electrode (GC-RDE,Φ=5 mm)in linear sweep voltammetry.The reference electrode(RE)was a platinum wire(Φ=0.38 mm)and the counter electrode (CE)was a platinum plate(1 cm×1 cm)in the voltammetry measurements.The linear sweep voltammograms and cyclic voltammograms were separately performed at a scan rate of 1 and 10 mV·s-1.The GCE was successively polished by α-Al2O3polishing powder with particle size of 1.5 μm,1 μm and 0.5 μm after an electrochemical measurement was completed.Then,the GCE was washed with ethanol and deionized water in turn,and finally dried with N2.
1.2 Preparation and characterization of gold deposits
In the electrodeposition,a gold plate(1.5 cm×1.5 cm)was used as the anode,and a nickel-plated copper foil(1.0 cm×1.0 cm)was the cathode.The distance between the anode and the cathode was retained at 3 cm.The current density was carried out 0.3 A·dm-2for electrodeposition in N2atmosphere.A magnetic stirrer at a rate of 500 r·min-1stirred the baths used in the electrodeposition at 328 K.After electrodeposition,the sample was successively rinsed with ethanol and deionized water to remove the remaining electrolyte,and then dried with N2.
The average grain size(t)of deposits was estimated using the Scherrer equation[24]:
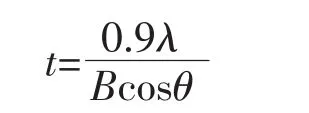
where λ is the wavelength of the incident X-rays,B is the peak half width at half maximum in radians,and θ is the Bragg angle.
The thickness of gold deposits and the cathodic current efficiency were calculated by the weight of gold deposits.The thicknesses of gold deposits were calculated according to the equation:
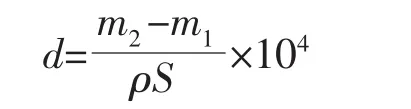
where d(μm)is the thickness of gold deposits,m1(g)and m2(g)are the weight of nickel-plated copper foils before and after the electrodeposition of gold,ρ is the density of gold(19.32 g·cm-3),and S(cm2)is the area of gold deposits.
The cathodic current efficiency was calculated according to the equation:
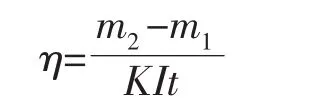
where η is the cathodic current efficiency,K is the electrochemical equivalent of Au3+(2.45 g·A-1·h-1),I(A)is the current in the electrodeposition process,and t(h)is the time of electrodeposition.
2 Results and discussion
2.1 Characterization of gold deposits
Gold deposits were obtained from [BMIm][BF4]solution without and with DMH,as well as the bath with DMH and cytosine.Fig.1 displays digital pictures of different gold deposits.
The gold deposit obtained in the basic bath is red-brown,whose surface is uneven and rough(Fig.1a).As displayed in Fig.1b,the gold deposit with a relatively uniform surface was obtained in bath with DMH,whereas the colour is slightly red.Compared to the gold deposits in Fig.1a and b,the appearance of the gold deposit in Fig.1c is optimal.The gold deposit is bright golden yellow,and the surface is uniform.It indicates that the addition of DMH as the complexing agent and cytosine as the additive to the bath can significantly improve the appearance of gold deposits.The thicknesses of the gold deposits displayed in Fig.1 are nearly 1.5 μm.The corresponding cathodic current efficiencies are 100%in the electrodeposition.The result indicates that the cathode reaction was the reduction of Au3+in bath with DMH and cytosine,and no other side reactions occurred.
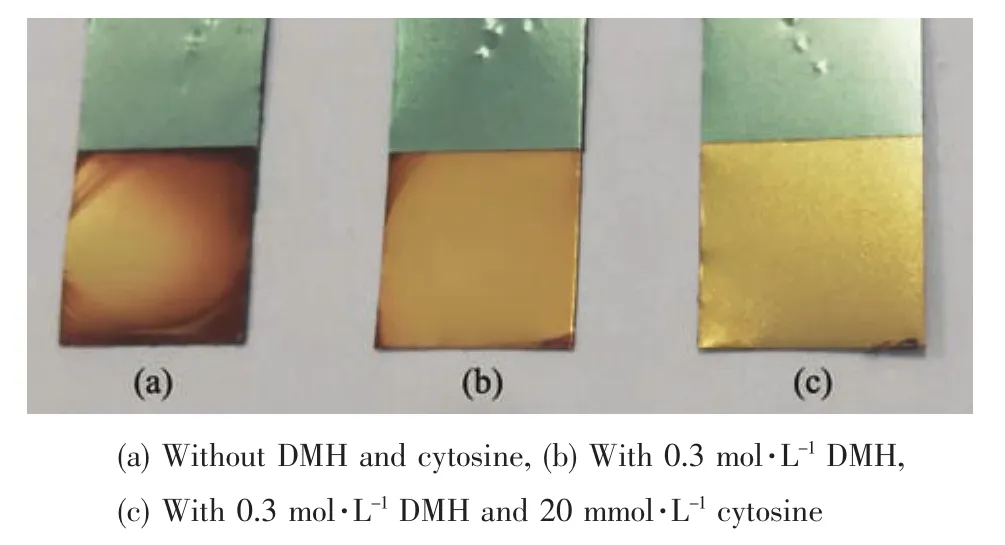
Fig.1 Digital pictures of gold deposits obtained from[BMIm][BF4]containing HAuCl4·3H2O

Fig.2 SEM images of gold deposits obtained from[BMIm][BF4]containing HAuCl4·3H2O
Fig.2 reveals the surface morphologies of the gold deposits obtained in the[BMIm][BF4]solution without and with DMH,as well as the bath with DMH and cytosine.As displayed in Fig.2a and b,the deposit obtained in the bath without DMH and cytosine is uneven,and many coarse crystal particles exist on the surface.The grains become smaller and the coarse crystal particles almost disappear on the surface,which is still uneven (Fig.2c and d).The gold deposit obtained in bath with DMH and cytosine is the smoothest,and the crystals are the smallest(Fig.2e and f).It can be concluded that gold deposits with a bright golden yellow appearance and refined grains can beobtained when DMH isadded asthe complexing agentand cytosine isadded asthe additive in the bath.
The XRD patterns of the gold deposits obtained in the above mentioned electrolytes are presented in Fig.3.The five peaks in Fig.3(a)and(b)and(c)at 2θ values of 38.2°,44.4°,64.6°,77.6°and 81.8°are indexed to the Au(111),(200),(220),(311)and(222)crystal face,respectively.The two indexed peaks in Fig.3(a)and(b)and(c)at 2θ values of 51.9°and 74.1°correspond to the nickel-plated copper foil,which was used as the substrate of the electrodeposition of gold.As shown in Fig.3,the intensity of the diffraction peak corresponding to the Au(111)crystal face is the largest in the five peaks.It indicates that the gold electrodeposits were preferentially deposited along the direction of the Au(111)crystal face.The tendency of preferred deposition along Au(111)crystal face direction is intenser with the addition of DMH and cytosine.The average size of crystal grains of gold deposits was calculated according to the Scherrer formula[24].The average size of crystal grains that Fig.3(a),(b)and(c)correspond to is 34.8,30.7 and 28.1 nm,respectively.The grains of the deposits obtained in bath with DMH and cytosine are the smallest.This result is consistent with the SEM measurements.
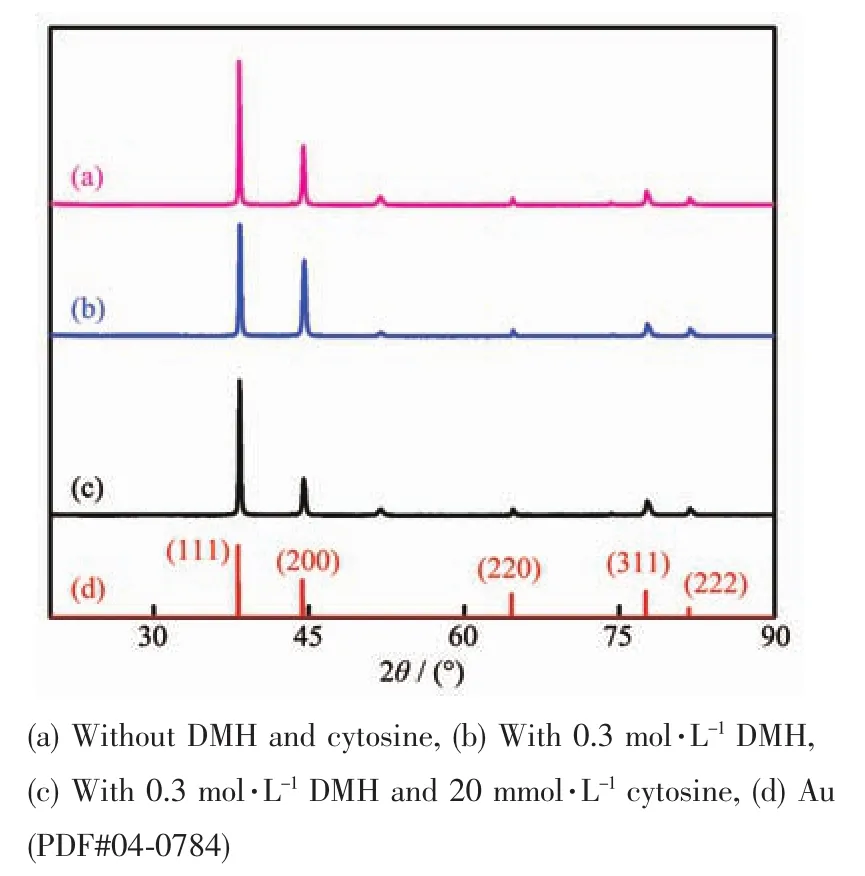
Fig.3 XRD patterns of gold deposits obtained from[BMIm][BF4]containing HAuCl4·3H2O
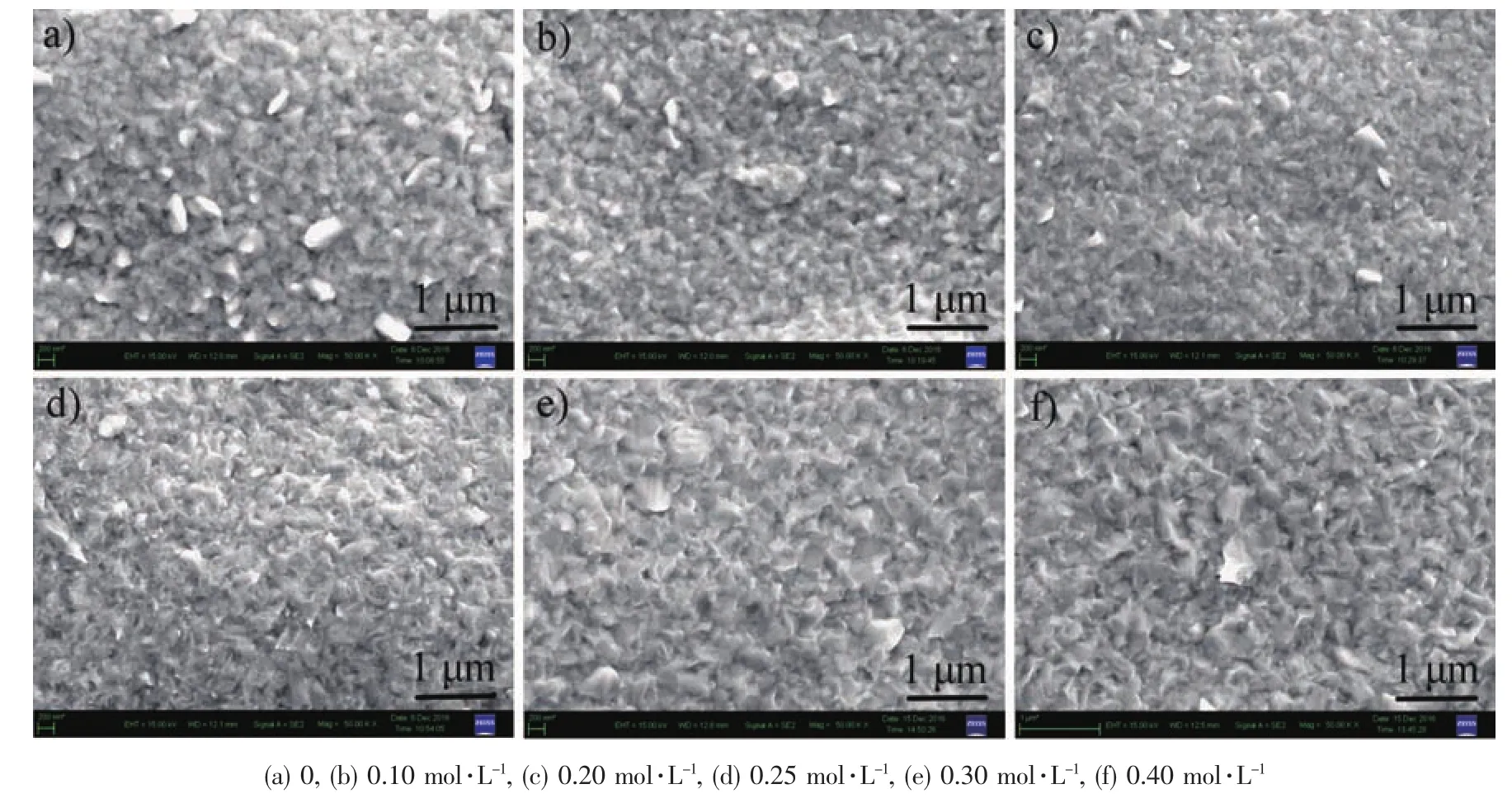
Fig.4 SEM images of gold deposits obtained from the bath containing 0.05 mol·L-1HAuCl4·3H2O with different concentrations of DMH
2.2 Influences of different components
In order to investigate the influences of DMH,the gold deposits were obtained from the different solution of 0.05 mol·L-1HAuCl4·3H2O in[BMIm][BF4]containing 0,0.10,0.20,0.25,0.3 and 0.40 mol·L-1DMH,respectively,and then the macroscopic conditions of the gold deposits were observed.The color of the gold deposits obtained from the bath without DMH is brown-yellow,and the surface is reddish.The gold deposits obtained from the bath with 0.10,0.20 and 0.25 mol·L-1DMH are all yellow,With adding DMH into the electrolytes,the appearances of gold deposits turn to better.The gold deposits obtained from the bath with 0.30 mol·L-1DMH are obviously optimal in appearance,which possess a brighter surface;the color of the deposits doesn′t change as the concentration of DMH continues to increase.
SEM measurements were performed to further investigate the influence of DMH on the surface morphologies of gold deposits.Fig.4 shows SEM images of the gold deposits obtained from the baths containing differentconcentrationsofDMH.The surface of the gold deposits is rough,and there are many coarse crystal grains on the gold deposits(Fig.4a).The smoothness of the gold deposits increases with the increased concentration of DMH,and the number of the coarse crystal grains is less and less.The gold deposits are the most smooth when the concentration of DMH is 0.30~0.40 mol·L-1,and the coarse crystal grains almost disappear.It was proved that DMH could brighten the gold deposits and refine the crystal grains with the contrast of the macroscopic states and surface morphologies of the deposits.
The influences of cytosine on the gold deposits were investigated on the basis of DMH in the baths.Cytosine with a concentration of 5,10,15,20 and 25 mmol·L-1was dissolved in the [BMIm][BF4]solution containing HAuCl4·3H2O and DMH.The gold deposits obtained in the baths with cytosine are brighter than those obtained in the bath without cytosine.The gold deposits obtained in the baths with cytosine are golden yellow,and almost no difference in appearance can be observed with the increase of the concentration of cytosine.
The surface morphologies of the gold deposits were characterized by SEM measurements to research the influences of cytosine.The crystal grains of the gold deposits can be refined with adding cytosine(Fig.5).The sizes of the crystal grains are gradually reduced with the concentration of cytosine increasing.The crystal grains of the gold deposits are the smallest,which were obtained in the bath with 20 mmol·L-1cytosine.SEM images indicated that cytosine could further refine the crystal grains of the gold deposits on the basis of DMH in the bath.Moreover,the addition of cytosine can enhance the brightness of the gold deposits through the contrast of the macroscopic states.

Fig.5 SEM images of gold deposits obtained from the bath containing 0.05 mol·L-1HAuCl4·3H2O and 0.3 mol·L-1 DMH with different concentrations of cytosine
2.3 Electrochemical measurements
As shown in Fig.6 the linear sweep voltammograms were measured with scan rate of 1 mV·s-1at 328 K in order to further investigate the influences of DMH and cytosine in the bath.The potential was scanned from-0.2 V,along the negative potential direction up to-1.8 V.Fig.6a is the linear sweep voltammogram of the pure ionic liquid [BMIm][BF4].The current density is less than 10-3mA·cm-2in the potential scan from-0.2 to-1.8 V.Therefore,[BMIm][BF4]is chemically stable in the above measurement conditions.The curves b,c and d are the voltammograms of the bath containing HAuCl4·3H2O without DMH and cytosine,with DMH,and with DMH and cytosine,respectively.
The current density of the curve b increases sharply from-0.45 V;with adding DMH,the potential shifts negatively to-0.53 V (Fig.6c).Besides,the current density of the curve c decreases with the contrast of curve b.The gold complexes[Au(DMH)4]-may be formed by Au3+and DMH in the bath,which can be formed in aqueous solutions[18-19].[Au(DMH)4]-inhibits the surface convert process in the reduction process,and it leads to the reduction of[Au(DMH)4]-occurring at more negative potential compared to Au3+.Therefore,the addition of DMH increases the cathodic polarization,resulting in brightening gold deposits and refining crystal grains of gold deposits.
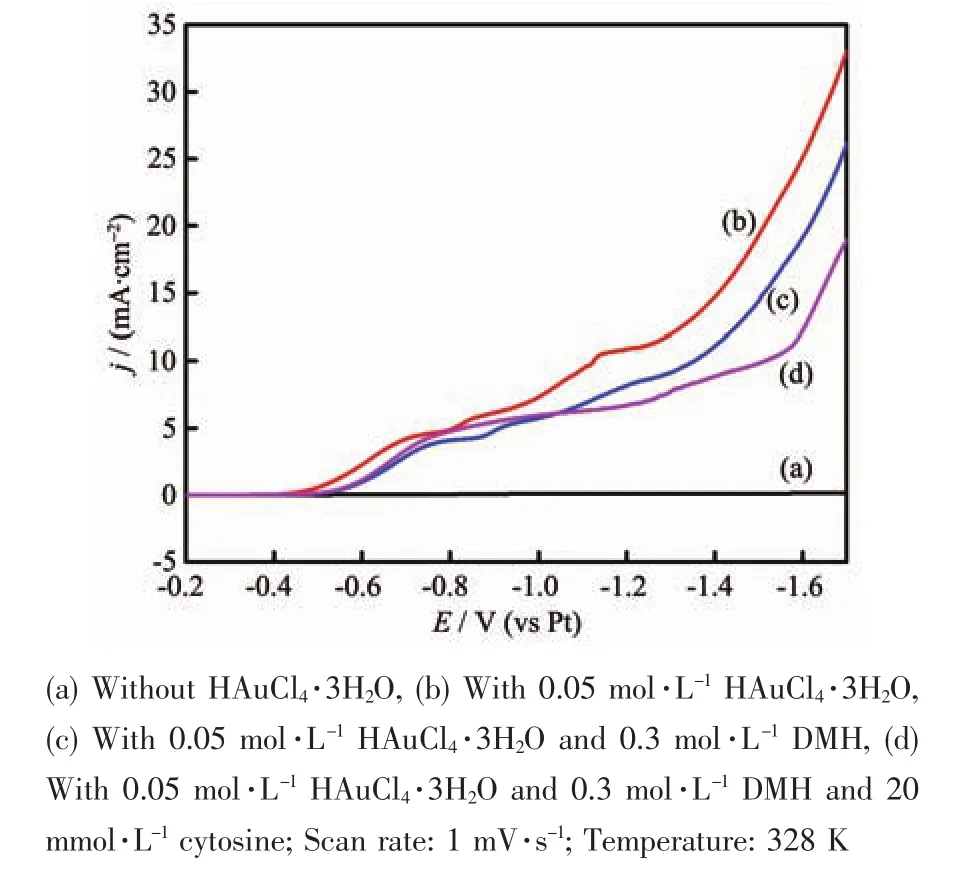
Fig.6 Linear sweep voltammograms recorded at the GCRDE in[BMIm][BF4]
The overpotential of curve d is not obviously changed with the addition of cytosine to the bath,but the cathodic current density is lower compared to the curve c when the potential exceeds-1.1 V.This period corresponds to the reduction of[Au(DMH)2]-to Au. (The detailed analysisofthe reactionsis investigated in Fig.8)Cytosine can be adsorbed on the generated Au nuclei[20-21], which is related to the growth rate of crystal nucleus and the nucleation rate of deposits.The addition of cytosine inhibits the reduction of[Au(DMH)2]-to Au,leading to the current density decreasing.Cytosine can be used as an additive to brighten the deposits and refine the grains,which has a synergistic effect with DMH.It accords with the results of digital images,SEM and XRD measurements.
The cyclic voltammogram recorded at the GCE of[BMIm][BF4]was performed with scan rate of 10 mV·s-1at 328 K to define the electrochemical window.As shown in Fig.7,the chemical properties of[BMIm][BF4]are stable at the range of-2.0 to 1.9 V in the above conditions.A cathode peak occurs at around-1.0 V in the voltammogram curve,which may be caused by the oxidation of the resulting reduzate during the potential scan along the negative direction.
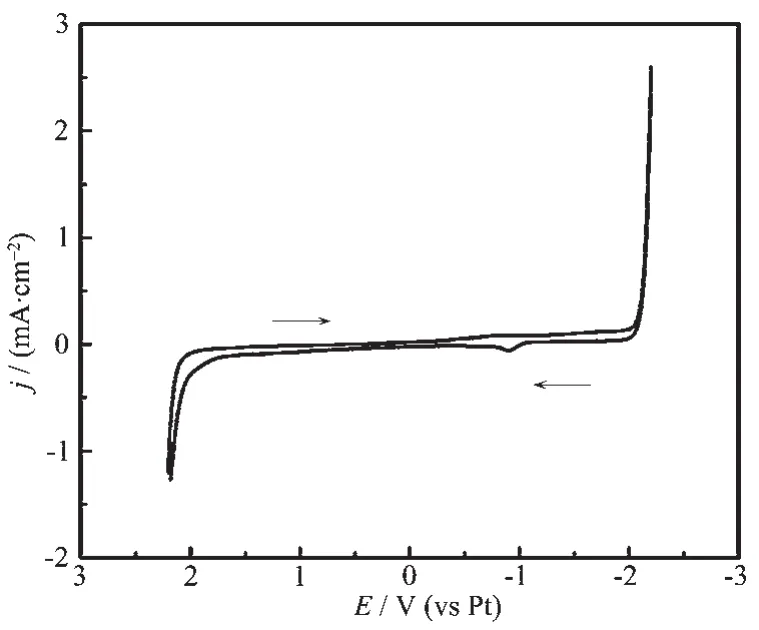
Fig.7 Cyclic voltammogram recorded at the GCE in[BMIm][BF4]with scan rate of 10 mV·s-1at 328 K
Cyclic voltammograms were performed to investigate the electrochemical behavior of the bath containing HAuCl4·3H2O in the absence or presence of DMH and cytosine,as shown in Fig.8.Fig.8A shows the cyclic voltammogram of the [BMIm][BF4]solution containing HAuCl4·3H2O.The potential was scanned from 0.6 V,along the negative potential direction up to-1.7 V,and then inverted to 0.6 V.Two reduction peaks (Fig.8A)are observed in the potential scan from 0.6 to-1.7 V vs Pt wire,at-0.59 V(c1)and-1.3 V(c1),respectively.The c1peak is attributed to the reduction of Au3+to Au+,and the c2peak is assigned to the reduction of Au+to Au.The reactions the two reduction peaks(c1and c2)represent are essentially consistent with the conclusions of previous authors[25-26].The curve A presents two oxidation peaks,a1and a2,in the reverse potential scan,which correspond to 0.27 and-0.36 V,respectively.
In order to investigate the reactions the two oxidation peaks(a1and a2)corresponded to separately,the curve A′were performed.The potential was scanned from 0.6 V,along the negative potential direction up to-0.63 V when the c1′peak just emerged,and then inverted to 0.6 V.The c1′peak represents the reduction of Au3+to Au+,which is as same as the c1peak shown in Fig.8A.It is attributed to that the potentials of the c1peak in Fig.8A and c1′peak in Fig.8A′are same in the completely same measurement conditions.Therefore,the a1′peak is assigned to the oxidation of Au+to Au3+.The a1peak and the a2peak separately correspond to the oxidation of Au+to Au3+,Au to Au+.Based on the above conclusions,a gold plate was used as the anode to ensure the purity of the bath and maintain a certain concentration of Au3+in the bath,which could be dissolved in the electrodeposition.
Fig.8B shows the cyclic voltammogram of the[BMIm][BF4]solution containing HAuCl4·3H2O and DMH.The corresponding potentials of the c1and c2peak are-0.72 V and-1.43 V(Fig.8B),respectively.The addition of DMH did not change the reactions in the bath,and the obvious difference is the negative shift of the two cathode peaks (c1and c2).DMH may complex with Au3+and Au+to form[Au(DMH)4]-and[Au(DMH)2]-in the bath,which inhibit the two reduction processes,respectively.The c1and c2peak correspond tothe reduction of[Au(DMH)4]-to[Au(DMH)2]-,[Au(DMH)2]-to Au(Fig.8B).DMH can increase the cathodic polari-zation,which is consistent with the results of Fig.6.
Fig.8C shows the cyclic voltammogram of the bath consisting of HAuCl4·3H2O and DMH and cytosine.Two cathodic peaks appear in the curve C,representing the same reduction reactions with those of the curve B,and the corresponding potentials are the same as those of the curve B.The comparison of the three curves indicates that no side reactions occurred when DMH and cytosine were added to the electrolyte,which was consistent with the cathodic current efficiency of 100%.
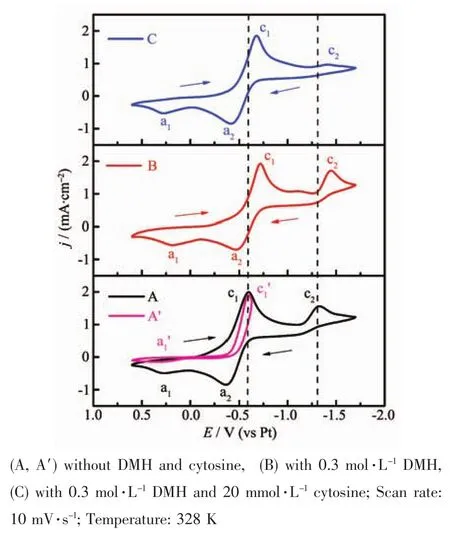
Fig.8 Cyclic voltammograms recorded at the GCE in a solution of 0.05 mol·L-1HAuCl4·3H2O in[BMIm][BF4]
3 Conclusions
It is first time that the golden-yellow gold deposit with a thickness of 1.5 μm can be obtained in ionic liquid [BMIm][BF4]containing HAuCl4·3H2O and DMH and cytosine.DMH and cytosine had obvious effects on brightening deposits and refining grains.The optimum concentrations of DMH and cytosine were 0.3 mol·L-1and 20 mmol·L-1,respectively.Linear sweep voltammogramsand cyclic voltammograms indicated that DMH may coordinate with Au3+and Au+,which increasesd the cathodic polarization,and the addition of cytosine on the basis of DMH could make the current density lower in the second renduction process,which was related to the adsorption of cytosine on the generated gold nuclei.The interaction between cytosine and DMH was synergism that made gold deposits bright and crystal grains refined.The cathodic current efficiency was 100%,indicating no side reactions occur,which can be confirmed by the cyclic voltammograms.Besides,cyclic voltammograms revealed that the reduction of Au3+to Au in the bath wasa two-step reduction process,including the reduction of Au3+to Au+,and further,Au+to Au.
[1]Dimitrijevic'S,Rajcˇic'-Vujasinovi M,Alagic'S,et al.Electrochim.Acta,2013,104:330-336
[2]Christie I R,Cameron B P.Gold Bull.,1994,27(1):12-20
[3]Watanabe H,Hayashi S,Honma H.J.Chem.Soc.,1999,146(2):574-579
[4]Kato M,Okinaka Y.Gold Bull.,2004,37(1/2):37-44
[5]Green T A.Gold Bull.,2007,40(2):105-114
[6]Sadyrbaeva T Z.Sep.Sci.Technol.,2012,86:262-265
[7]Seddon K R.J.Chem.Technol.Biotechnol.,1997,68(4):351-356
[8]Endres F,El Abedin S Z.Chem.Chem.Phys.,2006,8(18):2101-2116
[9]Abbott A P,McKenzie K.J.Phys.Chem.Chem.Phys.,2006,8(37):4265-4279
[10]Ueda M,Inaba R,Ohtsuka T.Electrochim.Acta,2013,100:281-284
[11]Shimamura O,Yoshimoto N,Matsumoto M,et al.J.Power Sources,2011,196(3):1586-1588
[12]Wibowo R,Aldous L,Rogers E I,et al.J.Phys.Chem.C,2010,114(8):3618-3626
[13]Endres F.ChemPhysChem,2002,3(2):144-154
[14]Yang P,Zhao Y,Su C,et al.Electrochim.Acta,2013,88:203-207
[15]Eugenio S,Rangel C M,Vilar R,et al.Electrochim.Acta,2011,56(28):10347-10352
[16]Pavlovich G Z,Luthy R G.Water Res.,1988,22(3):327-336
[17]Liu A,Ren X,An M,et al.Sci.Rep.,2014,4:3837
[18]Oyaizu K,Ohtani Y,Shiozawa A,et al.Inorg.Chem.,2005,44(20):6915-6917
[19]Ren X,Song Y,Liu A,et al.Electrochim.Acta,2015,176:10-17
[20]Lyu Z Y,Li A Q,Fei Y,et al.Electrochim.Acta,2013,109:136-144
[21]Zhang Q L,Zhou D L,Li Y F,et al.Microchim.Acta,2014,181(11/12):1239-1247
[22]De Sá A I,Eugénio S,Quaresma S,et al.Surf.Coat.Technol.,2013,232:645-651
[23]Katayama Y,Endo T,Miura T,et al.J.Electrochem.Soc.,2014,161(3):D87-D91
[24]Cullity B D.Element of X-ray Diffraction.Massachusetts:Addision-Wesley,1978:32-106
[25]Xu X H,Hussey C L.J.Electrochem.Soc.,1992,139(11):3103-3108
[26]Oyama T,Okajima T,Ohsaka T.J.Electrochem.Soc.,2007,154(6):D322-D327
Electrodeposition of Bright Gold Deposits in Ionic Liquid[BMIm][BF4]
SONG Yun-He YANG Pei-Xia LIAN Ye FENG Zhong-Bao ZHANG Jin-Qiu AN Mao-Zhong*
(State Key Laboratory of Urban Water Resource and Environment,School of Chemistry and Chemical Engineering,Harbin Institute of Technology,Harbin 150001,China)
A golden-yellow gold deposit with a thickness of 1.5 μm was electrodeposited from the bath with 5,5-dimethylhydantoin (DMH)as the complexing agent and cytosine as the additive in [BMIm][BF4]solution.The cathodic current efficiency was nearly 100%during electrodepositing.SEM and XRD results indicatd that DMH and cytosine had significant effects on the surface morphologies and phase structure of the deposits.The grain size and roughness of gold deposits decreased adding DMH and cytosine into the bath.Moreover,the Au(111)plane also became more obvious.The influences of DMH and cytosine were studied by the linear sweep voltammetry(LSV).The gold complexes[Au(DMH)4]-,[Au(DMH)2]-can be formed by DMH and Au3+,Au+,and the surface convert process was inhibited in the reduction process.The addition of DMH increased the cathodic polarization,which can brighten gold deposits and refine crystal grains of gold deposits.Cytosine was be absorbed on Au nuclei,and it had synergistic effects with DMH on brightening gold deposits and refining crystal grains.Cyclic voltammetry (CV)was used to investigated the electrochemical behavior and the results revealed that the reduction of Au3+to Au had a two-step reduction process.The first reduction process was Au3+to Au+,and the second process was Au+to Au,and the addition of DMH and cytosine did not bring side reactions.
gold deposit;ionic liquid;electrodeposition;5,5-dimethylhydantoin;cytosine
O614.123;TQ153.1
A
1001-4861(2018)01-0142-09
10.11862/CJIC.2018.020
2017-04-11。收修改稿日期:2017-09-04。
国家自然科学基金(No.51474080)资助项目。
*通信联系人。 E-mail:mzan@hit.edu.cn
