基于邻苯二甲酰-L-丙氨酸配体的两例Cuギ纯手性同质多晶配合物:合成与结构
2018-01-04李孟丽曾艳丽宋会花
李孟丽 常 旭 曾艳丽 宋会花*,
(1河北师范大学化学与材料科学学院,石家庄 050024)
(2深州市中学,深州 053800)
基于邻苯二甲酰-L-丙氨酸配体的两例Cuギ纯手性同质多晶配合物:合成与结构
李孟丽1,2常 旭1曾艳丽1宋会花*,1
(1河北师范大学化学与材料科学学院,石家庄 050024)
(2深州市中学,深州 053800)
合成了2例Cuギ的纯手性同质多晶[CuL2(Phen)](HL=邻苯二甲酰-L-丙氨酸,Phen=1,10-菲咯啉),并对其进行了表征。X射线单晶衍射测定表明,邻苯二甲酰-L-丙氨酸在2例配合物中采取了相同的配位方式,Cuギ原子具有变形的八面体配位环境,分别和2个邻苯二甲酰-L-丙氨酸离子上的2个羧基氧原子,1个1,10-菲咯啉的2个氮原子配位。不同的是这2例同质多晶显示出不同的颜色,并且配合物1属于单斜晶系,C2空间群,配合物2属于正交晶系,P212121空间群。配合物1通过π…π堆积作用形成一维链状结构,配合物2是简单小分子结构。此外,DFT理论研究表明,配合物1具有较低的能量,稳定性更高。
手性铜ギ配合物;晶体结构;同质多晶;异构体
0 Introduction
Concomitant polymorphism refers to the appearance of two or more polymorphs within the same crystal batch[1-2].Polymorphism is a well-established phenomenon with the same substance having different crystal forms[3-4].The crystallization process leading to a polymorph is generally sensitive to variation in the conditions of temperature,pressure and/or the manner in which the crystals are obtained[5-7].Supramolecular isomerism or polymorphism is of particular importance because the superstructure plays an essential role in determining the properties of crystalline materials[8].For example,in drug industry,the drug activity of a material might change abruptly from one polymorph to another[9].Therefore,the study of polymorphism has gained considerable impetus not only in fundamental research,but also in industrial interest,especially for pharmaceutical industry[10-13].The concomitant polymorphism are common in the synthesis of organics[14-17].Though organic matter concomitant polymorphs occur frequently,the observation of metal-organic complex concomitant polymorphs is not very common.Despite some scattered successful examples of polymorph have reported[18-19],it is also believed that the discovery of concomitant polymorphs is often serendipitous and gaining control over the crystallization process in order to selectively obtain a desired polymorph or suppress an undesired one is hard,and the success of controlling and obtaining a new polymorph remains a major challenge to this day.Subsequently,this may enable us to design and control crystallization of coordination networks by organic ligands and transmetal ions.
Coordination polymers by amino acid or amino acid derivatives are more importance in the bioorganic chemistry, due to the antimicrobial activities,antipyrotic activities,binding to DNA,and interaction with the cell membrane[20-21].N-phthalyl-L-alanine(HL,Scheme 1)is a derivative of amino acids,which holds diverse function groups and can give various possibilities to form coordination.Some coordination polymers by alanine and alanine derivatives have been obtained successfully[22-24].However,there is few report of coordination compound prepared with ligand HL[25].So we have prepared the ligand HL,and have been investigating its complexes with transition metals.The reaction of Cu(CH3COO)2·H2O,HL and 1,10-phenathroline(Phen)produced unexpectedly two novel concomitant isomers 1 and 2,namely,the simultaneous crystallization of different forms of the same species([CuL2(Phen)]).The complexeswere characterized by elemental analyses,IR and single crystal X-ray diffraction.Furthermore,the energy of them were carried with DFT theoretical calculation.

Scheme 1 Structure of N-phthalyl-L-alanine(HL)
1 Experimental
1.1 Materials and methods
The ligand HL was prepared by the reported method[26].Starting materials(phthalic anhydride and L-alanine)for the synthesis of the ligand HL were of reagent grade and were used without further purification.Other reagents and solvents for syntheses were purchased from commercial sources and were used without further purification.Elemental analyses(C,H and N)were performed on an Elemental Vario EL elemental analyzer.The infrared spectrum (400~4 000 cm-1)was recorded from KBr pellets on a FTIR-8900 spectrophotometer.
1.2 DFT calculations
Calculations were performed on polymorphs 1 and 2,taking molecular structures from the X-ray determined coordinates as the starting geometries.Then,equilibrium geometries were fully optimized at the DFT M06-2X/6-31G**level using the GAUSSIAN 09W program package[27].Harmonic frequencies were calculated based on the equilibrium geometries.
1.3 Synthesis of the complex[CuL2(Phen)]
A ethanol solution (5 mL)of HL (21.9 mg,0.1 mmol)and a methanol solution (5 mL)of 1,10-phenathroline (9.9 mg,0.05 mmol)were mixed by stirring.Cu(CH3COO)2·H2O(10 mg,0.05 mmol)in 5 mL H2O was added slowly to this reaction mixture with continuous stirring.Single-crystals were grown by slow evaporation at room temperature.Green and blue block shape crystals of 1 and 2 suitable for X-ray diffraction were obtained simultaneously within one week(Fig.1 and Fig.2).Complexes 1 and 2 possess the same chemical composition formulated as[CuL2(Phen)].Anal.Calcd.(%):C 60.04,H 3.56,N 8.24;Found(%):C 59.90,H 3.56,N 7.86 for complex 1;Found(%):C 59.84,H 3.59,N 5.24 for complex 2;IR for 1(cm-1):3 456(b),3 060(w),2 993(w),2 943(w),1 772~1 761(d),1 708(vs),1 603(vs),1 520(s),1 471~1 453(d),1 428(m),1 396(s),1 349(m),1 281(m),1 198(m),1 177(m),1 151(s),1 084(s),1 023(s),922(m),884(m),848(m),807(w),779(m),724(s),640(m),601(w),558(w),532(m),461(w).IR for 2(cm-1):3 449(b),2 991(w),2 942(w),1 772~1 760(sh),1 708(vs),1 626(vs),1 522(w),1 452(w),1 430(m),1 391(vs),1 342(m),1 278(m),1 175(w),1 153(m),1 074(sh),1 023(m),918(w),884(m),848(w),784(w),723(s),641(m),531(m),436(w).

Fig.1 Photograph of the two forms of[CuL2(Phen)]growing in MeOH/EtOH(1∶1,V/V)

Fig.2 Photograph of the two complex,1 gives the green diamond-like crystals and 2 is the blue diamond-like crystals
1.4 X-ray crystallography
Single-crystal X-ray diffraction data for all complexes were recorded on a Bruker SMART-CCD area detector diffractometer using graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 298(2)K by the ω-φ scan technique.The structures were solved by direct methods using the SHELXS-97 program,and all non-hydrogen atoms were refined anisotropically by full-matrix least-squares methods on F2using the SHELXL-97 program[28].Hydrogen atoms were added in geometrical positions and were not refined.A semiempirical absorption correction was applied to the intensity data using SADABS[29-30].A summary of the crystallographic data and refinement parameters is given in Table 1.Selected bond lengths and angles are given in Table 2 and 3.
CCDC:727552,1;727553,2.

Table1 Crystal data and structure refinement for complexes 1 and 2

Continued Table 1

Table2 Selected bond distances(nm)and angles(°)for complex 1

Table3 Selected bond distances(nm)and angles(°)for complex 2

Continued Table 3
2 Results and discussion
Co-crystallization of complexes 1 and 2 are obtained by the self-assembly reaction of Cu(CH3COO)2·H2O,HL and Phen simultaneously.The X-ray crystallographic determination reveal that 1 and 2 have the same composition formulated as[CuL2(Phen)].Nevertheless,they form different crystal structures.Complex 1 is a 1D supramolecular double chains formed by π … π interaction,but complex 2 is a separate mononuclear small molecule.
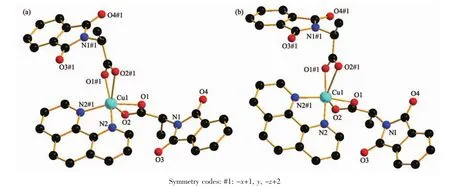
Fig.3 (a)View of the coordination of Cuギ ions in complex 1;(b)View of the coordination of Cuギ ions in complex 2
Complex 1 crystallizes in the monoclinic space group C2,and each asymmetry unit comprises of one Cuギion,two L ligands and one Phen molecule.Fig.3a shows the principal structural features with atomlabeling scheme.The central Cuギis in a slightly distorted octahedral geometry and coordinated by two pairs of oxygen atoms(O1,O1#1,O2,O2#1)from the carboxyl groups of two different L ligands and two nitrogen atoms (N2,N2#1)from one Phen.The O1 and N2#1 are in the axial positions and the O1#1,O2,O2#1 and N1 atoms are in the equatorial position.The Cu-O bond lengths are Cu(1)…O(1)0.197 50(19)nm,Cu(1)…O(1)#1 0.197 50(19)nm,Cu(1)…O(2)0.249 3(2)nm,Cu(1)…O(2)#1 0.249 3(2)nm,respectively(Table 2).All the Cu-N bond lengths are 0.200 8 nm.The angles around Cu center range from 57.57(7)°to 157.28(8)°.In complex 1,two L ligands adopt thesamecoordination modes(asymmetrical bidentate chel-ating mode)to coordinate the center Cuギion with two carboxylic oxygen atoms.Phen ligand presents symmetrical bidentate chelating mode.Fig.4 clearly shows that the adjacent unit connectes with each other through π … π stacking interactions between the aromatic rings moiety of the L ligands to form a 1D double chains,with the distance of face-toface about 0.366 4 nm.The adjacent Cu…Cu distance is 1.421 6 nm.
The molecular structure of complex 2 is shown in Fig.3b.The crystal of complex 2 belongs to orthorhombic,space group P212121.The Cuギion in complex 2 also displays six-coordinated octahedral geometry.The Cu-O bond lengths are Cu(1)…O(1)0.194 86(16)nm,Cu(1)…O(1)#1 0.194 86(16)nm,Cu(1)…O(2)0.261 18(19)nm,Cu(1)…O(2)#1 0.261 18(19)nm,respectively.The Cu-N bond length is 0.199 95(19)nm(Table 3).The angles around Cu center range from 55.52(6)°to 166.30(7)°.The Cu(1)… O(1)bond lengths of complex 1 are longer than complex 2,but Cu(1)…O(2)bond lengths of complex 1 are shorter than complex 2,while the Cu-N bond lengths of complex 1 are longer than 2.In addition,Cu(1)…O(2)bond lengths ofcomplex 1 and 2 are longer than Cu(1)…O(1)due to a Jahn-Teller effect[31].
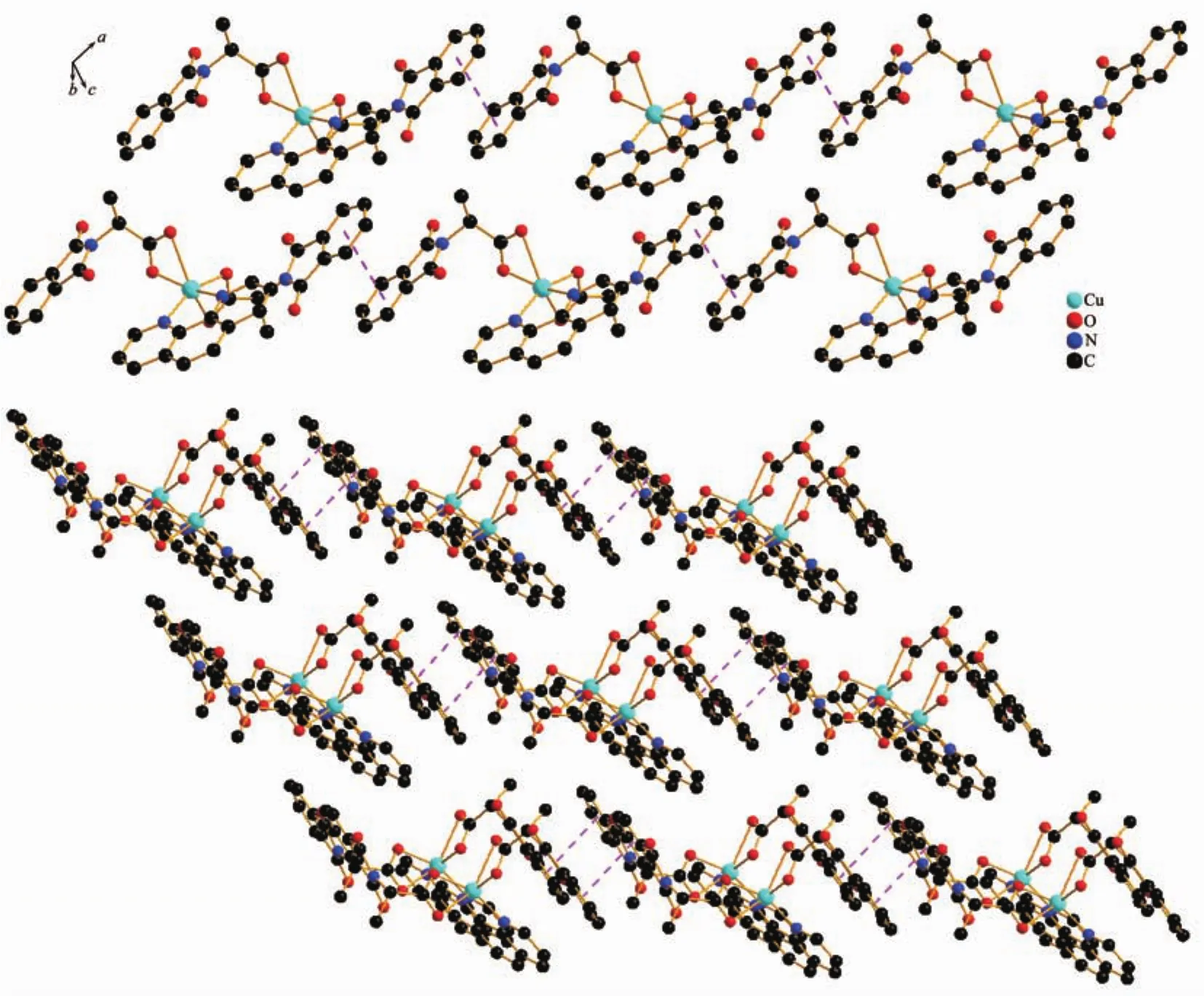
Fig.4 Packing diagram in complex 1
As mentioned earlier,complexes 1 and 2 are concomitant polymorphs of each other.Concomitant polymorphs arise when a material crystallizes in two or more forms out of the same reaction mixture[2].The concomitantpolymorphssometimeshavedifferent colors,but the origin of this phenomenon,called colour polymorphism or chromatomorphism,is not always clear and may be due to different reasons[32].There are at least three possible reasons for the different colors of the polymorphs:the first is different molecularconformations;the second isdifferent intermolecular interactions thatcan theoretically influence the electronic structure of molecules;the third is different molecular environment and crystal packing motifs.Through the careful analysis of the molecule structure of complexes 1 and 2 as stated above.The two forms have distinct structure,the adjacent unit of complex 1 connected with each other through π … π stacking interactions to form a 1D supramoleculardouble chains,butthere are no significant weak interaction been observed for complex 2.We hypothesized that the different intermolecular interactions influence the electronic structure of molecules,so the physical differences of the two polymorphs are revealed in their color[33].
Concomitant polymorphs can generally be described.Two or more polymorphs appear at the same time from the same mother liquor so-called concomitant polymorphs[2].Comparing the stability of complexes 1 and 2,we suppose that 1 has high relative stability,because complex 1 is a 1D supramolecular double chain,with the distance of face-toface about 0.3664 nm.While complex 2 is a separate mononuclear small molecule.The adjacent unit of complex 2 do not connect with each other through π…π stacking interaction between the aromatic rings moiety of the L ligands,with the distance of face-toface about 0.419 7 nm.The relative stability of the two polymorphs were test.The calculated densities of the two polymorphs are 1.536 and 1.491 g·cm-3,respectively,and according to the well known“density rule”[32],the polymorph 1 with the higher density is most likely to be more stable.Furthermore,all of the calculations in this work were carried out by using the GAUSSIAN 09W program package.Throughthe calculated energy comparison the two monomer between the complexes 1 and 2,-3 770.071 136 7 hartree and-3 770.067 595 7 hartree,respectively.It is obviously that complex 1 is lower in energy than 2 for about 0.003 541 hartree,suggesting that 2 is less stable than 1.In addition,the calculated interaction energy of the dimer of 1 without BBSE is 74.1 kJ·mol-1,and the interaction energy of the dimer of 2 with BBSE is 51.8 kJ·mol-1.On the basis of the above analysis,we find that the complex 1 is more stable than 2.The calculation and packing forms are also uniform with the “density rule”.The apparently high density of the complex 1 is consistent with its closest packing with significant π…π interactions between adjacent unit,which are obviously absent in complex 2[33].
Good quality crystals of ploymorphs 1 and 2 can be obtained from a solution of VCH3OH∶VC2H5OH∶VH2O=1∶1∶1 by a slow evaporation process.In addition,much effort had been made from hydrothermal synthesis method in different temperatures,solvents,metal salts and reaction time,but a blue precipitate was formed.In order to optimize synthetic methods and reaction conditions,a series of parallel experiments were conducted using the conventional solution method with different reaction conditions,such as nPhen∶nMギ∶nL,solvent ratio and metal salt.When the nPhen∶nMギ∶nLis 1∶1 ∶2 and VCH3OH∶VC2H5OH∶VH2Ois 1 ∶1 ∶1.Concomitant polymorphism are obtained.As is known to all,the water,ethanol and methanol are polar with different dipoles.These effects might also be related to the energy of the system.As mentioned above,Crystal structures are readily built-up,because they provide a facile means of reducing the energy of the system,rendering the crystalline state more stable.On the other hand,many parallel experiments were made using the different metal salts(Cu(NO3)2,CuSO4·5H2O,Cu(CH3COO)2·H2O),when the metal salt is replaced by Cu(CH3COO)2·H2O,the concomitant polymorphs have been obtained.It is worth noting that the presence of metal salt Cu(CH3COO)2·H2O in the system is necessary for successful preparation because no crystals are formed otherwise.The experiments results show that the CH3COO-plays an important role in the process of crystal formation.
The IR spectra (Fig.S1)of all complexes showed a distinct strong band appeared in the 3 460~3 440 cm-1range corresponding to the stretching vibration of the uncoordinated NH group of HL[34].In addition,the IR spectra of all complexes exhibited a strong band in the 1 630~1 600 cm-1range and is assigned to the stretching vibration of the coordinated carboxylate groups.Accordingly,the absence of any band in the 3 000~2 500 cm-1region in the IR spectra of the complexes suggests coordination of the COO-group of the N-protected amino acids to the central metal ions.In the case of two complexes,where the appearance of three bands at 1 772,1 760 and 1 708 cm-1reveals the incoordination of the benzoyl group of the N-phthalyl alanine acid and benzene ring.However,for all complexes the band appearing at 1 430~1 391 cm-1can likely be ascribed to the symmetric vibration of the coordinated carboxylate group[11].The Δν(COO)values(ν(COO)as-ν(COO)s< 200 cm-1)are consistent with the bidentate coordination of the carboxylate group of N-acetyl-derivatives of the amino acids[45].
3 Conclusions
In summary,two unusual concomitant polymorphs,[CuL2(Phen)],have observed unexpectedly.They can be discriminated by their visible appearance as they have different colors.We suppose that different colors are caused by different intermolecular interactions.The relative stability of the two polymorphs were tested.Our calculation results suggest that complex 1 is lower in energy and more stable than 2.Moreover,we optimize synthetic methods and reaction conditions(nPhen∶nMギ∶nL=1∶1∶2,VCH3OH∶VC2H5OH∶VH2O=1∶1∶1).Many parallel experiments were made using the different metal salts,and we found that two concomitant polymorphshave been detected only metalsalt Cu(CH3COO)2·H2O existing in the system.Although the CH3COO-is not coordinated with metal ion,the experiments result clearly reveal the significant effects of CH3COO-in the fabrication of crystals.It is also the subtlest and most challenging to control over the crystallization process in order to selectively obtain a desired polymorph.Polymorphs 1 and 2 had been obtained unexpectedly,which urge us to further extend this interesting system in the future.Furthermore,our report should enrich the crystal engineering approach for generating coordination polymers.
Supporting information is available at http://www.wjhxxb.cn
[1]Linas A,Burley J C,Prior T J,et al.Cryst.Growth Des.,2008,8:114-118
[2]Bernstein J,Davey R J,Henck J O.Angew.Chem.Int.Ed.,1999,38:3440-3461
[3]Braga D,Cojazzi G,Emiliani D,et al.CrystEngComm,2002,4:277-281
[4]Braga D,Cojazzi G,Paolucci D,et al.CrystEngComm,2001,38:1-3
[5]Anirban K,Rupam J S,Jubaraj B B,et al.Eur.J.Inorg.Chem.,2007,5:643-647
[6]Hu P,Yao Z J,Wang J Q,et al.Organometallics,2011,30:4935-4940
[7]Fromm K M,Doimeadios J L,Robin A Y,et al.Chem.Commun.,2005,36:4548-4550
[8]John S F,Lesibana P L,Orde Q M,et al.CrystEngComm,2008,10:740-747
[9]Mijanuddin M,Jana A D,Drew M G B,et al.Polyhedron,2009,28:665-672
[10]O′Donnell L J,Arvind A S,Haong P,et al.Gut,1992,33(7):947-949
[11]Bailey M A,Ingram M J,Naughton D P,et al.Transition Met.Chem.,2008,33:195-202
[12]Kukovec B M,Venter G A,Oliver C L.Cryst.Growth Des.,2012,12:456-465
[13]Yang C Q,Zhang Z W,Zeng Y L,et al.CrystEngComm,2012,14:2589-2594
[14]Du M,Zhang Z H,Wang X G,et al.Cryst.Growth Des.,2006,6:1867-1875
[15]Eccles K S,Deasy R E,Fábián L,et al.CrystEngComm,2011,13:6923-6925
[16]Munshi P,Venugopala K N,Jayashree B S,et al.Cryst.Growth Des.,2004,4:1105-1107
[17]Bond A D,Haynes D A,Pask C M,et al.Dalton Trans.,2002,12:2522-2531
[18]Field J S,Ledwaba L P,Munro O Q,et al.CrystEngComm,2008,10:740-747
[19]Procopio E Q,Mauro M,Panigati M,et al.J.Am.Chem.Soc.,2010,132:14397-14399
[20]Peresypkina E V,Bushuev M B,Virovets A V,et al.Acta Crystallogr.Sect.B:Struct.Sci.,2005,B61:164-173
[21]Das L K,Biswas A,Gomez-García C J,et al.Inorg.Chem.,2014,53:434-445
[22]Moreira V F,Ortego M L,Williams C F.Organometallics,2012,31:5950-5957
[23]Zhuang G L,Chen W X,Zeng G N.CrystEngComm,2012,14:679-683
[24]Chen Z C,Zhang C B,Liu X L,et al.Aust.J.Chem.,2012,65:1662-1666
[25]Santiago G R,Beatriz G,Milena R K,et al.J.Organomet.Chem.,2009,694:2191-2197
[26]Frisch M J T G,Schlegel H B,Scuseria G E,et al.Gaussian 09,Revision A02,Gaussian Inc.,Wallingford,2009.
[27]Ghazzali M,Al-Farhan K,El-Fahama A,et al.Polyhedron,2010,29:2829-2832
[28]Nuria A A,Marques-G P,Mirte K,et al.Inorg.Chem.,2010,49:9655-9663
[29]Sahoo S C,Kundu T,Banerjee R.J.Am.Chem.Soc.,2011,133:17950-17958
[30]Sarma D,Ramanujachary K V,Lofland S E,et al.Inorg.Chem.,2009,48:11660-11676
[31]Qu Z R,Zhao H,Wang Y P,et al.Chem.Eur.J.,2004,10:53-60
[32]Yu L,Stephenson G A,Mitchell C A,et al.J.Am.Chem.Soc.,2000,122:585-591
[33]Wang J,Ding L Y,Yang C Q.CrystEngComm,2007,9:591-594
[34]Dong L J,Chu W,Zhu Q L,et al.Cryst.Growth Des.,2011,11:93-99
[35]Abdel-Rahman L H,Nasser L A E.Transition Met.Chem.,2007,32:367-373
Two Concomitant Polymorphs of Homochiral CuギCoordination Compounds Based on N-phthalyl-L-alanine:Syntheses and Crystal Structures
LI Meng-Li1,2CHANG Xu1ZENG Yan-Li1SONG Hui-Hua*,1
(1College of Chemistry and Material Sciences,Hebei Normal University,Shijiazhuang 050024,China)(2Shenzhou City Middle School,Shenzhou,Hebei 053800,China)
Two isomers of novel homochiral coordination compound[CuL2(Phen)](HL=N-phthalyl-L-alanine,Phen=1,10-phenathroline)have been synthesized simultaneously and structurally characterized by elemental analyses,IR and single crystal X-ray diffraction.The two polymorphs 1 and 2 exhibit different colors.HL in these two complexes adopts same conformations,the molecular structures vary only slightly between the two forms.Cuギions are all distorted octahedral geometry and coordinated by two pairs of oxygen atoms from two L and two nitrogen atoms from one Phen in complexes 1 and 2,with a monoclinic space group C2 and an orthorhombic space group P212121,respectively.Complex 1 is self-assembled to form 1D double chains through π…π stacking interactions of the aromatic rings of L ligands from two adjacent structures,with face-to-face distances of ca.0.366 4 nm.There is no stacking in the structure of complex 2.Furthermore,the DFT theoretical calculation shows that complex 1 is lower in energy and more stable than 2.CCDC:727552,1;727553,2.
copperギcomplex;crystal structure;polymorphism;conformers;homochiral
O614.121
A
1001-4861(2018)01-0083-09
10.11862/CJIC.2018.001
2017-05-29。收修改稿日期:2017-10-25。
河北省自然科学基金(No.B2012205040)和河北师范大学基金(No.L2016K03)资助项目。
*通信联系人。 E-mail:songhuihua@mail.hebtu.edu.cn;会员登记号:S06N6160M1006。
